Characterizing Mechanical Changes in the Biceps Brachii Muscle in Mild Facioscapulohumeral Muscular Dystrophy Using Shear Wave Elastography
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement Setup
2.3. Measurement Protocol
2.4. Equipment
2.4.1. Elbow Flexion Moment
2.4.2. Sonography and Shear Wave Elastography (SWE) of the Biceps Brachii
2.4.3. Surface Electromyography of the Biceps Brachii (BB) and the Triceps Brachii
2.4.4. Data Acquisition
2.5. Statistics
3. Results
3.1. Participants, Anthropometrics, and Elbow Moment Performance
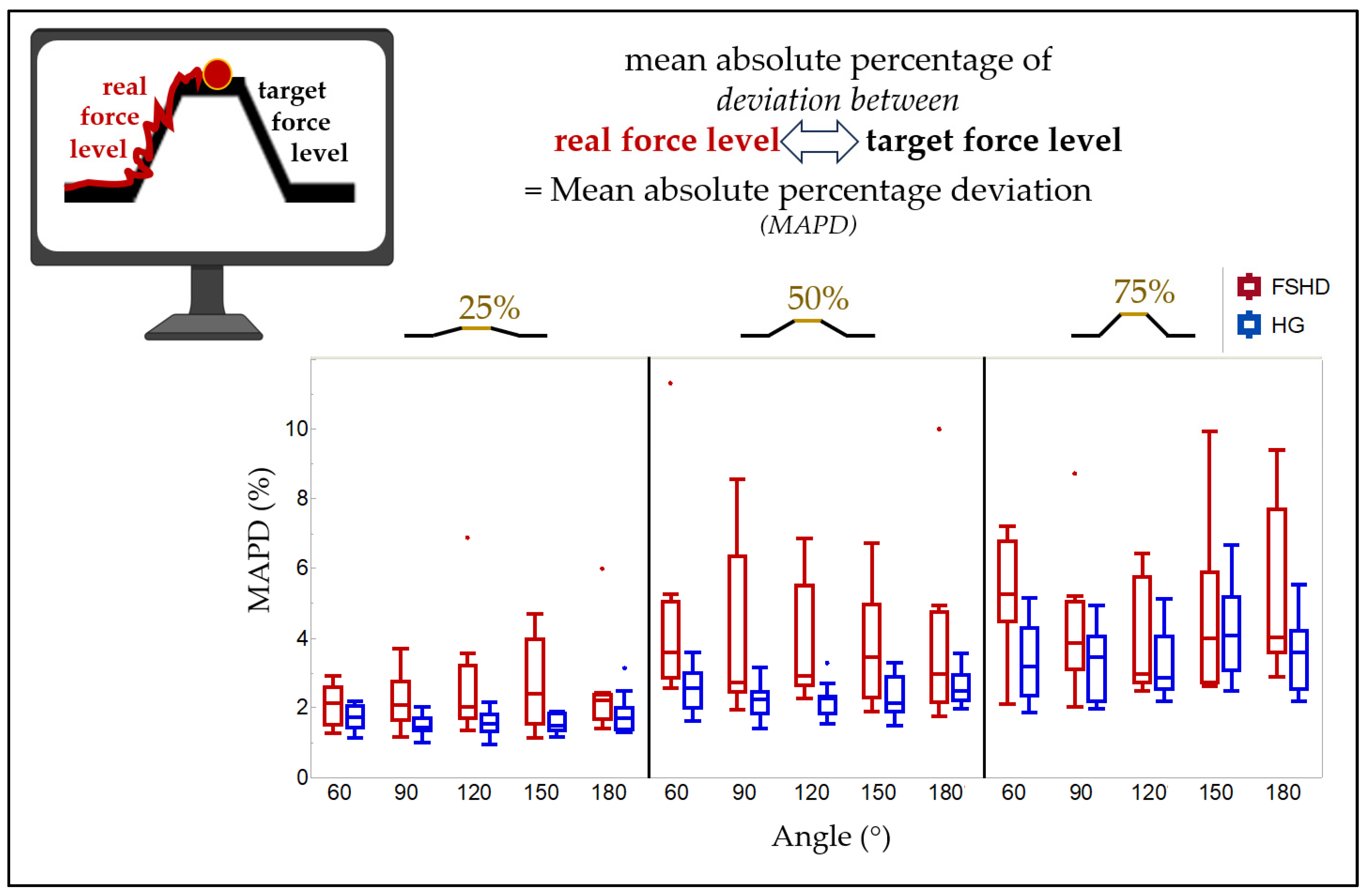
3.2. MAPD
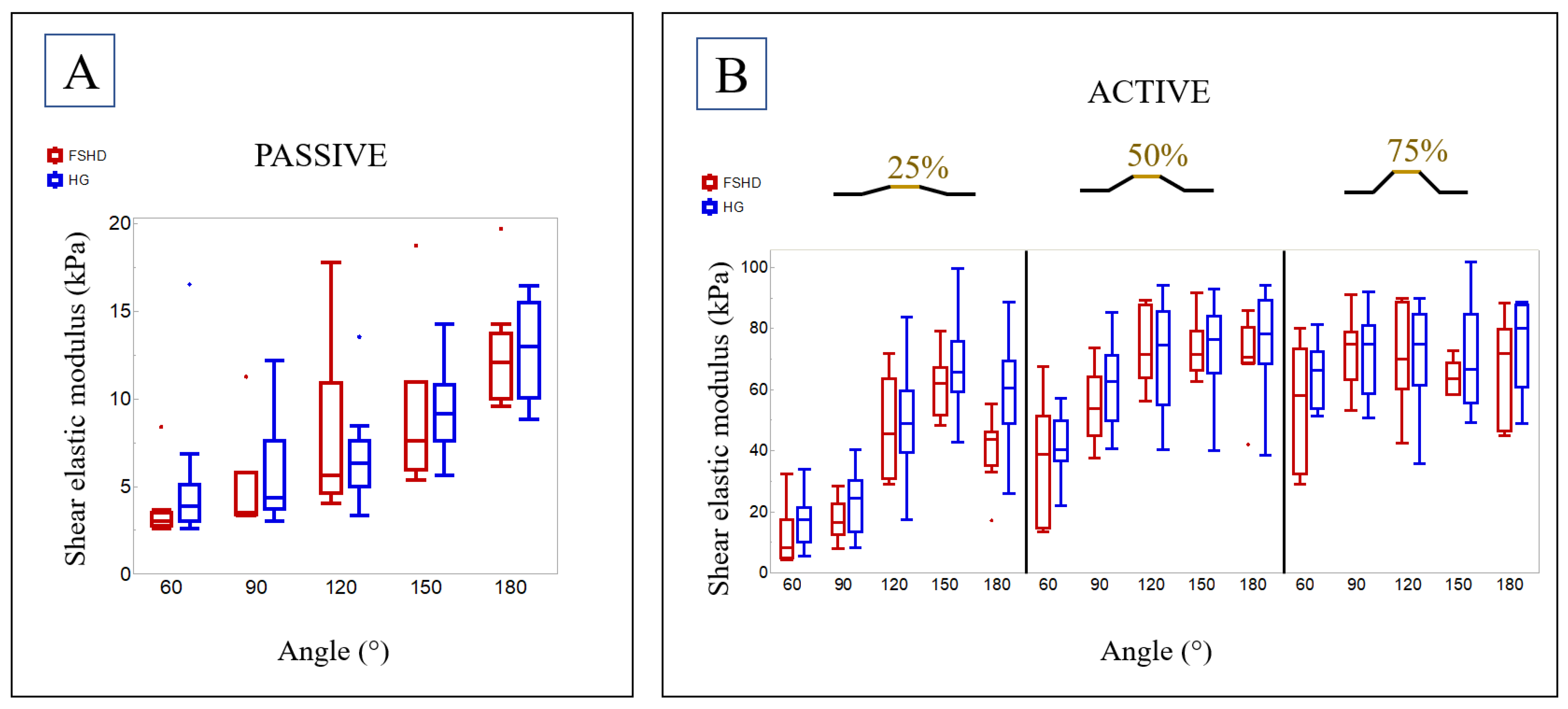
3.3. SWE
3.4. Comparison with a Healthy Group
4. Discussion
- (1)
- In muscle dystrophies such as FSHD, the shear elastic modulus depends on the joint angle and the percentage of muscle activation.
- (2)
- In contrast to previous studies on dystrophic muscle diseases [10,15,16,18,29], the shear elastic modulus in a passive state was not higher in the mild-FSHD group than in the HG, but careful considerations have to be made before concluding a potential non-existing difference between healthy and dystrophic altered muscles (see below).
- (3)
- Patients with FSHD could exhibit a lack of force control, indicated by a higher MAPD; in other words, they possibly deviated significantly more from the elbow moment they were instructed to follow during the ramp task than the HG did.
