Free-Running Cardiac and Respiratory Motion-Resolved Imaging: A Paradigm Shift for Managing Motion in Cardiac MRI?
Abstract
:1. Introduction
2. History and Rationale of Self-Gated Cardiac MRI
3. The Fully Automated Cardiac Free-Running Framework
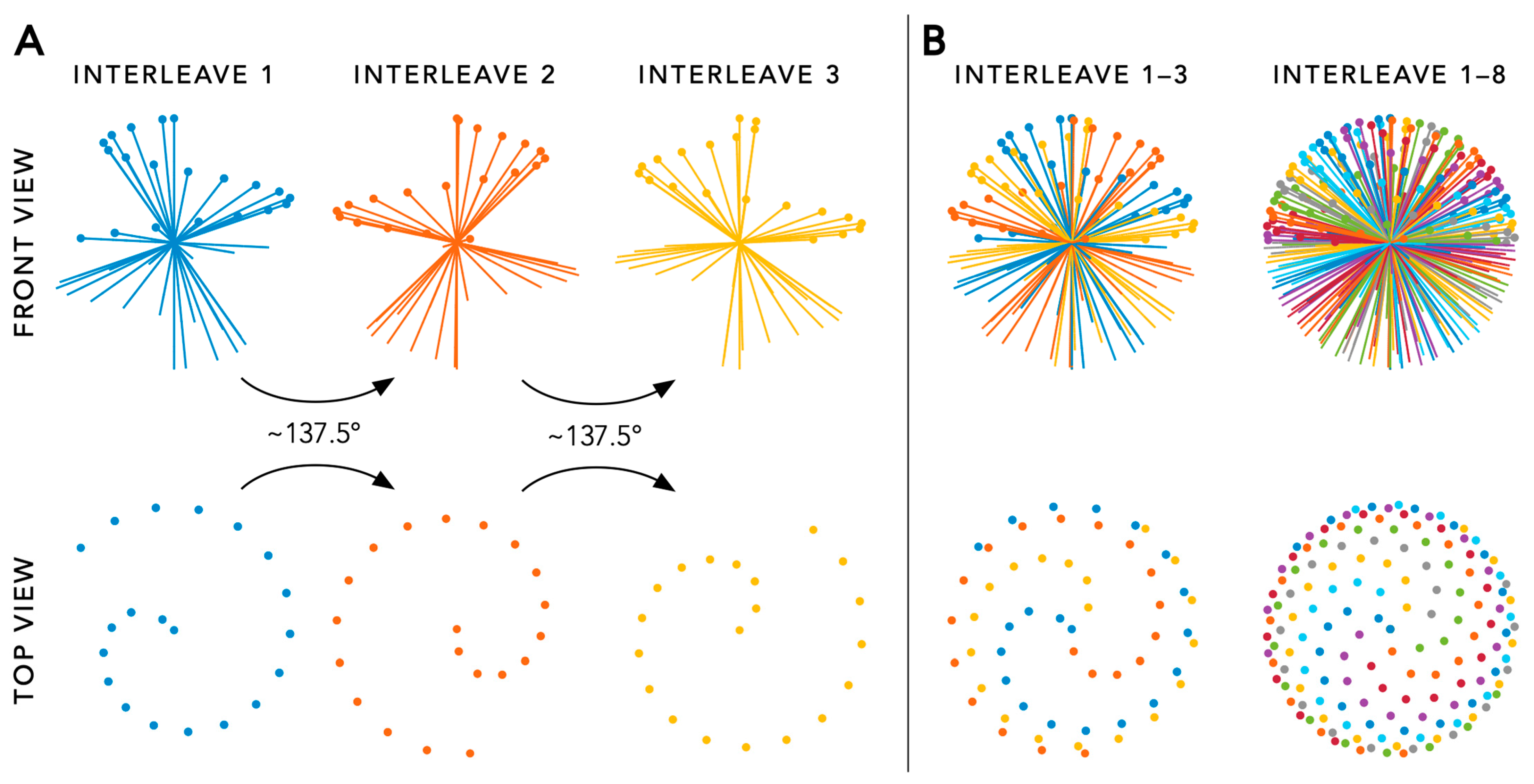
3.1. Acquisition
3.2. Reconstruction
3.3. Key Advantages
4. Recent Improvements to the Free-Running Framework
5. Clinical Applications
5.1. Anatomical Imaging and Coronary Angiography
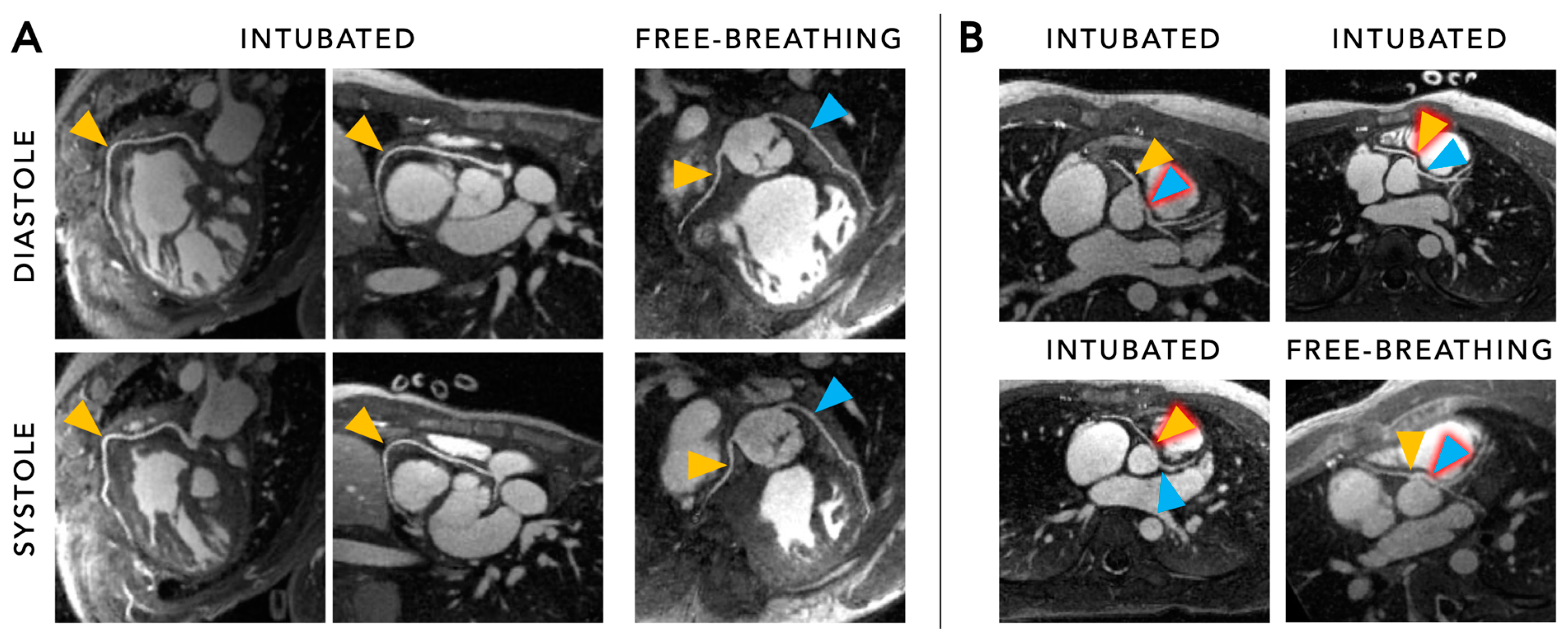
5.2. Functional Cine Imaging
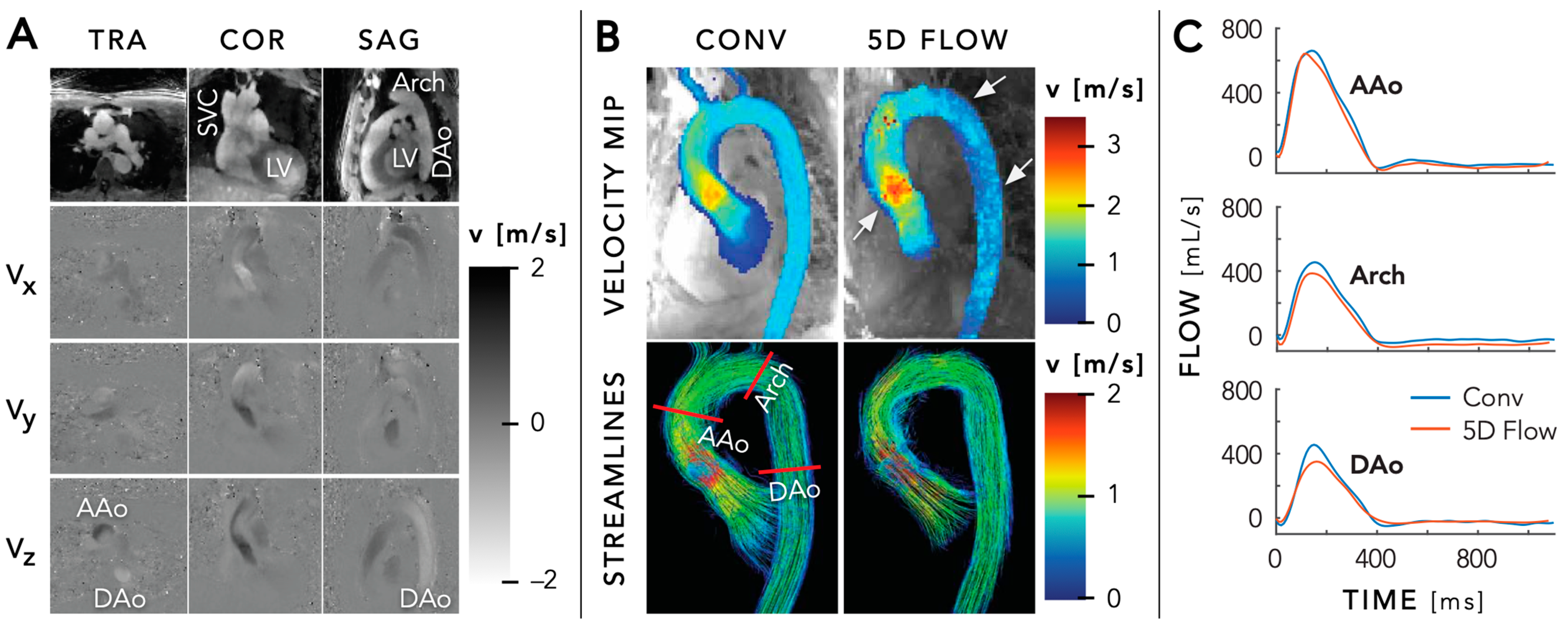
5.3. Flow Imaging
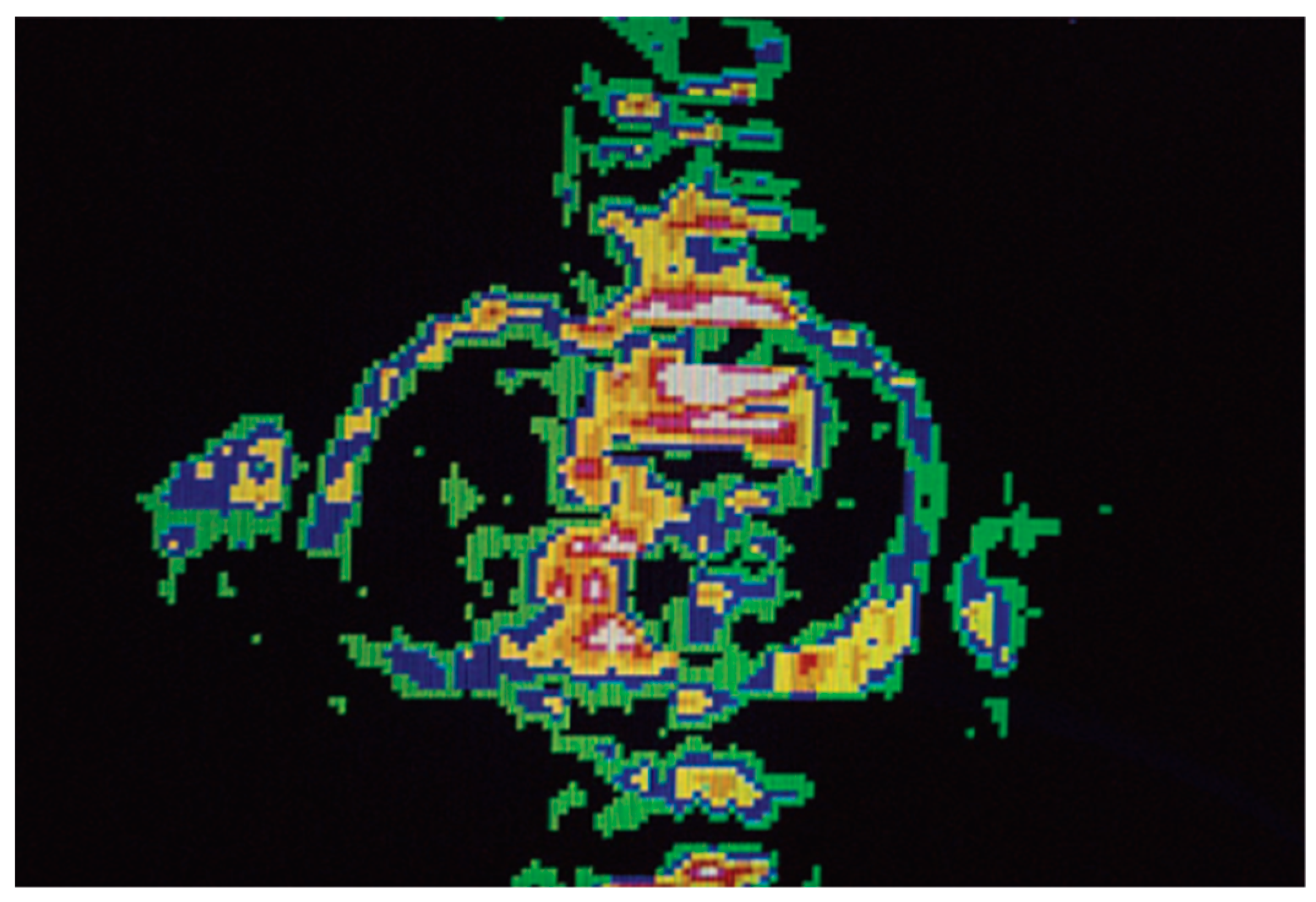
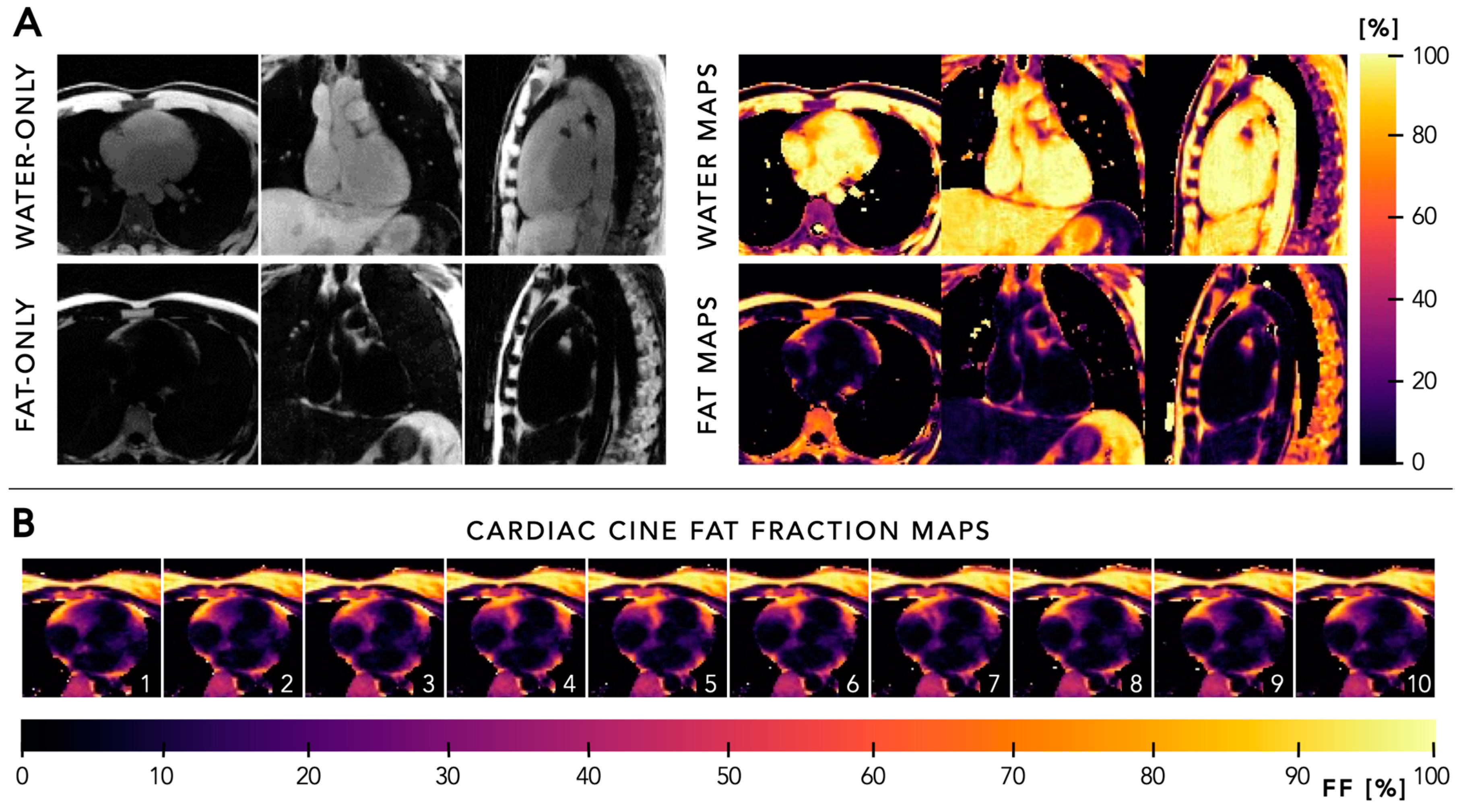
5.4. Quantitative Mapping
6. General Recommendations
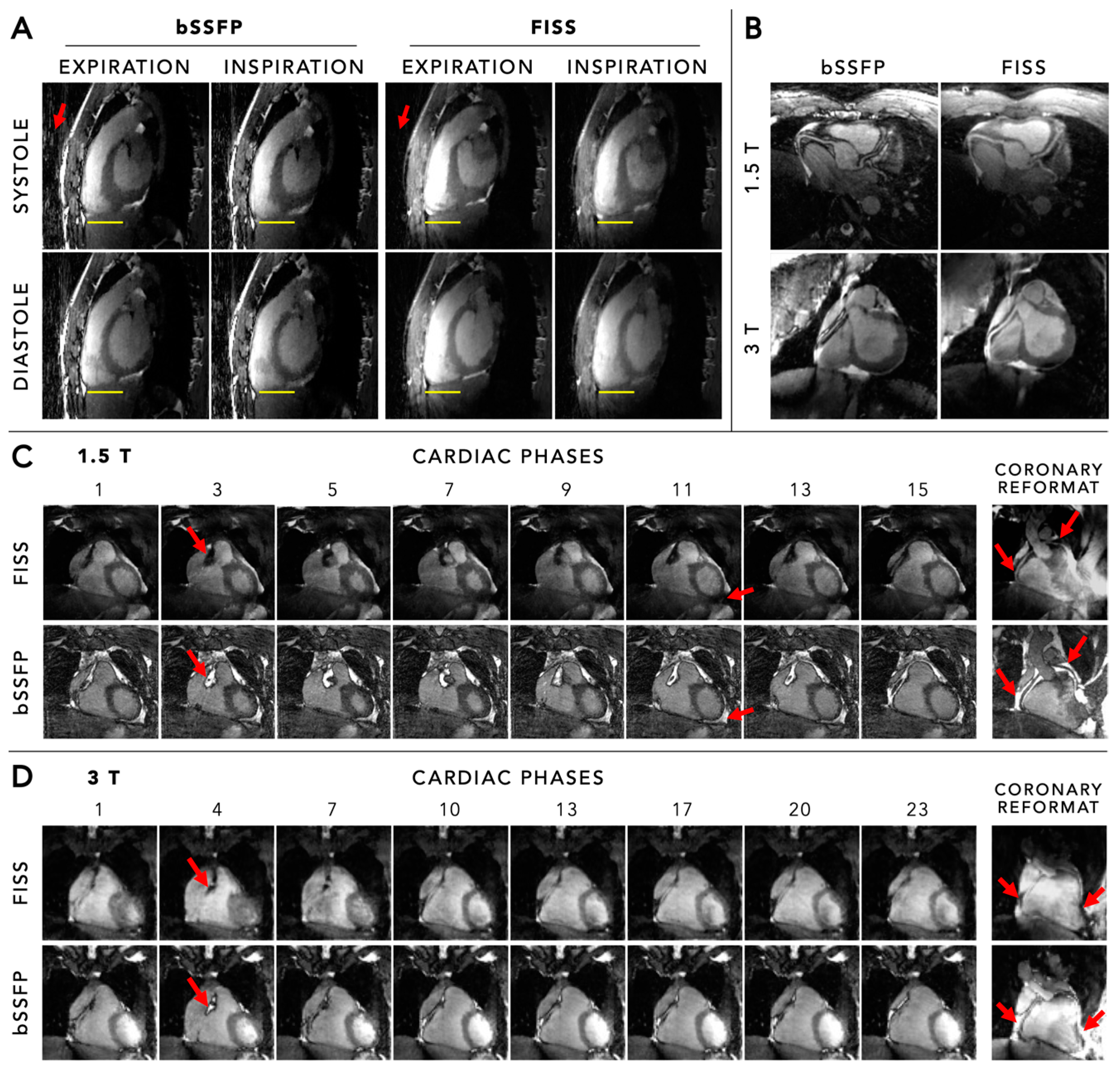
6.1. Fat Suppression
6.2. Field Strength Dependency
6.3. Role of Contrast Agents
7. Future Perspectives
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| AI | artificial intelligence |
| bSSFP | balanced steady-state free-precession |
| CAD | coronary artery disease |
| CAG | coronary angiography |
| CHD | congenital heart disease |
| CHESS | chemically selective saturation |
| CMRA | coronary magnetic resonance angiography |
| CS | compressed sensing |
| ECG | electrocardiogram |
| FISS | fast interrupted steady-state |
| fNAV | focused navigation |
| FRF | free-running framework |
| GIRF | gradient impulse response function |
| GRE | gradient echo |
| LIBRE | lipid-insensitive binomial off-resonant radiofrequency excitation |
| MPR | multiplanar reconstruction |
| MRI | magnetic resonance imaging |
| PCA | principal component analysis |
| PT | pilot tone |
| SG | self-gating/self-gated |
| SI | superior-inferior |
| SNR | signal-to-noise ratio |
| SIMBA | similarity-driven multi-dimensional binning algorithm |
| XD-GRASP | extra-dimensional golden-angle radial sparse parallel |
References
- Mallard, J.R. Magnetic resonance imaging-the Aberdeen perspective on developments in the early years. Phys. Med. Biol. 2006, 51, R45–R60. [Google Scholar] [CrossRef] [PubMed]
- Edelman, R.R.; Manning, W.J.; Burstein, D.; Paulin, S. Coronary arteries: Breath-hold MR angiography. Radiology 1991, 181, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Ehman, R.L.; Felmlee, J.P. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989, 173, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.C.; White, R.D.; Laub, G.; McVeigh, E.R.; Li, D.; Simonetti, O.P. Self-gated cardiac cine MRI. Magn. Reson. Med. 2004, 51, 93–102. [Google Scholar] [CrossRef]
