Controversies in Venous Thromboembolism Risk Assessment in Inflammatory Bowel Disease: A Narrative Review
Abstract
:1. Introduction
2. Epidemiology
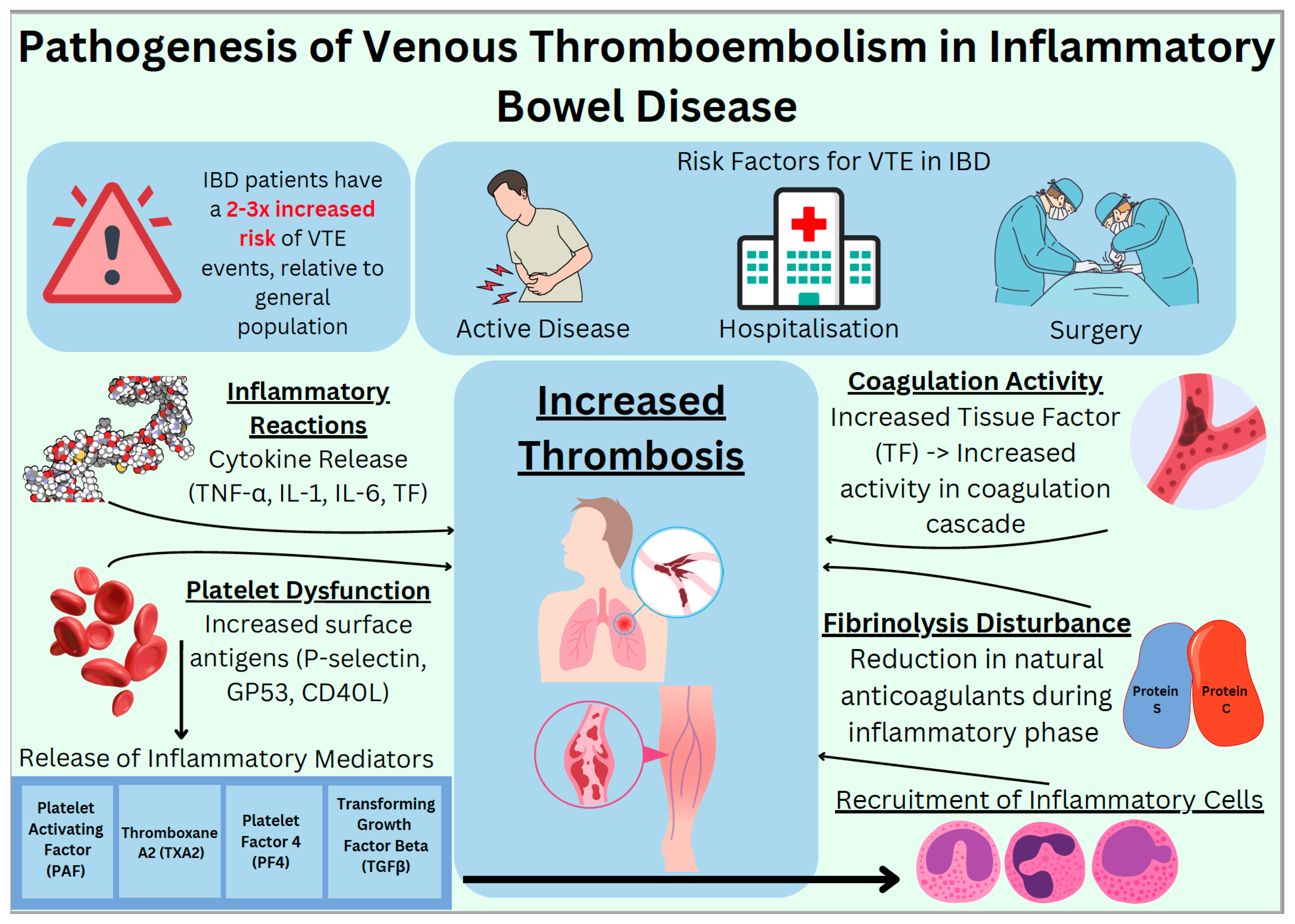
3. Pathophysiology and Risk Factors for VTE in IBD
4. Pregnancy
5. Post-Discharge Prophylaxis
6. Medication
6.1. 5-ASAs (5-Aminosalicylic Acid)
6.2. Corticosteroids
6.3. Thiopurines
6.4. Methotrexate
6.5. Tumour Necrosis Factor-Alpha Inhibitors (TNF-α)
6.6. Janus Kinase Inhibitors (JAKi)
6.7. Sphingosine 1-Phosphate (S1P) Receptor Modulators
6.8. Anti-Integrin Therapy
6.9. Anti IL-12 and Anti IL-23
7. Conclusions
8. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jimenez, K.M.; Gasche, C. Management of Iron Deficiency Anemia in Inflammatory Bowel Disease. Acta Haematol. 2019, 142, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.; Johansson, S.; Wallander, M.A.; García Rodríguez, L.A. Risk Factors and Short-Term Mortality of Venous Thromboembolism Diagnosed in the Primary Care Setting in the United Kingdom. Arch. Intern. Med. 2007, 167, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.; Millham, F. Increased Risk of Postoperative Deep Vein Thrombosis and Pulmonary Embolism in Patients with Inflammatory Bowel Disease: A Study of National Surgical Quality Improvement Program Patients. Arch. Surg. 2012, 147, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Faye, A.S. Venous Thromboembolism in Inflammatory Bowel Disease. World J. Gastroenterol. 2020, 26, 1231–1241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arvanitakis, K.D.; Arvanitaki, A.D.; Karkos, C.D.; Zintzaras, E.; Germanidis, G.S. The Risk of Venous Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Ann. Gastroenterol. 2021, 34, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Yuhara, H.; Steinmaus, C.; Corley, D.; Koike, J.; Igarashi, M.; Suzuki, T.; Mine, T. Meta-Analysis: The Risk of Venous Thromboembolism in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2013, 37, 953–962. [Google Scholar] [CrossRef]
- Grainge, M.J.; West, J.; Card, T.R. Venous Thromboembolism During Active Disease and Remission in Inflammatory Bowel Disease: A Cohort Study. Lancet 2010, 375, 657–663. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Horvath-Puho, E.; Sandler, R.S.; Rubin, D.T.; Ullman, T.A.; Pedersen, L.; Baron, J.A.; Sørensen, H.T. Thromboembolic Risk Among Danish Children and Adults with Inflammatory Bowel Diseases: A Population-Based Nationwide Study. Gut 2011, 60, 937–943. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Nugent, Z.; Singh, H. Persistently High Rate of Venous Thromboembolic Disease in Inflammatory Bowel Disease: A Population-Based Study. Am. J. Gastroenterol. 2021, 116, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Papa, A.; Saibeni, S.; Repici, A.; Malesci, A.; Vecchi, M. Inflammation and Coagulation in Inflammatory Bowel Disease: The Clot Thickens. Am. J. Gastroenterol. 2007, 102, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Kobbervig, C.E.; James, A.H.; Petterson, T.M.; Bailey, K.R.; Melton, L.J., 3rd. Trends in the Incidence of Venous Thromboembolism During Pregnancy or Postpartum: A 30-year Population-Based Study. Ann. Intern. Med. 2005, 143, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Virkus, R.A.; Løkkegaard, E.C.; Bergholt, T.; Mogensen, U.; Langhoff-Roos, J.; Lidegaard, Ø. Venous Thromboembolism in Pregnant and Puerperal Women in Denmark 1995–2005. A National Cohort Study. Thromb. Haemost. 2011, 106, 304–309. [Google Scholar] [CrossRef]