4.1. Shear Elastic Modulus in FSHD
4.2. Comparison to a Previously Published Healthy Group
4.3. Mean Absolute Percentage Deviation
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Appendix A
| 60° | 90° | 120° | 150° | 180° | |
|---|---|---|---|---|---|
| FSHD MAPD at 25% MVC (%) | 1.5/2.1/2.6 | 1.6/2.1/2.7 | 1.7/2.0/3.2 | 1.5/2.4/4.0 | 1.7/2.2/2.4 |
| (1.3–2.9) | (1.1–3.7) | (1.4–6.9) | (1.1–4.7) | (1.4–6.0) | |
| HG MAPD at 25% MVC (%) | 1.4/1.7/2.0 | 1.3/1.4/1.7 | 1.3/1.5/1.8 | 1.4/1.5/1.8 | 1.4/1.7/2.0 |
| (1.1–2.2) | (1.0–2.0) | (0.9–2.2) | (1.2–1.9) | (1.3–3.1) | |
| FSHD vs. HG | |||||
| p-value | 0.11 | 0.01 | 0.029 | 0.035 | 0.082 |
| corrected p-value | 0.157 | 0.03 | 0.062 | 0.066 | 0.137 |
| FSHD MAPD at 50% MVC (%) | 2.9/3.6/5.0 | 2.5/2.7/6.3 | 2.6/2.9/5.5 | 2.3/3.4/4.9 | 2.2/3.0/4.7 |
| (2.6–11.3) | (1.9–8.6) | (2.3–6.9) | (1.9–6.7) | (1.8–10.0) | |
| HG MAPD at 50% MVC (%) | 2.0/2.6/3.0 | 1.8/2.2/2.4 | 1.8/2.3/2.3 | 1.9/2.1/2.9 | 2.2/2.5/2.9 |
| (1.6–3.6) | (1.4–3.2) | (1.5–3.3) | (1.5–3.3) | (2.0–3.5) | |
| FSHD vs. HG | |||||
| p-value | 0.008 | 0.01 | 0.002 | 0.016 | 0.238 |
| corrected p-value | 0.03 | 0.03 | 0.03 | 0.04 | 0.275 |
| FSHD MAPD at 75% MVC (%) | 4.4/5.3/6.7 | 3.1/3.8/5.0 | 2.7/3.0/5.7 | 2.7/4.0/5.9 | 3.6/4.0/7.7 |
| (2.1–7.2) | (2.0–8.7) | (2.5–6.4) | (2.6–9.9) | (2.9–9.4) | |
| FSHD MAPD at 75% MVC (%) | 2.3/3.2/4.3 | 2.2/3.4/4.1 | 2.5/2.9/4.1 | 3.1/4.1/5.2 | 2.5/3.6/4.2 |
| (1.9–5.1) | (2.0–4.9) | (2.2–5.1) | (2.5–6.7) | (2.2–5.5) | |
| FSHD vs. HG | |||||
| p-value | 0.01 | 0.127 | 0.65 | 0.779 | 0.115 |
| corrected p-value | 0.03 | 0.159 | 0.696 | 0.779 | 0.157 |
| FSHD MVC moment (Nm) | 26.4/36.6/42.6 | 27.3/36.0/44.6 | 26.6/41.3/46.9 | 21.0/29.6/36.0 | 19.3/20.1/29.7 |
| (19.4–62.1) | (18.9–57.6) | (19.9–66.5) | (12.1–42.4) | (11.4–36.9) | |
| HG MVC moment (Nm) | 34.6/46.4/63.2 | 31.0/40.3/54.9 | 28.0/38.4/55.8 | 21.0/29.8/43.2 | 14.9/23.4/40.3 |
| (28.1–87.1) | (27.0–82.0) | (22.4–76.8) | (15.1–66.3) | (10.4–54.4) |
| 60° | 90° | 120° | 150° | 180° | |
|---|---|---|---|---|---|
| FSHD SWE passive (kPa) | 2.7/3.0/3.5 | 3.4/3.5/5.8 | 4.6/5.7/10.9 | 5.9/7.6/11.0 | 10.0/12.1/13.7 |
| (2.6–8.4) | (3.3–11.3) | (4.0–17.8) | (5.4–18.7) | (9.6–19.7) | |
| HG SWE passive (kPa) | 3.1/3.9/5.1 | 3.7/4.3/7.6 | 5.0/6.3/7.6 | 7.6/9.1/10.8 | 10.0/12.9/15.5 |
| (2.6–16.5) | (3.0–12.1) | (3.3–13.5) | (5.6–14.2) | (8.8–16.5) | |
| FSHD vs. HG | |||||
| p-value | 0.05 | 0.4 | 0.877 | 0.416 | 0.868 |
| corrected p-value | 0.583 | 0.8 | 0.973 | 0.8 | 0.973 |
| FSHD SWE at 25% MVC (kPa) | 4.7/8.3/17.3 | 12.5/16.4/22.5 | 30.8/45.5/63.4 | 51.5/61.8/67.2 | 35.1/43.7/46.2 |
| (4.2–32.3) | (7.7–28.2) | (28.9–71.6) | (48.1–78.9) | (17.1–55.4) | |
| HG SWE at 25% MVC (kPa) | 10.1/17.5/21.4 | 13.2/24.4/30.2 | 39.5/48.7/59.3 | 59.3/65.5/75.8 | 48.8/60.4/69.3 |
| (5.3–34.0) | (8.3–40.1) | (17.5–83.6) | (42.7–99.5) | (25.9–88.5) | |
| FSHD vs. HG | |||||
| p-value | 0.07 | 0.161 | 0.714 | 0.33 | 0.006 |
| corrected p-value | 0.583 | 0.671 | 0.945 | 0.8 | 0.15 |
| FSHD SWE at 50% MVC (kPa) | 14.4/38.7/51.2 | 44.7/53.7/64.1 | 63.8/71.5/87.5 | 66.1/71.4/78.9 | 68.6/70.5/80.3 |
| (13.2–67.5) | (37.7–73.8) | (56.0–89.0) | (62.4–91.5) | (42.0–85.9) | |
