Enhancing Cone-Beam CT Image Quality in TIPSS Procedures Using AI Denoising
Abstract
:1. Introduction
2. Methods
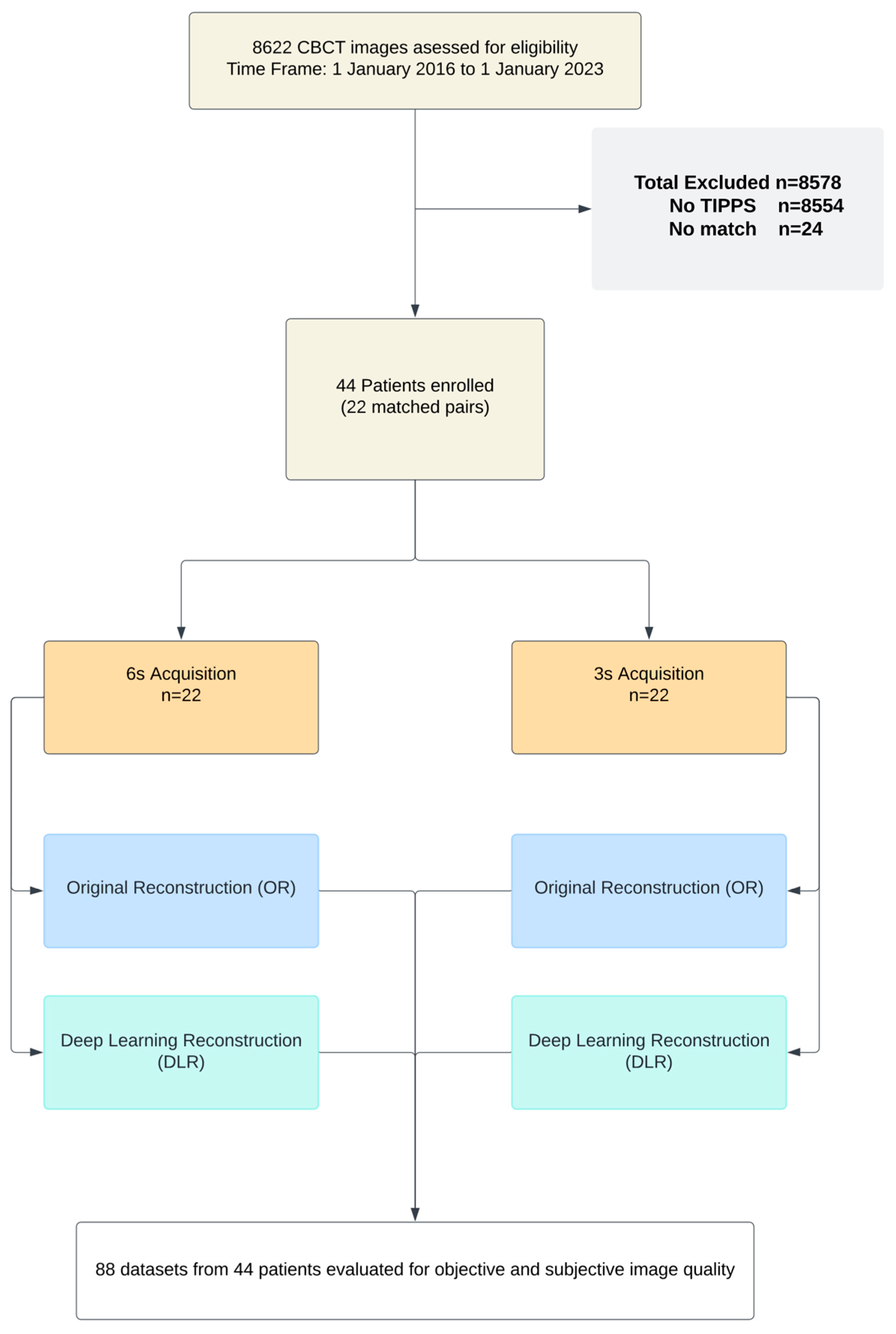
2.1. Study Population and Patient Characteristics
2.2. Image Acquisition, Reconstruction, and Postprocessing
2.3. Objective Image Quality
2.4. Subjective Image Quality
2.5. Statistical Analysis
3. Results
3.1. Study Population
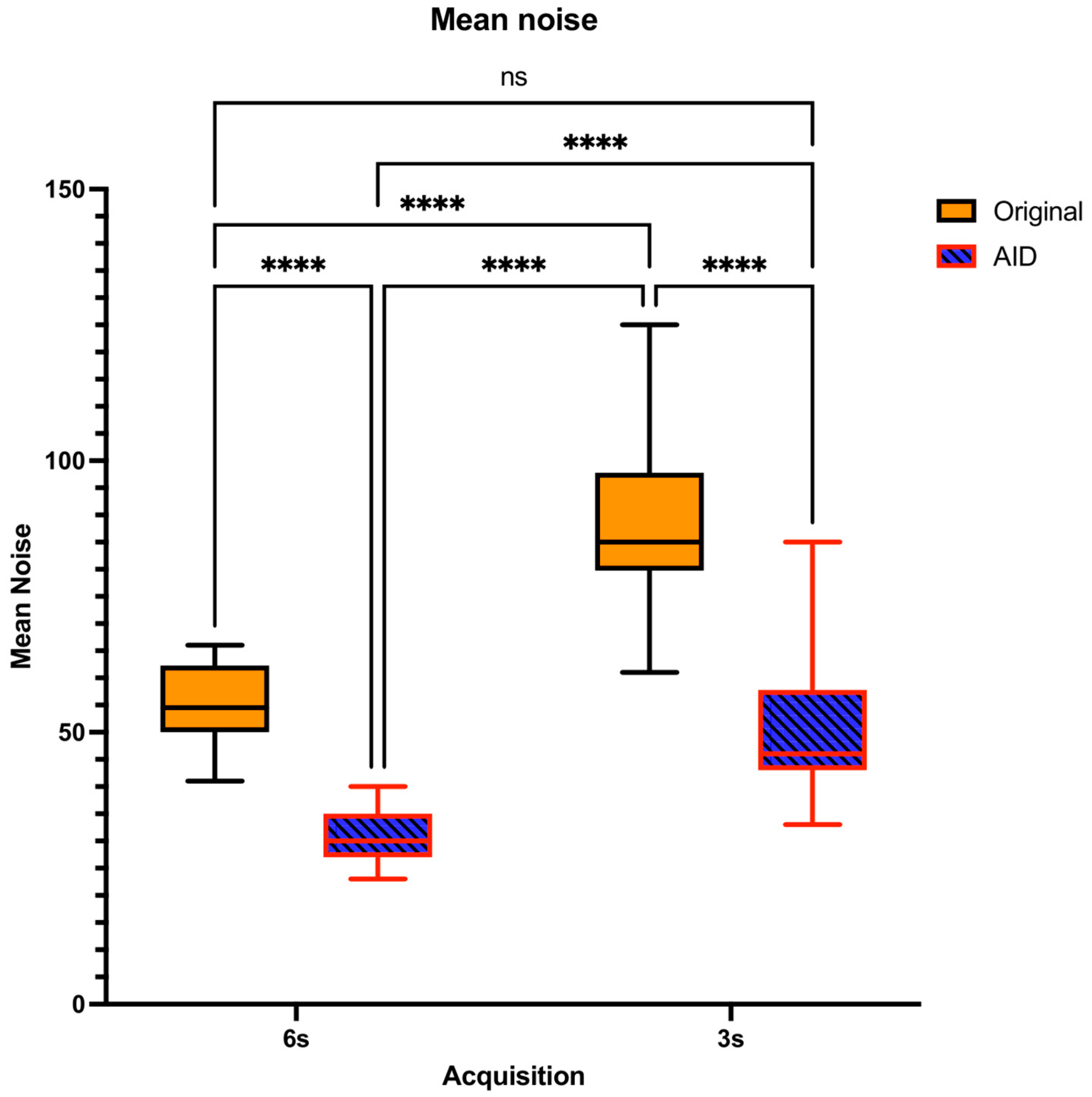
3.2. Objective Image Quality Assessment
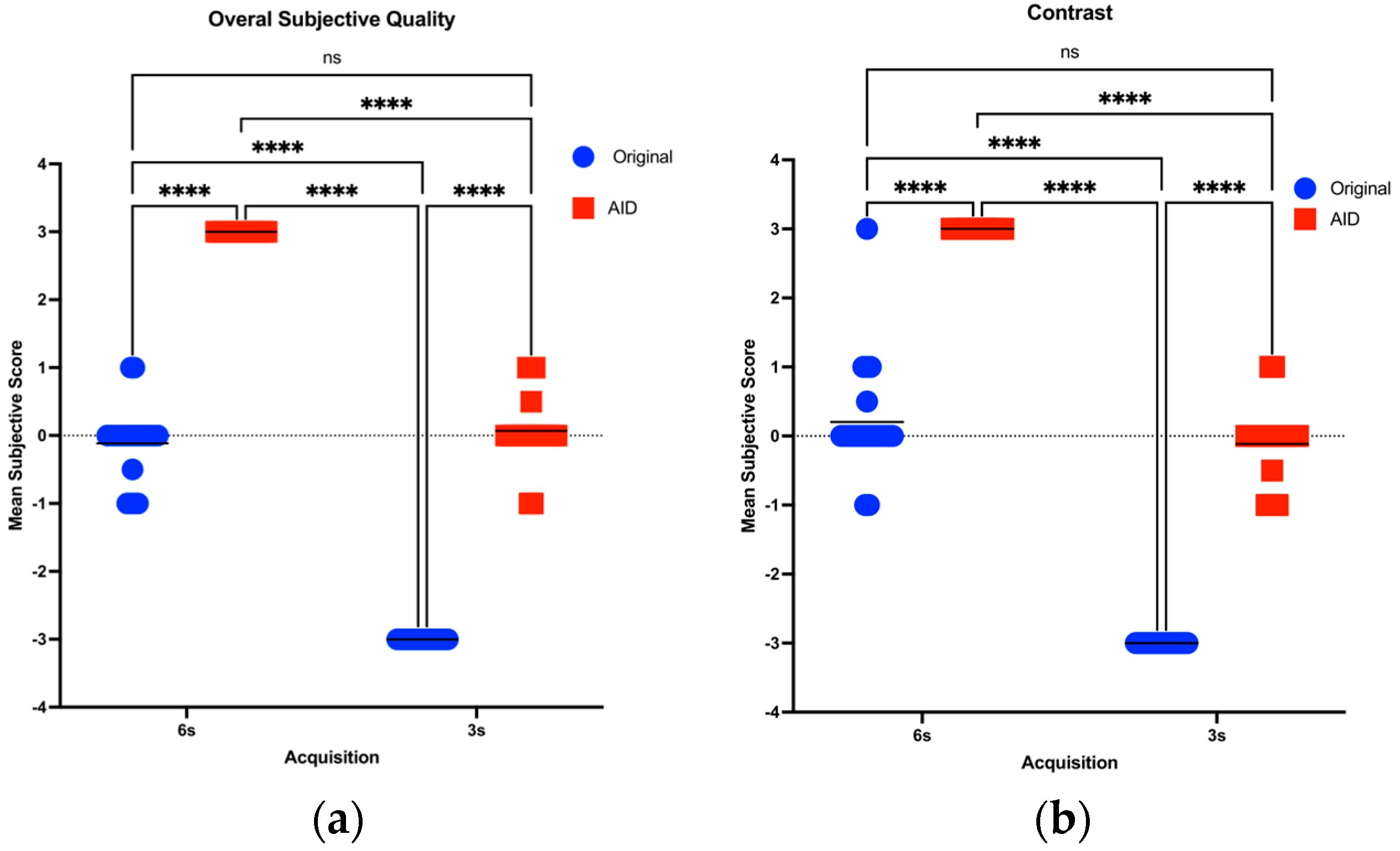
3.3. Subjective Image Quality Assessment
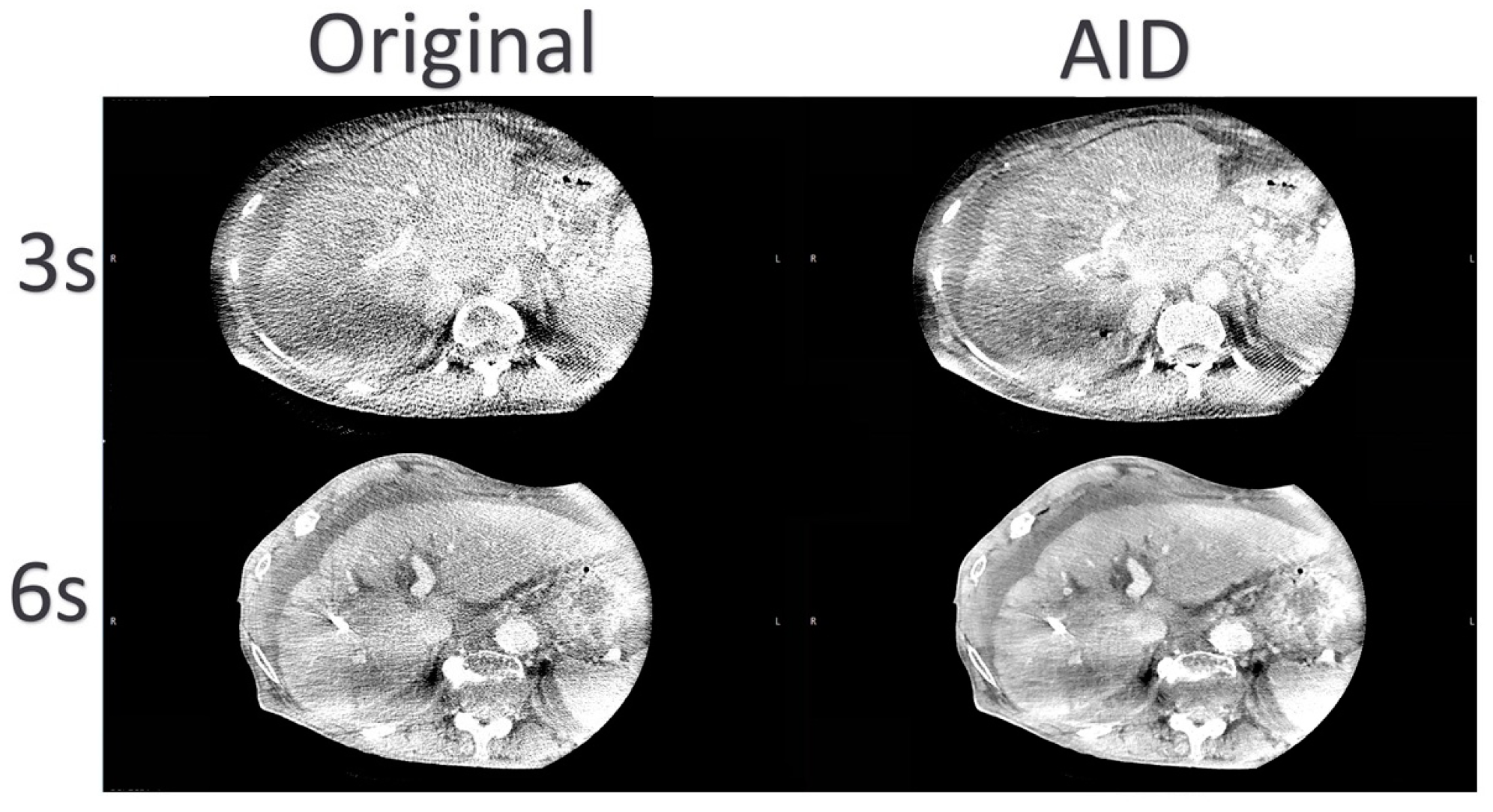
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PH | Portal hypertension |
| CLD | Chronic liver disease |
| TIPSS | Transjugular intrahepatic portosystemic shunt |
| CBCT | Cone-beam CT |
| FOV | Field of view |
| CNR | Contrast-to-Noise Ratio |
| AI | Artificial Intelligence |
| BMI | Body mass index |
| AID | AI denoising |
| ROI | Regions of interest |
| HU | Hounsfield Units |
| SD | Standard Deviation |
| Original | Original reconstruction |
| DAP | Dose area product |
| CNN | Convolutional neural network |
References
- Mauro, E.; Gadano, A. What’s new in portal hypertension? Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40 (Suppl. 1), 122–127. [Google Scholar] [CrossRef] [PubMed]
- Ketelsen, D.; Groezinger, G.; Maurer, M.; Lauer, U.M.; Grosse, U.; Horger, M.; Nikolaou, K.; Syha, R. Three-dimensional C-arm CT-guided transjugular intrahepatic portosystemic shunt placement: Feasibility, technical success and procedural time. Eur. Radiol. 