- Stehning, C.; Bornert, P.; Nehrke, K.; Eggers, H.; Stuber, M. Free-breathing whole-heart coronary MRA with 3D radial SSFP and self-navigated image reconstruction. Magn. Reson. Med. 2005, 54, 476–480. [Google Scholar] [CrossRef]
- Piccini, D.; Littmann, A.; Nielles-Vallespin, S.; Zenge, M.O. Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI. Magn. Reson. Med. 2011, 66, 1049–1056. [Google Scholar] [CrossRef]
- Piccini, D.; Feng, L.; Bonanno, G.; Coppo, S.; Yerly, J.; Lim, R.P.; Schwitter, J.; Sodickson, D.K.; Otazo, R.; Stuber, M. Four-dimensional respiratory motion-resolved whole heart coronary MR angiography. Magn. Reson. Med. 2017, 77, 1473–1484. [Google Scholar] [CrossRef]
- Piccini, D.; Monney, P.; Sierro, C.; Coppo, S.; Bonanno, G.; van Heeswijk, R.B.; Chaptinel, J.; Vincenti, G.; de Blois, J.; Koestner, S.C. Respiratory self-navigated postcontrast whole-heart coronary MR angiography: Initial experience in patients. Radiology 2014, 270, 378–386. [Google Scholar] [CrossRef]
- Coppo, S.; Piccini, D.; Bonanno, G.; Chaptinel, J.; Vincenti, G.; Feliciano, H.; van Heeswijk, R.B.; Schwitter, J.; Stuber, M. Free-running 4D whole-heart self-navigated golden angle MRI: Initial results. Magn. Reson. Med. 2015, 74, 1306–1316. [Google Scholar] [CrossRef]
- Feng, L.; Coppo, S.; Piccini, D.; Yerly, J.; Lim, R.P.; Masci, P.G.; Stuber, M.; Sodickson, D.K.; Otazo, R. 5D whole-heart sparse MRI. Magn. Reson. Med. 2018, 79, 826–838. [Google Scholar] [CrossRef]
- Feng, L.; Axel, L.; Chandarana, H.; Block, K.T.; Sodickson, D.K.; Otazo, R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2016, 75, 775–788. [Google Scholar] [CrossRef]
- Di Sopra, L.; Piccini, D.; Coppo, S.; Stuber, M.; Yerly, J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magn. Reson. Med. 2019, 82, 2118–2132. [Google Scholar] [CrossRef]
- Liu, J.; Nguyen, T.D.; Zhu, Y.; Spincemaille, P.; Prince, M.R.; Weinsaft, J.W.; Saloner, D.; Wang, Y. Self-gated free-breathing 3D coronary CINE imaging with simultaneous water and fat visualization. PLoS ONE 2014, 9, e89315. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Spincemaille, P.; Codella, N.C.; Nguyen, T.D.; Prince, M.R.; Wang, Y. Respiratory and cardiac self-gated free-breathing cardiac CINE imaging with multiecho 3D hybrid radial SSFP acquisition. Magn. Reson. Med. 2010, 63, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Larson, A.C.; Zhang, Q.; Simonetti, O.; Li, D. 4D radial coronary artery imaging within a single breath-hold: Cine angiography with phase-sensitive fat suppression (CAPS). Magn Reson Med. 2005, 54, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.; Ramsay, E.A.; Cunningham, C.H.; Plewes, D.B. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magn. Reson. Med. 2009, 61, 354–363. [Google Scholar] [CrossRef]
- Pang, J.; Sharif, B.; Fan, Z.; Bi, X.; Arsanjani, R.; Berman, D.S.; Li, D. ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function. Magn. Reson. Med. 2014, 72, 1208–1217. [Google Scholar] [CrossRef]
- Qi, H.; Jaubert, O.; Bustin, A.; Cruz, G.; Chen, H.; Botnar, R.; Prieto, C. Free-running 3D whole heart myocardial T(1) mapping with isotropic spatial resolution. Magn. Reson. Med. 2019, 82, 1331–1342. [Google Scholar] [CrossRef]
- Han, F.; Rapacchi, S.; Khan, S.; Ayad, I.; Salusky, I.; Gabriel, S.; Plotnik, A.; Finn, J.P.; Hu, P. Four-dimensional, multiphase, steady-state imaging with contrast enhancement (MUSIC) in the heart: A feasibility study in children. Magn. Reson. Med. 2015, 74, 1042–1049. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, F.; Rapacchi, S.; Nguyen, K.L.; Brunengraber, D.Z.; Kim, G.J.; Finn, J.P.; Hu, P. Accelerated ferumoxytol-enhanced 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) cardiovascular MRI: Validation in pediatric congenital heart disease. NMR Biomed. 2017, 30, e3663. [Google Scholar] [CrossRef]
- Moghari, M.H.; Barthur, A.; Amaral, M.E.; Geva, T.; Powell, A.J. Free-breathing whole-heart 3D cine magnetic resonance imaging with prospective respiratory motion compensation. Magn. Reson. Med. 2018, 80, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Ruijsink, B.; Nazir, M.S.; Cruz, G.; Prieto, C. Free breathing whole-heart 3D CINE MRI with self-gated Cartesian trajectory. Magn. Reson. Imaging. 2017, 38, 129–137. [Google Scholar] [CrossRef]
- Kustner, T.; Bustin, A.; Jaubert, O.; Hajhosseiny, R.; Masci, P.G.; Neji, R.; Botnar, R.; Prieto, C. Fully self-gated free-running 3D Cartesian cardiac CINE with isotropic whole-heart coverage in less than 2 min. NMR Biomed. 2021, 34, e4409. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef]
- Togawa, T.; Okai, O.; Oshima, M. Observation of blood flow E.M.F. in externally applied strong magnetic field by surface electrodes. Med. Biol. Eng. 1967, 5, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Ntsinjana, H.N.; Hughes, M.L.; Taylor, A.M. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J. Cardiovasc. Magn. Reson. 2011, 13, 51. [Google Scholar] [CrossRef]