| HG SWE at 50% MVC (kPa) | 36.7/40.3/49.5 | 49.5/62.4/71.2 | 54.9/74.4/85.7 | 65.4/76.5/84.0 | 68.4/78.1/89.0 |
| (22.1–57.0) | (40.7–85.0) | (40.2–94.0) | (40.0–92.7) | (38.3–94.0) | |
| FSHD vs. HG | |||||
| p-value | 0.57 | 0.238 | 0.868 | 0.616 | 0.238 |
| corrected p-value | 0.891 | 0.744 | 0.973 | 0.906 | 0.744 |
| FSHD SWE at 75% MVC (kPa) | 32.4/58.0/73.3 | 63.1/74.7/78.8 | 60.1/69.9/88.6 | 58.4/63.3/68.6 | 46.3/71.9/79.7 |
| (28.9–79.9) | (53.1–91.2) | (42.3–89.8) | (58.3–72.6) | (44.8–88.1) | |
| FSHD SWE at 75% MVC (kPa) | 53.6/66.2/72.2 | 58.6/74.8/80.9 | 61.3/74.7/84.4 | 55.4/66.6/84.7 | 60.5/80.1/87.7 |
| (51.2–81.3) | (50.8–91.9) | (35.6–89.7) | (49.1–101.8) | (48.6–88.6) | |
| FSHD vs. HG | |||||
| p-value | 0.473 | 0.973 | 0.967 | 0.718 | 0.275 |
| corrected p-value | 0.845 | 0.973 | 0.973 | 0.945 | 0.764 |
| FSHD SWE at MVC (kPa) | 47.1/63.6/84.0 | 56.4/71.1/75.3 | 56.9/65.3/77.5 | 48.8/53.5/72.9 | 57.0/67.7/75.3 |
| (28.1–88.7) | (47.7–77.6) | (30.9–82.6) | (42.1–90.8) | (33.6–76.3) | |
| HG SWE at MVC (kPa) | 47.0/60.1/65.2 | 48.4/53.7/70.4 | 47.2/53.4/63.0 | 46.0/57.5/69.0 | 49.2/52.6/77.6 |
| (37.7–78.5) | (30.3–78.6) | (43.0–81.4) | (23.0–80.3) | (44.7–82.6) | |
| FSHD vs. HG | |||||
| p-value | 0.374 | 0.127 | 0.095 | 0.92 | 0.525 |
| corrected p-value | 0.8 | 0.635 | 0.594 | 0.973 | 0.875 |
References
- Varma, A.; Weinstein, J.; Seabury, J.; Rosero, S.; Engebrecht, C.; Wagner, E.; Zizzi, C.; Luebbe, E.A.; Dilek, D.; McDermott, M.P.; et al. The Facioscapulohumeral Muscular Dystrophy-Health Index: Development and evaluation of a disease-specific outcome measure. Muscle Nerve 2023, 68, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, M.; Calanni, L.; Negro, M.; Ricci, G.; Bettio, C.; Barbero, M.; Berardinelli, A.; Siciliano, G.; Tupler, R.; Soldini, E.; et al. Increased resistance towards fatigability in patients with facioscapulohumeral muscular dystrophy. Eur. J. Appl. Physiol. 2021, 121, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Emerson, C.P.; Hayward, L.J. Outcome Measures in Facioscapulohumeral Muscular Dystrophy Clinical Trials. Cells 2022, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Mul, K.; Horlings, C.G.C.; Vincenten, S.C.C.; Voermans, N.C.; van Engelen, B.G.M.; van Alfen, N. Quantitative muscle MRI and ultrasound for facioscapulohumeral muscular dystrophy: Complementary imaging biomarkers. J. Neurol. 2018, 265, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Goselink, R.J.M.; Schreuder, T.H.A.; Mul, K.; Voermans, N.C.; Erasmus, C.E.; van Engelen, B.G.M.; van Alfen, N. Muscle ultrasound is a responsive biomarker in facioscapulohumeral dystrophy. Neurology 2020, 94, e1488–e1494. [Google Scholar] [CrossRef]
- Pillen, S.; Arts, I.M.P.; Zwarts, M.J. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 2008, 37, 679–693. [Google Scholar] [CrossRef]
- Wijntjes, J.; van Alfen, N. Muscle ultrasound: Present state and future opportunities. Muscle Nerve 2021, 63, 455–466. [Google Scholar] [CrossRef]
- Han, J.J.; De Bie, E.; Nicorici, A.; Abresch, R.T.; Bajcsy, R.; Kurillo, G. Reachable workspace reflects dynamometer-measured upper extremity strength in facioscapulohumeral muscular dystrophy. Muscle Nerve 2015, 52, 948–955. [Google Scholar] [CrossRef]
