Artificial Intelligence-Based Screening System for Diabetic Retinopathy in Primary Care
Abstract
:1. Introduction
2. Overview of the Published Articles
3. Subjects
3.1. Setting
3.2. Datasets
3.3. Design
3.4. Inclusion and Exclusion Criteria
3.4.1. Inclusion Criteria
- Type 2 diabetic patients included in the SIDIAP database for whom we had all the clinical and demographic variables necessary to feed the DRPA during the 11-year follow up study period.
- Type 2 diabetic patients from our database and Messidor-2 database with high-quality retinographies to feed the AIRS.
3.4.2. Exclusion Criteria
- Type 1 diabetic patients.
- Gestational diabetes.
- Patients who did not give informed consent.
- Type 2 diabetic patients with incomplete EHR or poor-quality retinographies.
4. Materials and Methods
4.1. Ethics and Consent
4.2. The Algorithms
4.2.1. Artificial-Intelligence-Based Reading System
Model Construction and Training
Validation
Testing
Diabetes Retinopathy Classification
4.2.2. Diabetic Retinopathy Prediction Algorithm
Model Construction and Training
Validation
Testing
4.3. Statistical Methods
5. Results
5.1. Testing the AIRS in Our Database
5.2. Testing the AIRS with Messidor-2
5.3. Testing the Diabetes Retinopathy Prediction Algorithm
5.3.1. Clinical and Demographic Data at Baseline
5.3.2. Performance of the Predictive Diabetic Retinopathy Algorithm
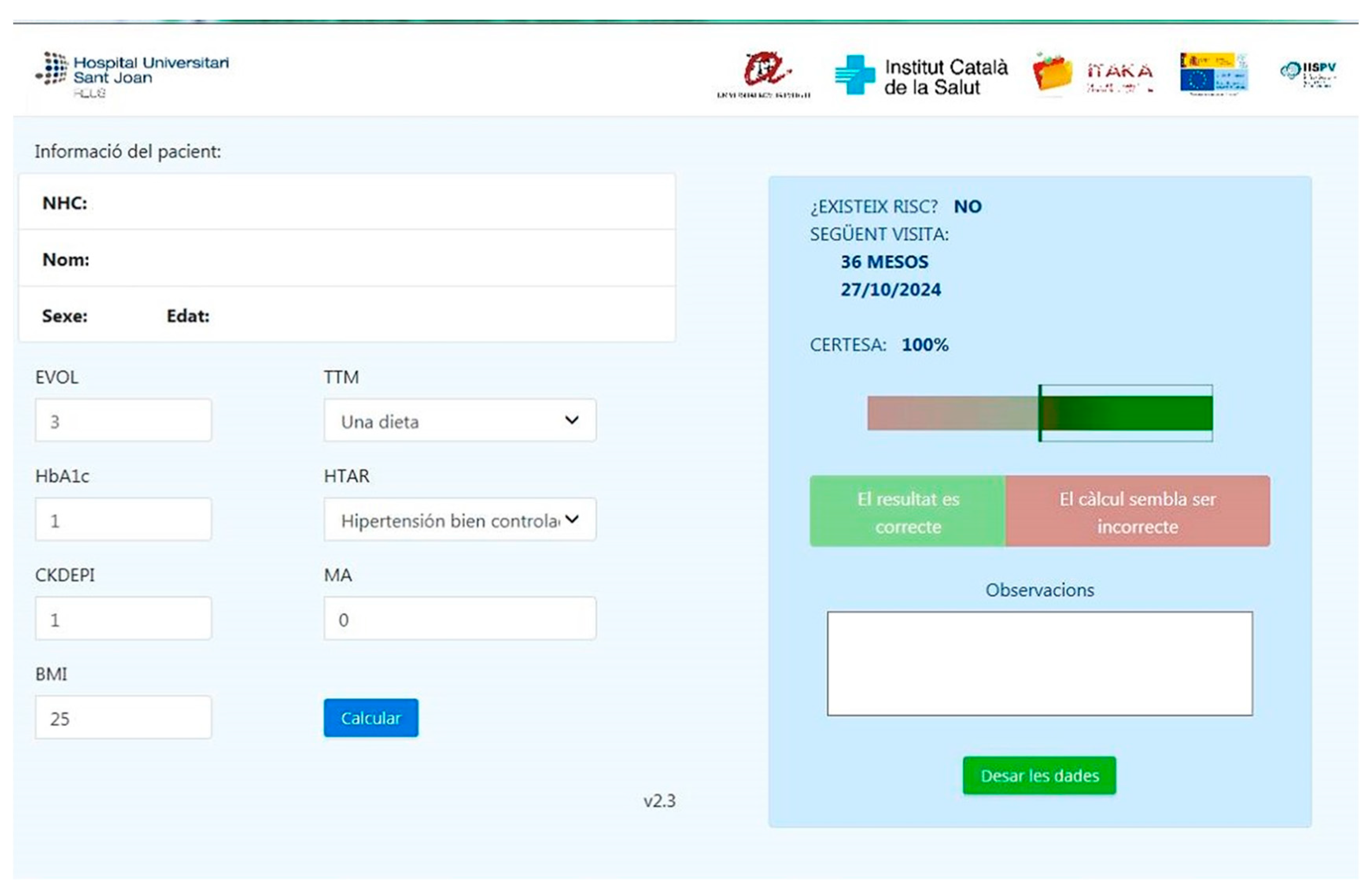
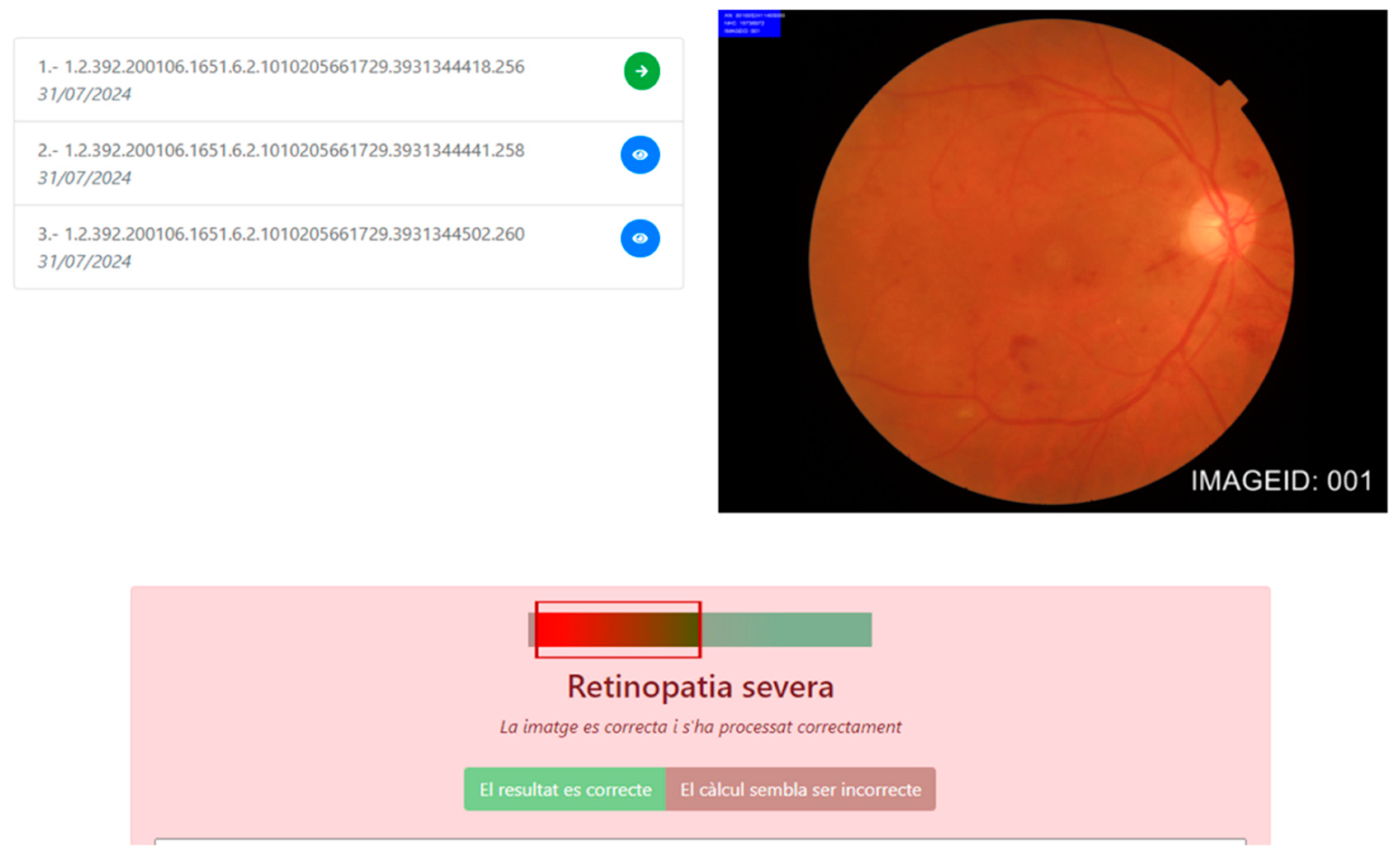
5.4. Patient Journey for the Early Detection of Diabetic Retinopathy in Primary Care
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; Da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The [email protected] Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Lee, L.J.; Yu, A.P.; Cahill, K.E.; Oglesby, A.K.; Tang, J.; Qiu, Y.; Birnbaum, H.G. Direct and indirect costs among employees with diabetic retinopathy in the United States. Curr. Med Res. Opin. 2008, 24, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Happich, M.; Reitberger, U.; Breitscheidel, L.; Ulbig, M.; Watkins, J. The economic burden of diabetic retinopathy in Germany in 2002. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ghanchi, F.; the Diabetic Retinopathy Guidelines Working Group. The Royal College of Ophthalmologists’ clinical guidelines for diabetic retinopathy: A summary. Eye 2013, 27, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Wu, A.M.; Young, B.K.; Wu, D.J.; Margo, C.E.; Greenberg, P.B. An Appraisal of Clinical Practice Guidelines for Diabetic Retinopathy. Am. J. Med Qual. 2016, 31, 370–375. [Google Scholar] [CrossRef]