2016, 26, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Vizzutti, F.; Schepis, F.; Arena, U.; Fanelli, F.; Gitto, S.; Aspite, S.; Turco, L.; Dragoni, G.; Laffi, G.; Marra, F. Transjugular intrahepatic portosystemic shunt (TIPS): Current indications and strategies to improve the outcomes. Intern. Emerg. Med. 2020, 15, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Fidelman, N.; Kwan, S.W.; LaBerge, J.M.; Gordon, R.L.; Ring, E.J.; Kerlan, R.K. The transjugular intrahepatic portosystemic shunt: An update. Am. J. Roentgenol. 2012, 199, 746–755. [Google Scholar] [CrossRef]
- Rossle, M.; Haag, K.; Ochs, A.; Sellinger, M.; Noldge, G.; Perarnau, J.-M.; Berger, E.; Blum, U.; Gabelmann, A.; Hauenstein, K.; et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N. Engl. J. Med. 1994, 330, 165–171. [Google Scholar] [CrossRef]
- Floridi, C.; Radaelli, A.; Abi-Jaoudeh, N.; Grass, M.; De Lin, M.; Chiaradia, M.; Geschwind, J.F.; Kobeiter, H.; Squillaci, E.; Maleux, G.; et al. C-arm cone-beam computed tomography in interventional oncology: Technical aspects and clinical applications. La Radiol. Medica 2014, 119, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Böning, G.; Lüdemann, W.M.; Chapiro, J.; Jonczyk, M.; Hamm, B.; Günther, R.W.; Gebauer, B.; Streitparth, F. Clinical Experience with Real-Time 3-D Guidance Based on C-Arm-Acquired Cone-Beam CT (CBCT) in Transjugular Intrahepatic Portosystemic Stent Shunt (TIPSS) Placement. Cardiovasc. Interv. Radiol. 2018, 41, 1035–1042. [Google Scholar] [CrossRef]