- Hug, F.; Tucker, K.; Gennisson, J.-L.; Tanter, M.; Nordez, A. Elastography for Muscle Biomechanics: Toward the Estimation of Individual Muscle Force. Exerc. Sport Sci. Rev. 2015, 43, 125–133. [Google Scholar] [CrossRef]
- Lacourpaille, L.; Hug, F.; Guével, A.; Péréon, Y.; Magot, A.; Hogrel, J.Y.; Nordez, A. Non-invasive assessment of muscle stiffness in patients with Duchenne muscular dystrophy. Muscle Nerve 2015, 51, 284–286. [Google Scholar] [CrossRef]
- Zimmer, M.; Kleiser, B.; Marquetand, J.; Ateş, F. Shear wave elastography characterizes passive and active mechanical properties of biceps brachii muscle in vivo. J. Mech. Behav. Biomed. Mater. 2022, 137, 105543. [Google Scholar] [CrossRef]
- Ateş, F.; Andrade, R.J.; Freitas, S.R.; Hug, F.; Lacourpaille, L.; Gross, R.; Yucesoy, C.A.; Nordez, A. Passive stiffness of monoarticular lower leg muscles is influenced by knee joint angle. Eur. J. Appl. Physiol. 2018, 118, 585–593. [Google Scholar] [CrossRef]
- Ateş, F.; Hug, F.; Bouillard, K.; Jubeau, M.; Frappart, T.; Couade, M.; Bercoff, J.; Nordez, A. Muscle shear elastic modulus is linearly related to muscle torque over the entire range of isometric contraction intensity. J. Electromyogr. Kinesiol. 2015, 25, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Bachasson, D.; Dubois, G.J.R.; Allenbach, Y.; Benveniste, O.; Hogrel, J.Y. Muscle Shear Wave Elastography in Inclusion Body Myositis: Feasibility, Reliability and Relationships with Muscle Impairments. Ultrasound Med. Biol. 2018, 44, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Pichiecchio, A.; Alessandrino, F.; Bortolotto, C.; Cerica, A.; Rosti, C.; Raciti, M.V.; Rossi, M.; Berardinelli, A.; Baranello, G.; Bastianello, S.; et al. Muscle ultrasound elastography and MRI in preschool children with Duchenne muscular dystrophy. Neuromuscul. Disord. 2018, 28, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Tsui, P.H.; Lu, C.H.; Hung, Y.H.; Tsai, M.R.; Shieh, J.Y.; Weng, W.C. Quantifying Lower Limb Muscle Stiffness as Ambulation Function Declines in Duchenne Muscular Dystrophy with Acoustic Radiation Force Impulse Shear Wave Elastography. Ultrasound Med. Biol. 2021, 47, 2880–2889. [Google Scholar] [CrossRef]
- Lin, C.-W.; Shieh, J.-Y.; Tsui, P.-H.; Chen, C.-L.; Lu, C.-H.; Hung, Y.-H.; Lee, H.-Y.; Weng, W.-C.; Gau, S.S.-F. Acoustic radiation force impulse shear wave elastography quantifies upper limb muscle in patients with Duchenne muscular dystrophy. Ultrason. Sonochem. 2023, 101, 106661. [Google Scholar] [CrossRef]
- Harada, R.; Taniguchi-Ikeda, M.; Nagasaka, M.; Nishii, T.; Inui, A.; Yamamoto, T.; Morioka, I.; Kuroda, R.; Iijima, K.; Nozu, K.; et al. Assessment of the upper limb muscles in patients with Fukuyama muscular dystrophy: Noninvasive assessment using visual ultrasound muscle analysis and shear wave elastography. Neuromuscul. Disord 2022, 32, 754–762. [Google Scholar] [CrossRef]
- Rassier, D.E.; MacIntosh, B.R.; Herzog, W. Length dependence of active force production in skeletal muscle. J. Appl. Physiol. 1999, 86, 1445–1457. [Google Scholar] [CrossRef]
- Le Sant, G.; Gross, R.; Hug, F.; Nordez, A. Influence of low muscle activation levels on the ankle torque and muscle shear modulus during plantar flexor stretching. J. Biomech. 2019, 93, 111–117. [Google Scholar] [CrossRef]
