Lower Late Development Rate of Acute Respiratory Distress Syndrome in Patients with Lower Mechanical Power or Driving Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure
2.2. Disease Definitions
2.3. Ventilator Settings and Weaning in Our Previous Cohort
- Mean blood pressure greater than 65 mmHg;
- Heart rate less than 140 beats per minute;
- SpO2 greater than 92%;
- PEEP less than 8 cm H2O;
- FiO2 less than 35%;
- Pressure support mode ventilation with pressure less than 10 cm H2O.
2.4. Data Utilized from Our Previous Cohort
2.5. MP Calculation
- DP (cm H2O) = Pplat − PEEP;
- MP (J/min) = 0.098 × RR × VT × (DP + PEEP).
2.6. Statistical Analysis
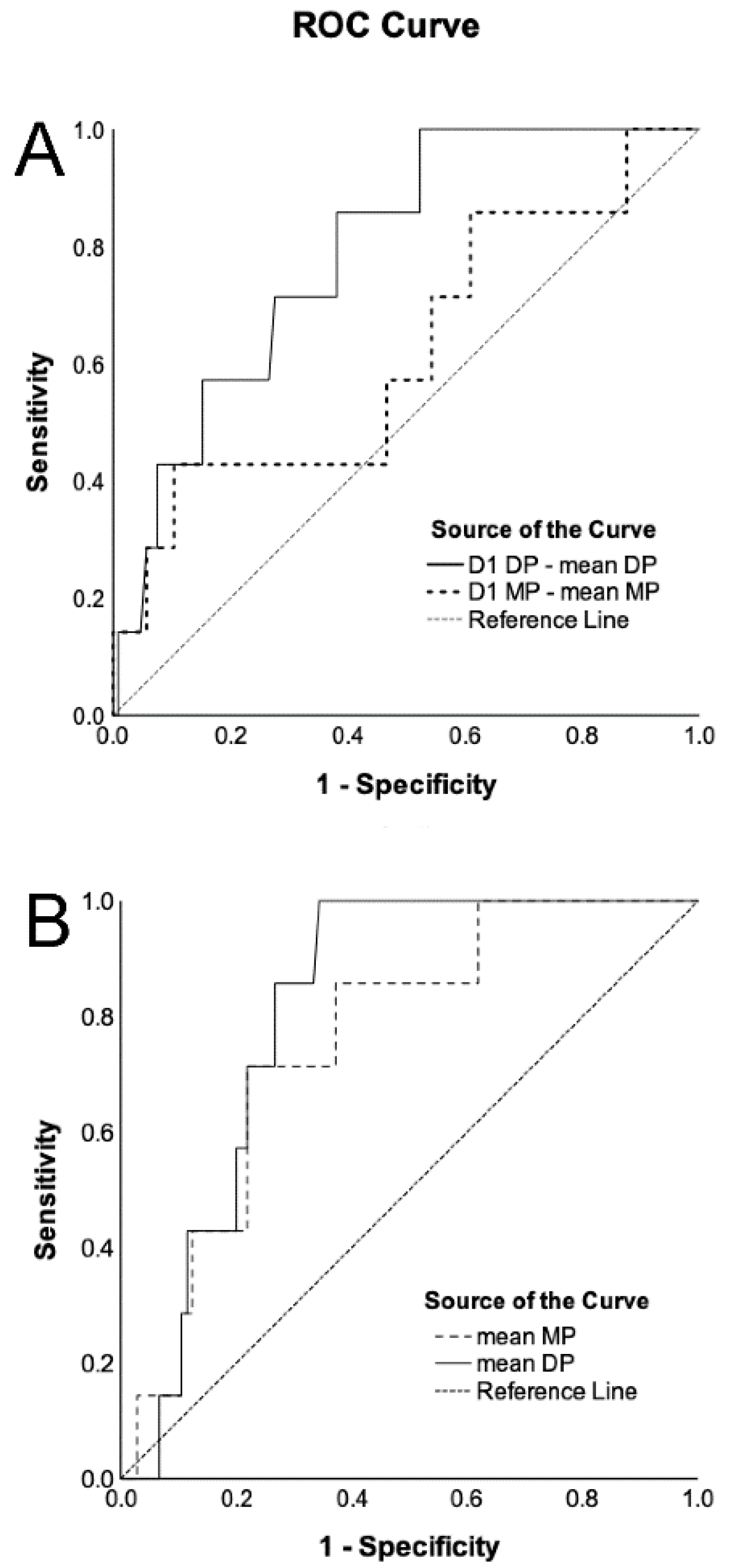
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | Acute respiratory distress syndrome |
| PBW | Predicted body weight |
| Pplat | Plateau pressure |
| PaO2 | Arterial partial pressure of oxygen |
| FiO2 | Fraction of inspired oxygen |
| MP | Mechanical power |
| VILI | Ventilator-induced lung injury |
| PEEP | Positive end-expiratory pressure |
| RR | Respiratory rate |
| ICU | Intensive care unit |
| DP | Driving pressure |
| KDIGO | Kidney Disease Improving Global Guidelines |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| SpO2 | Oxygen saturation by pulse oximetry |
| SBT | Spontaneous breathing trial |
| VT | Tidal volume |
| ROC | Receiver operating characteristic |
| CHF | Congestive heart failure |
| HR | Hazard ratio |
| CI | Confidence interval |
| AUROCs | Areas under the ROC curves |
References
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Writing Group for the PReVENT Investigators; Simonis, F.D.; Serpa Neto, A.; Binnekade, J.M.; Braber, A.; Bruin, K.C.M.; Determann, R.M.; Goekoop, G.J.; Heidt, J.; Horn, J.; et al. Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA 2018, 320, 1872–1880. [Google Scholar]
- Wu, H.P.; Chu, C.M.; Chuang, L.P.; Lin, S.W.; Leu, S.W.; Chang, K.W.; Chiu, L.C.; Liu, P.H.; Kao, K.C. The Association between Mechanical Power and Mortality in Patients with Pneumonia Using Pressure-Targeted Ventilation. Diagnostics 2021, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Roca, O.; Penuelas, O.; Muriel, A.; Garcia-de-Acilu, M.; Laborda, C.; Sacanell, J.; Riera, J.; Raymondos, K.; Du, B.; Thille, A.W.; et al. Driving Pressure Is a Risk Factor for ARDS in Mechanically Ventilated Subjects Without ARDS. Respir. Care 2021, 66, 1505–1513. [Google Scholar] [CrossRef]
- Yu, T.J.; Liu, Y.C.; Yu, C.C.; Tseng, J.C.; Hua, C.C.; Wu, H.P. Comparing hydrocortisone and methylprednisolone in patients with septic shock. Adv. Ther. 2009, 26, 728–735. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tseng, J.C.; Huang, C.Y.; Chu, C.M.; Wu, H.P. Mortality of severe septic patients between physician’s high and low care volumes. Biomed. J. 2017, 40, 226–231. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl. S2), S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Wu, H.P.; Liu, Y.C.; Lin, S.C.; Chien, M.Y.; Liao, F.C.; Chang, S.C.; Shieh, W.B. Comparison of respiratory parameters and plasma cytokine levels between treatment with Salmeterol/fluticasone and ipratropium/terbutaline/budesonide in mechanically ventilated COPD patients. Chang. Gung Med. J. 2012, 35, 373–381. [Google Scholar]