- Uludag, O.; Kaya, R.; Tutak, A.; Dogukan, M.; Celik, M.; Dumlupinar, E. Effect of Anesthesia Applied for Magnetic Resonance Imaging on the Body Temperature of Pediatric Patients. Cureus 2019, 11, e5705. [Google Scholar] [CrossRef]
- Fonseca, L.G.F.; Garbin, M.; Bertolizio, G. Anesthesia for pediatric magnetic resonance imaging: A review of practices and current pathways. Curr. Opin. Anaesthesiol. 2023, 36, 428–434. [Google Scholar] [CrossRef]
- Roy, C.W.; Di Sopra, L.; Whitehead, K.K.; Piccini, D.; Yerly, J.; Heerfordt, J.; Ghosh, R.M.; Fogel, M.A.; Stuber, M. Free-running cardiac and respiratory motion-resolved 5D whole-heart coronary cardiovascular magnetic resonance angiography in pediatric cardiac patients using ferumoxytol. J. Cardiovasc. Magn. Reson. 2022, 24, 39. [Google Scholar] [CrossRef]
- Roy, C.W.; Heerfordt, J.; Piccini, D.; Rossi, G.; Pavon, A.G.; Schwitter, J.; Stuber, M. Motion compensated whole-heart coronary cardiovascular magnetic resonance angiography using focused navigation (fNAV). J. Cardiovasc. Magn. Reson. 2021, 23, 33. [Google Scholar] [CrossRef]
- Luo, J.; Addy, N.O.; Ingle, R.R.; Baron, C.A.; Cheng, J.Y.; Hu, B.S.; Nishimura, D.G. Nonrigid Motion Correction With 3D Image-Based Navigators for Coronary MR Angiography. Magn. Reson. Med. 2017, 77, 1884–1893. [Google Scholar] [CrossRef]
- Falcao, M.B.L.; Rossi, G.M.C.; Rutz, T.; Prsa, M.; Tenisch, E.; Ma, L.; Weiss, E.K.; Baraboo, J.J.; Yerly, J.; Markl, M.; et al. Focused navigation for respiratory-motion-corrected free-running radial 4D flow MRI. Magn. Reson. Med. 2023, 90, 117–132. [Google Scholar] [CrossRef]
- Heerfordt, J.; Whitehead, K.K.; Bastiaansen, J.A.M.; Di Sopra, L.; Roy, C.W.; Yerly, J.; Milani, B.; Fogel, M.A.; Stuber, M.; Piccini, D. Similarity-driven multi-dimensional binning algorithm (SIMBA) for free-running motion-suppressed whole-heart MRA. Magn. Reson. Med. 2021, 86, 213–229. [Google Scholar] [CrossRef]
- Romanin, L.; Milani, B.; Roy, C.W.; Yerly, J.; Bustin, A.; Si-Mohamed, S.; Prsa, M.; Rutz, T.; Tenisch, E.; Schwitter, J.; et al. Similarity-driven motion-resolved reconstruction for ferumoxytol-enhanced whole-heart MRI in congenital heart disease. PLoS ONE 2024, 19, e0304612. [Google Scholar] [CrossRef]