- Zimmer, M.; Kleiser, B.; Marquetand, J.; Ates, F. Characterization of Muscle Weakness Due to Myasthenia Gravis Using Shear Wave Elastography. Diagnostics 2023, 13, 1108. [Google Scholar] [CrossRef]
- Cipriano, K.J.; Wickstrom, J.; Glicksman, M.; Hirth, L.; Farrell, M.; Livinski, A.A.; Esfahani, S.A.; Maldonado, R.J.; Astrow, J.; Berrigan, W.A.; et al. A scoping review of methods used in musculoskeletal soft tissue and nerve shear wave elastography studies. Clin. Neurophysiol. 2022, 140, 181–195. [Google Scholar] [CrossRef]
- Romano, A.; Staber, D.; Grimm, A.; Kronlage, C.; Marquetand, J. Limitations of Muscle Ultrasound Shear Wave Elastography for Clinical Routine-Positioning and Muscle Selection. Sensors 2021, 21, 8490. [Google Scholar] [CrossRef] [PubMed]
- MRC. Aids to the Examination of the Peripheral Nervous System; HMSO: London, UK, 1976. [Google Scholar]
- Compston, A. Aids to the Investigation of Peripheral Nerve Injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty’s Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with Aids to the Examination of the Peripheral Nervous System. By Michael O’Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain 2010, 133, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Cerone, G. Tutorial. Surface EMG detection, conditioning and pre-processing: Best practices. J. Electromyogr. Kinesiol. 2020, 54, 102440. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Moreta, M.C.; Fleet, A.; Reebye, R.; McKernan, G.; Berger, M.; Farag, J.; Munin, M.C. Reliability and Validity of the Modified Heckmatt Scale in Evaluating Muscle Changes with Ultrasound in Spasticity. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100071. [Google Scholar] [CrossRef]
- Yu, H.K.; Liu, X.; Pan, M.; Chen, J.W.; Liu, C.; Wu, Y.; Li, Z.B.; Wang, H.Y. Performance of Passive Muscle Stiffness in Diagnosis and Assessment of Disease Progression in Duchenne Muscular Dystrophy. Ultrasound Med. Biol. 2022, 48, 414–421. [Google Scholar] [CrossRef]
- Bouillard, K.; Hug, F.; Guével, A.; Nordez, A. Shear elastic modulus can be used to estimate an index of individual muscle force during a submaximal isometric fatiguing contraction. J. Appl. Physiol. (1985) 2012, 113, 1353–1361. [Google Scholar] [CrossRef]
- Sasaki, K.; Toyama, S.; Ishii, N. Length-force characteristics of in vivo human muscle reflected by supersonic shear imaging. J. Appl. Physiol. 2014, 117, 153–162. [Google Scholar] [CrossRef]
- Chernak, L.A.; DeWall, R.J.; Lee, K.S.; Thelen, D.G. Length and activation dependent variations in muscle shear wave speed. Physiol. Meas. 2013, 34, 713. [Google Scholar] [CrossRef]
- Bernabei, M.; Lee, S.S.M.; Perreault, E.J.; Sandercock, T.G. Shear wave velocity is sensitive to changes in muscle stiffness that occur independently from changes in force. J. Appl. Physiol. (1985) 2020, 128, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Crutison, J.; Sun, M.; Royston, T.J. The combined importance of finite dimensions, anisotropy, and pre-stress in acoustoelastography. J. Acoust. Soc. Am. 2022, 151, 2403. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Smerilli, G.; Cipolletta, E.; Wakefield, R.J.; De Angelis, R.; Risa, A.M.; Salaffi, F.; Farah, S.; Villota-Eraso, C.; Maccarrone, V.; et al. Muscle involvement in systemic lupus erythematosus: Multimodal ultrasound assessment and relationship with physical performance. Rheumatology 2022, 61, 4775–4785. [Google Scholar] [CrossRef] [PubMed]