- López, M.I.; Díez, A.; Velilla, S.; Rueda, A.; Alvarez, A.; Pastor, J.C. Prevalence of diabetic retinopathy and eye care in a rural area of Spain. Ophthalmic Epidemiol. 2002, 9, 205–214. [Google Scholar] [CrossRef]
- Wong, T.Y.; Bressler, N.M. Artificial intelligence with deep learning technology looks into diabetic retinopathy screening. JAMA 2016, 316, 2366–2367. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; Yeo, I.Y.S.; Lee, S.Y.; et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Lavin, P.T.; Birch, M.; Shah, N.; Folk, J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit. Med. 2018, 1, 39. [Google Scholar] [CrossRef] [PubMed]
- Scotland, G.S.; McNamee, P.; Philip, S.; Fleming, A.D.; Goatman, K.A.; Prescott, G.J.; Fonseca, S.; Sharp, P.F.; Olson, J.A. Cost-effectiveness of implementing automated grading within the national screening programme for diabetic retinopathy in Scotland. Br. J. Ophthalmol. 2007, 91, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.; Rudisill, C.; Egan, C.; Kapetanakis, V.V.; Salas-Vega, S.; Owen, C.G.; Lee, A.; Louw, V.; Anderson, J.; Liew, G.; et al. Automated Diabetic Retinopathy Image Assessment Software: Diagnostic Accuracy and Cost-Effectiveness Compared with Human Graders. Ophthalmology 2017, 124, 343–351. [Google Scholar] [CrossRef] [PubMed]
- O’keeffe, D.; Riordan, F.; Harkins, V.; Kearney, P.; Mc Hugh, S. Predictors of attendance at diabetic retinopathy screening among people with type 2 diabetes: Secondary analysis of data from primary care. Prim. Care Diabetes 2021, 15, 1086–1094. [Google Scholar] [CrossRef]
- Van Der Heijden, A.A.; Abramoff, M.D.; Verbraak, F.; Van Hecke, M.V.; Liem, A.; Nijpels, G. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 2018, 96, 63–68. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef]
- Bhaskaranand, M.; Ramachandra, C.; Bhat, S.; Cuadros, J.; Nittala, M.G.; Sadda, S.R.; Solanki, K. The value of automated diabetic retinopathy screening with the EyeArt system: A study of more than 100,000 consecutive encounters from people with diabetes. Diabetes Technol. Ther. 2019, 21, 635–643. [Google Scholar] [CrossRef]
- Ribeiro, M.L.; Nunes, S.G.; Cunha-Vaz, J.G. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care 2013, 36, 1254–1259. [Google Scholar] [CrossRef]
- González-Gonzalo, C.; Sánchez-Gutiérrez, V.; Hernández-Martínez, P.; Contreras, I.; Lechanteur, Y.T.; Domanian, A.; van Ginneken, B.; Sánchez, C.I. Evaluation of a deep learning system for the joint automated detection of diabetic retinopathy and age-related macular degeneration. Acta Ophthalmol. 2020, 98, 368–377. [Google Scholar] [CrossRef]