- Braak, S.; Meijer, E.; Mali, W. Operator Radiation Exposure in Cone-Beam Computed Tomography Guidance. J. Belg. Soc. Radiol. 2016, 100, 140. [Google Scholar]
- Estler, A.; Herrmann, J.; Artzner, C.; Hoffmann, R.; Nikolaou, K.; Hepp, T.; Grosse, U.; Schempf, U.; Seith, F.; Groezinger, G. Use of Cone-Beam Computed Tomography (CBCT) for Targeting the Portal Vein in Transjugular Intrahepatic Portosystemic Shunt (TIPS) Procedures: Comparison of Low-Dose with Standard-Dose CBCT. Iran. J. Radiol. 2021, 18, 111704. [Google Scholar] [CrossRef]
- Brendlin, A.S.; Estler, A.; Plajer, D.; Lutz, A.; Grözinger, G.; Bongers, M.N.; Tsiflikas, I.; Afat, S.; Artzner, C.P. AI Denoising Significantly Enhances Image Quality and Diagnostic Confidence in Interventional Cone-Beam Computed Tomography. Tomography 2022, 8, 933–947. [Google Scholar] [CrossRef]
- Hong, J.H.; Park, E.-A.; Lee, W.; Ahn, C.; Kim, J.-H. Incremental Image Noise Reduction in Coronary CT Angiography Using a Deep Learning-Based Technique with Iterative Reconstruction. Korean J. Radiol. 2020, 21, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Brendlin, A.S.; Dehdab, R.; Stenzl, B.; Mueck, J.; Ghibes, P.; Groezinger, G.; Kim, J.; Afat, S.; Artzner, C. Novel Deep Learning Denoising Enhances Image Quality and Lowers Radiation Exposure in Interventional Bronchial Artery Embolization Cone Beam CT. Acad. Radiol. 2023, 30, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, S.H.; Kim, S. Self-supervised denoising of projection data for low-dose cone-beam CT. Med. Phys. 2023, 50, 6319–6333. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Lange, M.; Lenga, L.; Yel, I.; Koch, V.; Booz, C.; Martin, S.; Bernatz, S.; Vogl, T.; Albrecht, M.; et al. Salvaging low contrast abdominal CT studies using noise-optimised virtual monoenergetic image reconstruction. BJR Open 2022, 4, 20220006. [Google Scholar] [CrossRef]
- Lau, B.K.F.; Dillon, O.; Vinod, S.K.; O’Brien, R.T.; Reynolds, T. Faster and lower dose imaging: Evaluating adaptive, constant gantry velocity and angular separation in fast low-dose 4D cone beam CT imaging. Med. Phys. 2024, 51, 1364–1382. [Google Scholar] [CrossRef]
- Yuasa, Y.; Shiinoki, T.; Fujimoto, K.; Hanazawa, H.; Uehara, T.; Koike, M.; Shibuya, K. Effect of gantry speed on accuracy of extracted target motion trajectories and image quality in 4D-CBCT: Phantom study. Biomed. Phys. Eng. Express 2017, 3, 067001. [Google Scholar] [CrossRef]
- Kember, S.A.; Hansen, V.N.; Fast, M.F.; Nill, S.; McDonald, F.; Ahmed, M.; Thomas, K.; McNair, H.A. Evaluation of three presets for four-dimensional cone beam CT in lung radiotherapy verification by visual grading analysis. Br. J. Radiol. 2016, 89, 20150933. [Google Scholar] [CrossRef]
- Zhao, X.; Du, Y.; Yue, H.; Wang, R.; Zhou, S.; Wu, H.; Wang, W.; Peng, Y. Deep learning-based projection synthesis for low-dose conebeam computed tomography imaging in image-guided radiotherapy. Quant. Imaging Med. Surg. 2024, 14, 231–250. [Google Scholar] [CrossRef]