- Alfuraih, A.M.; O’Connor, P.; Tan, A.L.; Hensor, E.M.A.; Ladas, A.; Emery, P.; Wakefield, R.J. Muscle shear wave elastography in idiopathic inflammatory myopathies: A case-control study with MRI correlation. Skeletal. Radiol. 2019, 48, 1209–1219. [Google Scholar] [CrossRef]
- Wang, L.H.; Tawil, R. Facioscapulohumeral Dystrophy. Curr. Neurol. Neurosci. Rep. 2016, 16, 66. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.-k.; Sheng, S.-y.; Liang, S.-m.; Lu, H.; Chen, R.-y.; Pan, M.; Wen, Z.-b. Quantitative evaluation of passive muscle stiffness by shear wave elastography in healthy individuals of different ages. Eur. Radiol. 2021, 31, 3187–3194. [Google Scholar] [CrossRef]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; LeBrasseur, N.K.; An, K.N. Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Wakefield, R.J. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res. 2019, 31, 1755–1763. [Google Scholar] [CrossRef]
- Şendur, H.N.; Cindil, E.; Cerit, M.N.; Kılıç, P.; Gültekin, I.; Oktar, S. Evaluation of effects of aging on skeletal muscle elasticity using shear wave elastography. Eur. J. Radiol. 2020, 128, 109038. [Google Scholar] [CrossRef]
- Akagi, R.; Yamashita, Y.; Ueyasu, Y. Age-Related Differences in Muscle Shear Moduli in the Lower Extremity. Ultrasound Med. Biol. 2015, 41, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Walton, J.N. The pathology of the muscle spindle. A study of biopsy material in various muscular and neuromuscular diseases. J. Neurol. Sci. 1968, 7, 15–70. [Google Scholar] [CrossRef] [PubMed]
- Kararizou, E.G.; Manta, P.; Kalfakis, N.; Gkiatas, K.A.; Vassilopoulos, D. Morphologic and morphometrical study of the muscle spindle in muscular dystrophy. Anal. Quant. Cytol. Histol. 2007, 29, 148–152. [Google Scholar]
- Kröger, S.; Watkins, B. Muscle spindle function in healthy and diseased muscle. Skelet. Muscle 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Restuccia, D.; Servidei, S.; Nardone, R.; Oliviero, A.; Profice, P.; Mangiola, F.; Tonali, P.; Rothwell, J.C. Functional involvement of cerebral cortex in Duchenne muscular dystrophy. Muscle Nerve 1998, 21, 662–664. [Google Scholar] [CrossRef]
- Lv, S.Y.; Zou, Q.H.; Cui, J.L.; Zhao, N.; Hu, J.; Long, X.Y.; Sun, Y.C.; He, J.; Zhu, C.Z.; He, Y.; et al. Decreased gray matter concentration and local synchronization of spontaneous activity in the motor cortex in Duchenne muscular dystrophy. AJNR Am. J. Neuroradiol. 2011, 32, 2196–2200. [Google Scholar] [CrossRef]
- Quarantelli, M.; Lanzillo, R.; Del Vecchio, W.; Mollica, C.; Prinster, A.; Iadicicco, L.; Iodice, V.; Santoro, L.; Salvatore, M. Modifications of brain tissue volumes in facioscapulohumeral dystrophy. Neuroimage 2006, 32, 1237–1242. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Tonali, P.A.; Felicetti, L.; De Marco, M.B.; Saturno, E.; Pilato, F.; Pescatori, M.; Dileone, M.; Pasqualetti, P.; et al. Changes in motor cortex excitability in facioscapulohumeral muscular dystrophy. Neuromuscul. Disord. 2004, 14, 39–45. [Google Scholar] [CrossRef]