- Villar, J.; Martin-Rodriguez, C.; Dominguez-Berrot, A.M.; Fernandez, L.; Ferrando, C.; Soler, J.A.; Diaz-Lamas, A.M.; Gonzalez-Higueras, E.; Nogales, L.; Ambros, A.; et al. A Quantile Analysis of Plateau and Driving Pressures: Effects on Mortality in Patients With Acute Respiratory Distress Syndrome Receiving Lung-Protective Ventilation. Crit. Care Med. 2017, 45, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; van der Staay, M.; Schadler, D.; Frerichs, I.; Weiler, N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019, 45, 1321–1323. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Carter, C.; Lefebvre, P.; Raut, M.; Vekeman, F.; Duh, M.S.; Shorr, A.F. Red blood cell transfusions and the risk of acute respiratory distress syndrome among the critically ill: A cohort study. Crit. Care 2007, 11, R63. [Google Scholar] [CrossRef]
- Serpa Neto, A.; Cardoso, S.O.; Manetta, J.A.; Pereira, V.G.; Esposito, D.C.; Pasqualucci Mde, O.; Damasceno, M.C.; Schultz, M.J. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA 2012, 308, 1651–1659. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Slutsky, A.S.; Brochard, L.J.; Brower, R.; Serpa-Neto, A.; Cavalcanti, A.B.; Mercat, A.; Meade, M.; Morais, C.C.A.; Goligher, E.; et al. Ventilatory Variables and Mechanical Power in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2021, 204, 303–311. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Wu, H.P.; Leu, S.W.; Lin, S.W.; Hung, C.Y.; Chen, N.H.; Hu, H.C.; Huang, C.C.; Kao, K.C. Role of Changes in Driving Pressure and Mechanical Power in Predicting Mortality in Patients with Acute Respiratory Distress Syndrome. Diagnostics 2023, 13, 1226. [Google Scholar] [CrossRef]
- van Meenen, D.M.P.; Algera, A.G.; Schuijt, M.T.U.; Simonis, F.D.; van der Hoeven, S.M.; Neto, A.S.; Abreu, M.G.; Pelosi, P.; Paulus, F.; Schultz, M.J.; et al. Effect of mechanical power on mortality in invasively ventilated ICU patients without the acute respiratory distress syndrome: An analysis of three randomised clinical trials. Eur. J. Anaesthesiol. 2023, 40, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Dianti, J.; Matelski, J.; Tisminetzky, M.; Walkey, A.J.; Munshi, L.; Del Sorbo, L.; Fan, E.; Costa, E.L.; Hodgson, C.L.; Brochard, L.; et al. Comparing the Effects of Tidal Volume, Driving Pressure, and Mechanical Power on Mortality in Trials of Lung-Protective Mechanical Ventilation. Respir. Care 2021, 66, 221–227. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | ARDS Development (n = 7) | No ARDS Development (n = 105) |
|---|---|---|
| Age, years | 70.3 ± 19.5 | 77.4 ± 10.8 |