- Falcao, M.B.L.; Di Sopra, L.; Ma, L.; Bacher, M.; Yerly, J.; Speier, P.; Rutz, T.; Prsa, M.; Markl, M.; Stuber, M.; et al. Pilot tone navigation for respiratory and cardiac motion-resolved free-running 5D flow MRI. Magn. Reson. Med. 2022, 87, 718–732. [Google Scholar] [CrossRef]
- Vahle, T.; Bacher, M.; Rigie, D.; Fenchel, M.; Speier, P.; Bollenbeck, J.; Schafers, K.P.; Kiefer, B.; Boada, F.E. Respiratory Motion Detection and Correction for MR Using the Pilot Tone: Applications for MR and Simultaneous PET/MR Examinations. Invest. Radiol. 2020, 55, 153–159. [Google Scholar] [CrossRef]
- Speier, P.; Fenchel, M.; Rehner, R. PT-Nav: A novel respiratory navigation method for continuous acquisitions based on modulation of a pilot tone in the MR-receiver. Proc. ESMRMB 2015, 129, 97–98. [Google Scholar]
- Roy, C.W.; Milani, B.; Yerly, J.; Si-Mohamed, S.; Romanin, L.; Bustin, A.; Tenisch, E.; Rutz, T.; Prsa, M.; Stuber, M. Intra-bin correction and inter-bin compensation of respiratory motion in free-running five-dimensional whole-heart magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2024, 26, 101037. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Qureshi, I.M.; Shah, J.A.; Zaheer, M. Motion correction based reconstruction method for compressively sampled cardiac MR imaging. Magn. Reson. Imaging. 2017, 36, 159–166. [Google Scholar] [CrossRef]
- Di Carli, M.F.; Geva, T.; Davidoff, R. The Future of Cardiovascular Imaging. Circulation 2016, 133, 2640–2661. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Weberling, L.D.; Lossnitzer, D.; Frey, N.; Andre, F. Coronary Computed Tomography vs. Cardiac Magnetic Resonance Imaging in the Evaluation of Coronary Artery Disease. Diagnostics 2022, 13, 125. [Google Scholar] [CrossRef]
- Prince, M.R.; Zhang, H.L.; Chabra, S.G.; Jacobs, P.; Wang, Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J. X-ray Sci. Technol. 2003, 11, 231–240. [Google Scholar]
- Finn, J.P.; Nguyen, K.L.; Han, F.; Zhou, Z.; Salusky, I.; Ayad, I.; Hu, P. Cardiovascular MRI with ferumoxytol. Clin. Radiol. 2016, 71, 796–806. [Google Scholar] [CrossRef]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.S.; Nielsen, J.F.; Bernstein, M.A.; Markl, M.; Gatehouse, P.D.; Botnar, R.M.; Saloner, D.; Lorenz, C.; Wen, H.; Hu, B.S.; et al. Cardiovascular magnetic resonance phase contrast imaging. J. Cardiovasc. Magn. Reson. 2015, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Markl, M.; Frydrychowicz, A.; Kozerke, S.; Hope, M.; Wieben, O. 4D flow MRI. J. Magn. Reson. Imaging. 2012, 36, 1015–1036. [Google Scholar] [CrossRef]