- Wong, D.C.S.; Kiew, G.; Jeon, S.; Ting, D.; Wong, D.; Kiew, G. Singapore Eye Lesions Analyzer (SELENA): The Deep Learning System for Retinal Diseases. In Artificial Intelligence in Ophthalmology, 1st ed.; Grzybowski, A., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 177–185. [Google Scholar]
- Li, Z.; Keel, S.; Liu, C.; He, Y.; Meng, W.; Scheetz, J.; Lee, P.Y.; Shaw, J.; Ting, D.; Wong, T.Y.; et al. An automated grading System for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care 2018, 41, 2509–2516. [Google Scholar] [CrossRef]
- Ruamviboonsuk, P.; Krause, J.; Chotcomwongse, P.; Sayres, R.; Raman, R.; Widner, K.; Campana, B.J.L.; Phene, S.; Hemarat, K.; Tadarati, M.; et al. Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. NPJ Digit. Med. 2019, 2, 25. [Google Scholar] [CrossRef]
- Soliz, P. Multicenter, head-to-head, real-world validation study of seven automated artificial intelligence diabetic retinopathy screening systems. Diabetes Care 2021, 44, 1168–1175. [Google Scholar] [CrossRef]
- Aspelund, T.; Þórisdóttir, Ó.; Ólafsdottir, E.; Gudmundsdottir, A.; Einarsdóttir, A.B.; Mehlsen, J.; Einarsson, S.; Pálsson, Ó.; Einarsson, G.; Bek, T.; et al. Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia 2011, 54, 2525–2532. [Google Scholar] [CrossRef]
- Soto-Pedre, E.; Pinies, J.A.; Hernaez-Ortega, M.C. External validation of a risk assessment model to adjust the frequency of eye-screening visits in patients with diabetes mellitus. J. Diabetes Its Complicat. 2015, 29, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.H.; Aspelund, T.; Kirby, P.; Russell, G.; Einarsson, S.; Palsson, O.; Stefánsson, E. Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: A scientific approach to reducing healthcare costs. Br. J. Ophthalmol. 2016, 100, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H.; Aldington, S.J.; Leal, J.; Luengo-Fernandez, R.; Oke, J.; Sivaprasad, S.; Gazis, A.; Stratton, I.M. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Heal. Technol. Assess. 2015, 19, 1–116. [Google Scholar] [CrossRef]
- Broadbent, D.M.; Sampson, C.J.; Wang, A.; Howard, L.; Williams, A.E.; Howlin, S.U.; Appelbe, D.; Moitt, T.; Cheyne, C.P.; Rahni, M.M.; et al. Individualised screening for diabetic retinopathy: The ISDR study—rationale, design and methodology for a randomised controlled trial comparing annual and individualised risk-based variable-interval screening. BMJ Open 2019, 9, e025788. [Google Scholar] [CrossRef]