- Mesin, L.; Cescon, C.; Gazzoni, M.; Merletti, R.; Rainoldi, A. A bi-dimensional index for the selective assessment of myoelectric manifestations of peripheral and central muscle fatigue. J. Electromyogr. Kinesiol. 2009, 19, 851–863. [Google Scholar] [CrossRef]

| Parameters | Patients with FSHD | Healthy Volunteers |
|---|---|---|
| Sex | 3 female, 5 male | 7 female, 7 male |
| Handedness | 2 left, 6 right | 13 right, 1 both |
| Age (years) | 27/44/52 (24–61) | 24/28/31 (22–39) |
| Body mass index (kg/m2) | 21.7/26.7/32.6 (21.3–35.2) | 21.3/23.1/28.4 (19.4–31.1) |
| Upper arm circumference (cm) | 29/32/35 (27–38) | 27/28/30 (26–41) |
| ID | FSHD Type | Genetic | Weakness According to MRC | Modified Heckmatt Scale | Highest MVC Elbow Flexion Torque in Nm and Respective Elbow Angle | |
|---|---|---|---|---|---|---|
| 1 | 1 | D4Z4 repeats of 20 and 18 kb | 5/5/5/5/5 | 1 | 41.1 | 120° |
| 2 | 1 | D4Z4 repeats of 33 and 30 kb | 5/5/5/4+/4+ | 2 | 38.6 | 120° |
| 3 | 1 | D4Z4 repeats of 36 and 33 kb | 4+/4+/4+/5/5 | 1 | 43.2 | 60° |
| 4 | 1 | p13E-11/EcoRI DNA fragments with 15kb/3 Kpn1 copies | 4+/4+/4+/4+/4+ | 3 | 25.5 | 90° |
| 5 | 1 | D4Z4 repeat of 37 and 34 kb | 4/4−/4+/4/4 | 1 | 42.9 | 120° |
| 6 | 1 | D4Z4 repeats of 30 and 27 kb | 5/5/5/5/5 | 1 | 66.5 | 120° |
| 7 | 1 | Abbreviated fragments D4Z4 repeats, exact length not available | 4/4−/3/4+/4− | 2 | 22.7 | 120° |
| 8 | 1 | D4Z4 repeats of 33 and 30 kb | 5/5/5/5/5 | 1 | 48.2 | 120° |
| Elbow Angle | 60° | 90° | 120° | 150° | 180° | p-Value |
|---|---|---|---|---|---|---|
| Length of BB (cm) | 12.3/13.5/13.9 (12.0–14.0) | 14.0/14.3/15.3 (13.0–15.5) | 15.0/16.3/16.7 (14.5–17.0) | 15.9/17.3/18.9 (15.2–19.5) | 18.3/19.5/21.1 (17.2–21.5) | <0.001 |
| CSA of BB (cm2) | 7.5/9.2/12.1
(7.1–12.9) | 7.8/8.7/12.0
(6.4–12.7) | 7.4/8.2/11.8
(5.0–12.2) | 7.4/8.8/10.9
(4.9–12.4) | 7.7/9.2/11.0
(5.3–12.9) | 0.531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleiser, B.; Zimmer, M.; Ateş, F.; Marquetand, J. Characterizing Mechanical Changes in the Biceps Brachii Muscle in Mild Facioscapulohumeral Muscular Dystrophy Using Shear Wave Elastography. Diagnostics 2024, 14, 1985. https://doi.org/10.3390/diagnostics14171985
Kleiser B, Zimmer M, Ateş F, Marquetand J. Characterizing Mechanical Changes in the Biceps Brachii Muscle in Mild Facioscapulohumeral Muscular Dystrophy Using Shear Wave Elastography. Diagnostics. 2024; 14(17):1985. https://doi.org/10.3390/diagnostics14171985
Chicago/Turabian StyleKleiser, Benedict, Manuela Zimmer, Filiz Ateş, and Justus Marquetand. 2024. "Characterizing Mechanical Changes in the Biceps Brachii Muscle in Mild Facioscapulohumeral Muscular Dystrophy Using Shear Wave Elastography" Diagnostics 14, no. 17: 1985. https://doi.org/10.3390/diagnostics14171985
APA StyleKleiser, B., Zimmer, M., Ateş, F., & Marquetand, J. (2024). Characterizing Mechanical Changes in the Biceps Brachii Muscle in Mild Facioscapulohumeral Muscular Dystrophy Using Shear Wave Elastography. Diagnostics, 14(17), 1985. https://doi.org/10.3390/diagnostics14171985