- Bissell, M.M.; Raimondi, F.; Ait Ali, L.; Allen, B.D.; Barker, A.J.; Bolger, A.; Burris, N.; Carhall, C.J.; Collins, J.D.; Ebbers, T.; et al. 4D Flow cardiovascular magnetic resonance consensus statement: 2023 update. J. Cardiovasc. Magn. Reson. 2023, 25, 40. [Google Scholar] [CrossRef]
- Markl, M.; Schnell, S.; Wu, C.; Bollache, E.; Jarvis, K.; Barker, A.J.; Robinson, J.D.; Rigsby, C.K. Advanced flow MRI: Emerging techniques and applications. Clin. Radiol. 2016, 71, 779–795. [Google Scholar] [CrossRef]
- Wei, Z.; Whitehead, K.K.; Khiabani, R.H.; Tree, M.; Tang, E.; Paridon, S.M.; Fogel, M.A.; Yoganathan, A.P. Respiratory Effects on Fontan Circulation During Rest and Exercise Using Real-Time Cardiac Magnetic Resonance Imaging. Ann. Thorac. Surg. 2016, 101, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.E.; Yerly, J.; Piccini, D.; Di Sopra, L.; Roy, C.W.; Carr, J.C.; Rigsby, C.K.; Kim, D.; Stuber, M.; Markl, M. 5D Flow MRI: A Fully Self-gated, Free-running Framework for Cardiac and Respiratory Motion-resolved 3D Hemodynamics. Radiol. Cardiothorac. Imaging. 2020, 2, e200219. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yerly, J.; Di Sopra, L.; Piccini, D.; Lee, J.; DiCarlo, A.; Passman, R.; Greenland, P.; Kim, D.; Stuber, M.; et al. Using 5D flow MRI to decode the effects of rhythm on left atrial 3D flow dynamics in patients with atrial fibrillation. Magn. Reson. Med. 2021, 85, 3125–3139. [Google Scholar] [CrossRef]
- Reeder, S.B.; Hu, H.H.; Sirlin, C.B. Proton density fat-fraction: A standardized MR-based biomarker of tissue fat concentration. J. Magn. Reson. Imaging. 2012, 36, 1011–1014. [Google Scholar] [CrossRef]
- Bray, T.J.; Chouhan, M.D.; Punwani, S.; Bainbridge, A.; Hall-Craggs, M.A. Fat fraction mapping using magnetic resonance imaging: Insight into pathophysiology. Br. J. Radiol. 2018, 91, 20170344. [Google Scholar] [CrossRef]
- Chen, O.; Sharma, A.; Ahmad, I.; Bourji, N.; Nestoiter, K.; Hua, P.; Hua, B.; Ivanov, A.; Yossef, J.; Klem, I.; et al. Correlation between pericardial, mediastinal, and intrathoracic fat volumes with the presence and severity of coronary artery disease, metabolic syndrome, and cardiac risk factors. Eur. Heart J. Cardiovasc. Imaging. 2015, 16, 37–46. [Google Scholar] [CrossRef]
- Sironi, A.M.; Petz, R.; De Marchi, D.; Buzzigoli, E.; Ciociaro, D.; Positano, V.; Lombardi, M.; Ferrannini, E.; Gastaldelli, A. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabet. Med. 2012, 29, 622–627. [Google Scholar] [CrossRef]