- Decencière, E.; Zhang, X.; Cazuguel, G.; Lay, B.; Cochener, B.; Trone, C.; Gain, P.; Ordonez, R.; Massin, P.; Erginay, A.; et al. feedback on a publicly distributed image database: The Messidor database. Image Anal. Ster. 2014, 33, 231. [Google Scholar] [CrossRef]
- Cuadros, J.; Bresnick, G. EyePACS: An adaptable telemedicine system for diabetic retinopathy screening. J. Diabetes Sci. Technol. 2009, 3, 509–516. [Google Scholar] [CrossRef]
- de la Torre, J.; Puig, D.; Valls, A. Weighted kappa loss function for multi-class classification of ordinal data in deep learning. Pattern Recognit. Lett. 2018, 105, 144–154. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Verges-Puig, R.; de la Torre, J.; Valls, A.; Relaño-Barambio, N.; Puig, D.; Baget-Bernaldiz, M. Validation of a Deep Learning Algorithm for Diabetic Retinopathy. Telemed. e-Health 2020, 26, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P.; Valls, A.; Moreno, A.; Sagarra-Alamo, R.; Basora-Gallisa, J.; Saleh, E.; Baget-Bernaldiz, M.; Puig, D. A Clinical Decision Support System for Diabetic Retinopathy Screening: Creating a Clinical Support Application. Telemed. e-Health 2019, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P.; Verges-Pujol, R.; Santos-Blanco, E.; Maarof, N.; Valls, A.; Mundet, X.; Moreno, A.; Galindo, L.; Baget-Bernaldiz, M. Validation of a diagnostic support system for diabetic retinopathy based on clinical parameters. Transl. Vis. Sci. Technol. 2021, 10, 17. [Google Scholar] [CrossRef]
- Ribeiro, L.; Oliveira, C.M.; Neves, C.; Ramos, J.D.; Ferreira, H.; Cunha-Vaz, J. Screening for diabetic retinopathy in the central region of portugal. added value of automated ‘disease/no disease’ grading. Ophthalmologica 2015, 233, 96–103. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, A.A.W.A.; Walraven, I.; Riet, E.V.; Aspelund, T.; Lund, S.H.; Elders, P.; Polak, B.C.P.; Moll, A.C.; Keunen, J.E.E.; Dekker, J.M.; et al. Validation of a model to estimate personalised screening frequency to monitor diabetic retinopathy. Diabetologia 2014, 57, 1332–1338. [Google Scholar] [CrossRef] [PubMed]

| Algorithm | Tech | Year | Training Set | Validation Set | Testing Set | DR Classification | Detection | AUC | S (%) | SP (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| IDX-DR | CNN | 2018 | N/A | N/A | Messidor-2 | FPRC | RDR | N/A | 96.8 | 59.4 |
| EyeArt 2.0 | Image analysis technology | 2019 | EyePACS | N/A | 850,908 | ICDR | RDR | 0.96 | 91.3 | 91.1 |
| Retmarker | Recognition of lesions | 2011 | N/A | N/A | 21,514 | No RD/RDR | DR/no DR | 0.84 | 95.8 | 63.2 |