- Beaudry, J.; Esquinas, P.L.; Shieh, C.-C. Learning from our neighbours: A novel approach on sinogram completion using bin-sharing and deep learning to reconstruct high quality 4DCBCT. In Proceedings of the Medical Imaging 2019: Physics of Medical Imaging, San Diego, CA, USA, 17–20 February 2019; Volume 10948, pp. 1025–1035. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.; Kim, W.; Kang, S.; Park, C.; Cho, H.; Lim, Y.; Kim, G.; Park, S.; Lim, H.; et al. Four-Dimensional CBCT Reconstruction Based on a Residual Convolutional Neural Network for Improving Image Quality. J. Korean Phys. Soc. 2019, 75, 73–79. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Z.; Chang, Y.; Ge, Y.; Yin, F.-F.; Ren, L. Enhancement of 4-D Cone-Beam Computed Tomography (4D-CBCT) Using a Dual-Encoder Convolutional Neural Network (DeCNN). IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Liu, K.; Zhang, L. Research on improved black widow algorithm for medical image denoising. Sci. Rep. 2024, 14, 2514. [Google Scholar] [CrossRef] [PubMed]




| Sex | Dataset | n | Age | Height | Weight | DAP | BMI |
|---|---|---|---|---|---|---|---|
| Female | 3 s | 9 | 40.89 ± 19.93 | 160.44 ± 8.13 | 62.44 ± 12.10 | 2368.11 ± 501.41 | 24.23 ± 3.88 |
| 6 s | 11 | 53.91 ± 16.78 | 165.82 ± 4.56 | 59.55 ± 7.61 | 4678.56 ± 644.74 | 21.67 ± 2.86 | |
| Overall | 20 | 48.05 ± 18.97 | 163.40 ± 6.80 | 60.85 ± 9.71 | 3638.86 ± 1309.73 | 22.82 ± 3.51 | |
| Male | 3 s | 13 | 54.23 ± 22.97 | 172.39 ± 18.72 | 70.80 ± 19.97 | 2615.11 ± 801.48 | 23.09 ± 4.36 |
| 6 s | 11 | 51.27 ± 20.45 | 165.55 ± 23.42 | 74.51 ± 23.81 | 5598.44 ± 1675.99 | 26.42 ± 6.87 | |
| Overall | 24 | 52.88 ± 21.43 | 169.25 ± 20.82 | 72.50 ± 21.41 | 3982.47 ± 1965.22 | 24.62 ± 5.77 | |