- Mackowiak, A.L.C.; Roy, C.W.; Yerly, J.; Falcao, M.B.L.; Bacher, M.; Speier, P.; Piccini, D.; Stuber, M.; Bastiaansen, J.A.M. Motion-resolved fat-fraction mapping with whole-heart free-running multiecho GRE and pilot tone. Magn. Reson. Med. 2023, 90, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Daude, P.; Troalen, T.; Mackowiak, A.L.C.; Royer, E.; Piccini, D.; Yerly, J.; Pfeuffer, J.; Kober, F.; Gouny, S.C.; Bernard, M.; et al. Trajectory correction enables free-running chemical shift encoded imaging for accurate cardiac proton-density fat fraction quantification at 3T. J. Cardiovasc. Magn. Reson. 2024, 26, 101048. [Google Scholar] [CrossRef]
- Addy, N.O.; Wu, H.H.; Nishimura, D.G. Simple method for MR gradient system characterization and k-space trajectory estimation. Magn. Reson. Med. 2012, 68, 120–129. [Google Scholar] [CrossRef]
- Vannesjo, S.J.; Haeberlin, M.; Kasper, L.; Pavan, M.; Wilm, B.J.; Barmet, C.; Pruessmann, K.P. Gradient system characterization by impulse response measurements with a dynamic field camera. Magn. Reson. Med. 2013, 69, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bustin, A.; Cruz, G.; Jaubert, O.; Chen, H.; Botnar, R.M.; Prieto, C. Free-running simultaneous myocardial T1/T2 mapping and cine imaging with 3D whole-heart coverage and isotropic spatial resolution. Magn. Reson. Imaging. 2019, 63, 159–169. [Google Scholar] [CrossRef]
- Shaw, J.L.; Yang, Q.; Zhou, Z.; Nguyen, C.; Li, D.; Christodoulou, A.G. Free-breathing, non-ECG, continuous myocardial T(1) mapping with cardiovascular magnetic resonance multitasking. Magn. Reson. Med. 2019, 81, 2450–2463. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, A.G.; Shaw, J.L.; Nguyen, C.; Yang, Q.; Xie, Y.; Wang, N.; Li, D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat. Biomed. Eng. 2018, 2, 215–226. [Google Scholar] [CrossRef]
- Phair, A.; Cruz, G.; Qi, H.; Botnar, R.M.; Prieto, C. Free-running 3D whole-heart T(1) and T(2) mapping and cine MRI using low-rank reconstruction with non-rigid cardiac motion correction. Magn. Reson. Med. 2023, 89, 217–232. [Google Scholar] [CrossRef]
- Bastiaansen, J.A.M.; Stuber, M. Flexible water excitation for fat-free MRI at 3T using lipid insensitive binomial off-resonant RF excitation (LIBRE) pulses. Magn. Reson. Med. 2018, 79, 3007–3017. [Google Scholar] [CrossRef]