| SELENA+ | CNN | 2019 | ImageNet | N/A | 3556 | ICDR | RDR | 0.95 | 91.8 | 98.7 |
| LabelMe | CNN | N/A | 71,043 | 35,201 | N/A | N/A | RDR | 0.95 | 92.5 | 98.5 |
| ARDA | CNN | N/A | 130,000 | Messidor-2 EyePACS | N/A | N/A | RDR | 0.99 | 87 | 98.5 |
| MIRA | CNN | 2019 | Own dataset EyePACS | Own dataset 5000 | Own data Messidor-2 | Messidor-2 | RDR | 0.92–0.958 | 94.6–96.7 | 99.1–99.8 |
| Algorithm | Age | Sex | DM Duration | DM Treatment | HbA1c | HTA | eGFR | Protein in Urine | BMI | Type DM | Cholesterol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspelund | X | X | X | X | X | ||||||
| Scanlon | X | X | X | ||||||||
| Broadbent | X | X | |||||||||
| Retiprogram | X | X | X | X | X | X | X | X | X |
| Author | Testing Place (Group) | Number of Patients | AUC |
|---|---|---|---|
| Aspelund | Spain (Soto-Pedre) | 508 | 0.74 |
| UK (Lund) | 9690 | 0.83 | |
| Scanlon | Ireland (Smith) | 2929 | 0.77 |
| Broadbent | UK | 4460 | 0.88 |
| Retiprogram | Catalonia (Romero) | 40,129 | 0.92–0.96 |
| DR Grade | Microaneurysms (μA) | Hemorrhages (H) | Neovascularization |
|---|---|---|---|
| No DR | 0 | 0 | 0 |
| Mild DR | ≤5 | 0 | 0 |
| Moderate DR | 5 < μA < 15 | 0 < H < 5 | 0 |
| Severe DR | μA ≥ 15 | H ≥ 5 | 0/1 |
| Variable | Without DR | With DR | p |
|---|---|---|---|
| Age (y) | 68.53 ± 11.06 (30–99) | 69.89 ± 9.89 (33–98) | 0.684 1 |
| Female (%) | 46.67 | 48.40 | 0.3802 2 |
| Diabetes duration (y) | 7.25 ± 5.20 (0.2–56.99) | 11.15 ± 6.90 (0.2–48.87) | <0.001 1 |
| HbA1c (%) | 7.22 ± 1.26 (3.5–16.5) | 7.82 ± 1.45 (3.8–18.50) | <0.001 1 |
| Microalbuminuria (mg) | 34.73 ± 132.65 (0–59.75) | 81.08 ± 250.74 (16.24–2999.75) | <0.001 1 |
| Body mass index | 30.21 ± 5 (16–38.91) | 30.15 ± 5.15 (16.24–40.75) | 0.004 1 |
| Creatinine | 1.13 ± 0.23 (0.87–1.22) | 1.16 ± 0.35 (0.87–1.23) | <0.001 1 |
| eGFR (CKD-EPI) | 60.62 ± 7.56 (60.05–69.84) | 58.55 ± 9.54 (58.53–69.77) | <0.001 1 |
| Arterial hypertension (%) | 33 | 39 | <0.001 2 |
| Cholesterol total | 196 ± 41.3 (165–258) | 198 ± 43.4 (168–261) | 0.883 1 |
| Triglycerides | 168 ± 122 (42–298) | 168 ± 125 (40–301) | 0.386 1 |
| Predictions Given by the AIRS | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Classification provided by Ophthalmologists | 0 | 12.71 | 12 | 0 | 0 |
| 1 | 41 | 892 | 5 | 0 | |
| 2 | 9 | 35 | 1.065 | 0 | |
| 3 | 0 | 17 | 64 | 641 | |
| Total | 12.621 | 956 | 1.129 | 641 | |
| Predictions Given by the AIRS | |||
|---|---|---|---|
| Nonreferable DR | Referable DR | ||