| Overall | 3 s | 22 | 48.77 ± 22.32 | 167.50 ± 16.17 | 67.38 ± 17.36 | 2514.06 ± 691.59 | 23.56 ± 4.12 |
| 6 s | 22 | 52.59 ± 18.31 | 165.68 ± 16.47 | 67.03 ± 18.87 | 5138.50 ± 1325.57 | 24.05 ± 5.68 | |
| Overall | 44 | 50.68 ± 20.26 | 166.59 ± 16.16 | 67.20 ± 17.92 | 3826.28 ± 1689.29 | 23.80 ± 4.91 |
| Parameter | Dataset | Reconstruction | Mean ± SD | Adjusted Two-Tailed Pairwise Comparisons (p) | |||
|---|---|---|---|---|---|---|---|
| 3 s-AID | 3 s-Original | 6 s-AID | 6 s-Original | ||||
| CNR | 3 s | AID | 3.81 ± 0.86 | n/a | <0.0001 | <0.0001 | 0.9968 |
| Original | 2.15 ± 0.40 | <0.0001 | n/a | <0.0001 | <0.0001 | ||
| 6 s | AID | 6.45 ± 1.23 | <0.0001 | <0.0001 | n/a | <0.0001 | |
| Original | 3.65 ± 0.67 | 0.9968 | <0.0001 | <0.0001 | n/a | ||
| Contrast | 3 s | AID | 190.99 ± 44.69 | n/a | >0.9999 | >0.9999 | 0.9998 |
| Original | 191.91 ± 42.94 | >0.9999 | n/a | >0.9999 | >0.9999 | ||
| 6 s | AID | 195.16 ± 35.88 | >0.9999 | >0.9999 | n/a | 0.9554 | |
| Original | 200.34 ± 38.67 | 0.9998 | >0.9999 | 0.9554 | n/a | ||
| Mean Noise | 3 s | AID | 51.23 ± 12.11 | n/a | <0.0001 | <0.0001 | 0.9423 |
| Original | 89.64 ± 14.44 | <0.0001 | n/a | <0.0001 | <0.0001 | ||
| 6 s | AID | 30.59 ± 4.60 | <0.0001 | <0.0001 | n/a | <0.0001 | |
| Original | 55.18 ± 7.06 | 0.9423 | <0.0001 | <0.0001 | n/a | ||
| Parameter | Run | Reconstruction | Rater 1 Mean ± SD | Rater 2 Mean ± SD | Spearman’s Rho |
|---|---|---|---|---|---|
| Overall Subjective image quality | 3 s | AID | 0.011 ± 0.750 | 0.023 ± 0.758 | 0.990 |
| Original | −0.750 ± 0.435 | −0.750 ± 0.435 | 1 | ||