- Koktzoglou, I.; Edelman, R.R. Radial fast interrupted steady-state (FISS) magnetic resonance imaging. Magn. Reson. Med. 2018, 79, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Masala, N.; Bastiaansen, J.A.M.; Di Sopra, L.; Roy, C.W.; Piccini, D.; Yerly, J.; Colotti, R.; Stuber, M. Free-running 5D coronary MR angiography at 1.5T using LIBRE water excitation pulses. Magn. Reson. Med. 2020, 84, 1470–1485. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen, J.A.M.; Piccini, D.; Di Sopra, L.; Roy, C.W.; Heerfordt, J.; Edelman, R.R.; Koktzoglou, I.; Yerly, J.; Stuber, M. Natively fat-suppressed 5D whole-heart MRI with a radial free-running fast-interrupted steady-state (FISS) sequence at 1.5T and 3T. Magn. Reson Med. 2020, 83, 45–55. [Google Scholar] [CrossRef]
- Campbell-Washburn, A.E.; Varghese, J.; Nayak, K.S.; Ramasawmy, R.; Simonetti, O.P. Cardiac MRI at Low Field Strengths. J. Magn. Reson. Imaging. 2024, 59, 412–430. [Google Scholar] [CrossRef]
- Uhlig, J.; Al-Bourini, O.; Salgado, R.; Francone, M.; Vliegenthart, R.; Bremerich, J.; Lotz, J.; Gutberlet, M. Gadolinium-based Contrast Agents for Cardiac MRI: Use of Linear and Macrocyclic Agents with Associated Safety Profile from 154 779 European Patients. Radiol. Cardiothorac. Imaging. 2020, 2, e200102. [Google Scholar] [CrossRef] [PubMed]
- Paiman, E.H.M.; Lamb, H.J. When should we use contrast material in cardiac MRI? J. Magn. Reson. Imaging. 2017, 46, 1551–1572. [Google Scholar] [CrossRef] [PubMed]
- Bruder, O.; Wagner, A.; Lombardi, M.; Schwitter, J.; van Rossum, A.; Pilz, G.; Nothnagel, D.; Steen, H.; Petersen, S.; Nagel, E.; et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry--multi national results from 57 centers in 15 countries. J. Cardiovasc. Magn. Reson. 2013, 15, 9. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtackers, R.J.; Stuber, M. Free-Running Cardiac and Respiratory Motion-Resolved Imaging: A Paradigm Shift for Managing Motion in Cardiac MRI? Diagnostics 2024, 14, 1946. https://doi.org/10.3390/diagnostics14171946
Holtackers RJ, Stuber M. Free-Running Cardiac and Respiratory Motion-Resolved Imaging: A Paradigm Shift for Managing Motion in Cardiac MRI? Diagnostics. 2024; 14(17):1946. https://doi.org/10.3390/diagnostics14171946
Chicago/Turabian StyleHoltackers, Robert J., and Matthias Stuber. 2024. "Free-Running Cardiac and Respiratory Motion-Resolved Imaging: A Paradigm Shift for Managing Motion in Cardiac MRI?" Diagnostics 14, no. 17: 1946. https://doi.org/10.3390/diagnostics14171946