| Classification provided by ophthalmologists | Nonreferable DR | 13,463 | 64 |
| Referable DR | 58 | 1706 | |
| 13,521 | 1770 | ||
| Predictions Given by the AIRS | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Classification provided by MESSIDOR-2 | 0 | 610 | 35 | 0 | 0 |
| 1 | 13 | 143 | 7 | 0 | |
| 2 | 2 | 15 | 116 | 10 | |
| 3 | 0 | 4 | 7 | 238 | |
| Total | 625 | 197 | 130 | 248 | |
| Predictions Given by the AIRS | |||
|---|---|---|---|
| Nonreferable DR | Referable DR | ||
| Classification provided by MESSIDOR-2 | Non-referable DR | 809 | 5 |
| Referable DR | 13 | 373 | |
| 822 | 378 | ||
| Independent Variables | Data |
|---|---|
Gender
| 22,859 (58%) 17,270 (42%) |
| Age (years) | 68.12 ± 10.39 (33–99) |
| BMI (kg/m2) | 27.28 ± 5.2 (18–36.81) |
Blood pressure control:
| 27,288 (68%) 12,841 (32%) |
| Glycosylated hemoglobin (%) Diabetes duration (years) | 7.76 ± 1.61 (4.8–16.6) 9.21 ± 5.51 (0.9–55.98) |
| Microalbuminuria (mg/24 h) Diabetes treatment | 257.3 ± 122.83 (16.23–3155.72) |
| 4419 (10.1%) 31,019 (77.3%) 4691 (12.6%) |
| eGFR | 73.09 ± 15.24 (60.05–83.25) |
| Type of DR at Baseline | Type of DR at the End of the Study | |||
|---|---|---|---|---|
| 0 | 36,758 | (91.6%) | 33,898 | (85.5%) |
| 1 | 3371 | (8.4%) | 4293 | (11.7%) |
| 2 | 1398 | (3.5%) | ||
| 3 | 199 | (0.5%) | ||
| 4 | 164 | (0.29%) | ||
| 5 | 241 | (1.05%) | ||
| Patients with any DR | 6295 | (15.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baget-Bernaldiz, M.; Fontoba-Poveda, B.; Romero-Aroca, P.; Navarro-Gil, R.; Hernando-Comerma, A.; Bautista-Perez, A.; Llagostera-Serra, M.; Morente-Lorenzo, C.; Vizcarro, M.; Mira-Puerto, A. Artificial Intelligence-Based Screening System for Diabetic Retinopathy in Primary Care. Diagnostics 2024, 14, 1992. https://doi.org/10.3390/diagnostics14171992
Baget-Bernaldiz M, Fontoba-Poveda B, Romero-Aroca P, Navarro-Gil R, Hernando-Comerma A, Bautista-Perez A, Llagostera-Serra M, Morente-Lorenzo C, Vizcarro M, Mira-Puerto A. Artificial Intelligence-Based Screening System for Diabetic Retinopathy in Primary Care. Diagnostics. 2024; 14(17):1992. https://doi.org/10.3390/diagnostics14171992
Chicago/Turabian StyleBaget-Bernaldiz, Marc, Benilde Fontoba-Poveda, Pedro Romero-Aroca, Raul Navarro-Gil, Adriana Hernando-Comerma, Angel Bautista-Perez, Monica Llagostera-Serra, Cristian Morente-Lorenzo, Montse Vizcarro, and Alejandra Mira-Puerto. 2024. "Artificial Intelligence-Based Screening System for Diabetic Retinopathy in Primary Care" Diagnostics 14, no. 17: 1992. https://doi.org/10.3390/diagnostics14171992