| 6 s | AID | 0.750 ± 0.435 | 0.750 ± 0.435 | 1 | |
| Original | −0.023 ± 0.758 | −0.034 ± 0.765 | 0.990 | ||
| Contrast | 3 s | AID | −0.023 ± 0.758 | −0.034 ± 0.765 | 0.990 |
| Original | −0.750 ± 0.435 | −0.750 ± 0.435 | 1 | ||
| 6 s | AID | 0.750 ± 0.435 | 0.750 ± 0.435 | 1 | |
| Original | 0.045 ± 0.757 | 0.057 ± 0.764 | 0.990 | ||
| Motion Artifacts | 3 s | AID | 0.557 ± 0.522 | 0.580 ± 0.519 | 0.954 |
| Original | 0.330 ± 0.656 | 0.330 ± 0.690 | 0.888 | ||
| 6 s | AID | −0.352 ± 0.644 | −0.409 ± 0.600 | 0.839 | |
| Original | −0.580 ± 0.496 | −0.545 ± 0.501 | 0.794 | ||
| Sharpness | 3 s | AID | 0.023 ± 0.727 | 0.011 ± 0.735 | 0.990 |
| Original | −0.750 ± 0.435 | −0.750 ± 0.435 | 1 | ||
| 6 s | AID | 0.750 ± 0.435 | 0.750 ± 0.435 | 1 | |
| Original | −0.023 ± 0.727 | −0.011 ± 0.735 | 0.990 |
| Parameter | Dataset | Recon | Mean ± SD | Adjusted Two-Tailed Pairwise Comparisons (p) | |||
|---|---|---|---|---|---|---|---|
| 3 s AID | 3 s Original | 6 s AID | 6 s Original | ||||
| Overall Subjective image quality | 3 s | AID | 0.017 ± 0.752 | n/a | <0.0001 | <0.0001 | >0.9999 |
| Original | −0.750 ± 0.435 | <0.0001 | n/a | <0.0001 | <0.0001 | ||
| 6 s | AID | 0.750 ± 0.435 | <0.0001 | <0.0001 | n/a | <0.0001 | |
| Original | −0.028 ± 0.759 | >0.9999 | <0.0001 | <0.0001 | n/a | ||
| Contrast | 3 s | AID | −0.028 ± 0.759 | n/a | <0.0001 | <0.0001 | 0.9971 |
| Original | −0.750 ± 0.435 | <0.0001 | n/a | <0.0001 | <0.0001 | ||
| 6 s | AID | 0.750 ± 0.435 | <0.0001 | <0.0001 | n/a | <0.0001 | |
| Original | 0.051 ± 0.758 | 0.9971 | <0.0001 | <0.0001 | n/a | ||