| APACHE II score | 29.4 ± 7.1 | 25.1 ± 6.7 |
| Sex | ||
| Male | 5 (71.4) | 64 (61.0) |
| Female | 2 (28.6) | 41 (39.0) |
| History | ||
| COPD | 1 (14.3) | 33 (31.4) |
| CHF | 3 (42.9) | 10 (9.5) * |
| Liver cirrhosis | 0 (0.0) | 4 (3.8) |
| Hemodialysis | 1 (14.3) | 8 (7.6) |
| Diabetes mellitus | 4 (57.1) | 36 (34.3) |
| Murray score on Day 1 | 1.4 ± 0.3 | 1.3 ± 0.3 |
| PaO2/FiO2 ratio (mm Hg) | 414.0 ± 96.8 | 465.5 ± 200.3 |
| Positive end expiratory pressure (cm H2O) | 7.1 ± 1.9 | 6.5 ± 1.7 |
| Dynamic lung compliance (mL/cm H2O) | 29.1 ± 9.6 | 28.6 ± 8.3 |
| Chest radiography (quadrants infiltrated) | 2.0 ± 0.8 | 1.8 ± 0.8 |
| Adverse events | ||
| Shock | 4 (57.1) | 30 (28.6) |
| Stage 2 or 3 acute kidney injury | 3 (42.9) | 36 (34.3) |
| GI bleeding | 0 (0.0) | 9 (8.6) |
| Thrombocytopenia | 2 (28.6) | 26 (24.8) |
| Jaundice | 0 (0.0) | 0 (0.0) |
| Ventilator settings on Day 1 | ||
| FiO2 (%) | 60.0 ± 25.5 | 50.8 ± 22.4 |
| Driving pressure (cm H2O) | 18.3 ± 3.9 | 19.5 ± 3.5 |
| Vt/PBW (mL/kg) | 9.5 ± 1.6 | 10.5 ± 2.5 |
| Respiratory rate (/min) | 22.1 ± 7.0 | 20.0 ± 6.3 |
| Mechanical power (J/min) | 26.6 ± 10.0 | 26.6 ± 10.3 |
| Mean Vt/PBW (mL/kg) | 10.0 ± 1.0 | 9.7 ± 1.8 |
| Mean mechanical power (J/min) | 24.9 ± 4.4 | 20.0 ± 5.7 * |
| Mean driving pressure (cm H2O) | 18.0 ± 1.6 | 15.1 ± 3.3 * |
| Variables | Univariate HR (95% CI) | p Value | Model 1 * | Model 2 † | ||
|---|---|---|---|---|---|---|
| Multivariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value | |||
| CHF | 6.435 (1.439–28.789) | 0.015 | 8.064 (1.736–37.456) | 0.008 | 6.523 (1.446–29.432) | 0.015 |
| Mean MP (J/min) | 1.149 (1.010–1.307) | 0.035 | 1.177 (1.021–1.358) | 0.025 | ||
| Mean DP (cm H2O) | 1.208 (1.017–1.435) | 0.031 | 1.226 (1.016–1.479) | 0.034 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Liu, P.-H.; Lin, S.-W.; Yu, C.-C.; Chu, C.-M.; Wu, H.-P. Lower Late Development Rate of Acute Respiratory Distress Syndrome in Patients with Lower Mechanical Power or Driving Pressure. Diagnostics 2024, 14, 1969. https://doi.org/10.3390/diagnostics14171969
Lee Y-C, Liu P-H, Lin S-W, Yu C-C, Chu C-M, Wu H-P. Lower Late Development Rate of Acute Respiratory Distress Syndrome in Patients with Lower Mechanical Power or Driving Pressure. Diagnostics. 2024; 14(17):1969. https://doi.org/10.3390/diagnostics14171969
Chicago/Turabian StyleLee, Ya-Chi, Pi-Hua Liu, Shih-Wei Lin, Chung-Chieh Yu, Chien-Ming Chu, and Huang-Pin Wu. 2024. "Lower Late Development Rate of Acute Respiratory Distress Syndrome in Patients with Lower Mechanical Power or Driving Pressure" Diagnostics 14, no. 17: 1969. https://doi.org/10.3390/diagnostics14171969
APA StyleLee, Y.-C., Liu, P.-H., Lin, S.-W., Yu, C.-C., Chu, C.-M., & Wu, H.-P. (2024). Lower Late Development Rate of Acute Respiratory Distress Syndrome in Patients with Lower Mechanical Power or Driving Pressure. Diagnostics, 14(17), 1969. https://doi.org/10.3390/diagnostics14171969