| Motion Artifacts | 3 s | AID | 0.568 ± 0.515 | n/a | 0.0751 | <0.0001 | <0.0001 |
| Original | 0.330 ± 0.656 | 0.0751 | n/a | <0.0001 | <0.0001 | ||
| 6 s | AID | −0.381 ± 0.587 | <0.0001 | <0.0001 | n/a | 0.1182 | |
| Original | −0.562 ± 0.472 | <0.0001 | <0.0001 | 0.1182 | n/a | ||
| Sharpness | 3 s | AID | 0.017 ± 0.729 | n/a | <0.0001 | <0.0001 | 0.9994 |
| Original | −0.750 ± 0.435 | <0.0001 | n/a | <0.0001 | <0.0001 | ||
| 6 s | AID | 0.750 ± 0.435 | <0.0001 | <0.0001 | n/a | <0.0001 | |
| Original | −0.017 ± 0.729 | 0.9994 | <0.0001 | <0.0001 | n/a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehdab, R.; Brendlin, A.S.; Grözinger, G.; Almansour, H.; Brendel, J.M.; Gassenmaier, S.; Ghibes, P.; Werner, S.; Nikolaou, K.; Afat, S. Enhancing Cone-Beam CT Image Quality in TIPSS Procedures Using AI Denoising. Diagnostics 2024, 14, 1989. https://doi.org/10.3390/diagnostics14171989
Dehdab R, Brendlin AS, Grözinger G, Almansour H, Brendel JM, Gassenmaier S, Ghibes P, Werner S, Nikolaou K, Afat S. Enhancing Cone-Beam CT Image Quality in TIPSS Procedures Using AI Denoising. Diagnostics. 2024; 14(17):1989. https://doi.org/10.3390/diagnostics14171989
Chicago/Turabian StyleDehdab, Reza, Andreas S. Brendlin, Gerd Grözinger, Haidara Almansour, Jan Michael Brendel, Sebastian Gassenmaier, Patrick Ghibes, Sebastian Werner, Konstantin Nikolaou, and Saif Afat. 2024. "Enhancing Cone-Beam CT Image Quality in TIPSS Procedures Using AI Denoising" Diagnostics 14, no. 17: 1989. https://doi.org/10.3390/diagnostics14171989