- Kim, Y.H.; Pfaller, B.; Marson, A.; Yim, H.W.; Huang, V.; Ito, S. The Risk of Venous Thromboembolism in Women with Inflammatory Bowel Disease During Pregnancy and the Postpartum Period: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e17309. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Navi, B.B.; Sriram, N.; Hovsepian, D.A.; Devereux, R.B.; Elkind, M.S.V. Risk of a Thrombotic Event After the 6-Week Postpartum Period. N. Engl. J. Med. 2014, 370, 1307–1315. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Rehman, A.; Sina, C.; Gavrilova, O.; Hasler, R.; Ott, S.; Baines, J.F.; Schreiber, S.; Rosenstiel, P. Nod2 Is Essential for Temporal Development of Intestinal Microbial Communities. Gut 2011, 60, 1354–1362. [Google Scholar] [CrossRef]
- Gala, D.; Newsome, T.; Roberson, N.; Lee, S.M.; Thekkanal, M.; Shah, M.; Kumar, V.; Bandaru, P.; Gayam, V. Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview. Diseases 2022, 10, 73. [Google Scholar] [CrossRef]
- Meiring, M.; Allers, W.; Le Roux, E. Tissue Factor: A Potent Stimulator of von Willebrand Factor Synthesis by Human Umbilical Vein Endothelial Cells. Int. J. Med. Sci. 2016, 13, 759–764. [Google Scholar] [CrossRef]
- Yoshida, H.; Granger, D.N. Inflammatory Bowel Disease: A Paradigm for the Link Between Coagulation and Inflammation. Inflamm. Bowel Dis. 2009, 15, 1245–1255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, C.E.; Rampton, D.S. Platelet Dysfunction: A New Dimension in Inflammatory Bowel Disease. Gut 1995, 36, 5–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harries, A.D.; Beeching, N.J.; Rogerson, S.J.; Nye, F.J. The Platelet Count as a Simple Measure to Distinguish Inflammatory Bowel Disease from Infective Diarrhoea. J. Infect. 1991, 22, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Katz, J.A.; Saibeni, S.; Papa, A.; Gasbarrini, A.; Vecchi, M.; Fiocchi, C. Activated Platelets are the Source of Elevated Levels of Soluble CD40 Ligand in the Circulation of Inflammatory Bowel Disease Patients. Gut 2003, 52, 1435–1441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhaliwal, G.; Patrone, M.V.; Bickston, S.J. Venous Thromboembolism in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2023, 13, 251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johns, D.R. Cerebrovascular Complications of Inflammatory Bowel Disease. Am. J. Gastroenterol. 1991, 86, 367–370 PMID: 1998321. [Google Scholar] [PubMed]
- Landman, C.; Nahon, S.; Cosnes, J.; Bouhnik, Y.; Brixi-Benmansour, H.; Bouguen, G.; Colombel, J.F.; Savoye, G.; Coffin, B.; Abitbol, V.; et al. Portomesenteric Vein Thrombosis in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, J.H.; Sharpe, J.A.; Sutton, D.M. Cerebral and Retinal Vascular Complications of Inflammatory Bowel Disease. Ann. Neurol. 1979, 5, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Papay, P.; Miehsler, W.; Tilg, H.; Petritsch, W.; Reinisch, W.; Mayer, A.; Haas, T.; Kaser, A.; Feichtenschlager, T.; Fuchssteiner, H.; et al. Clinical Presentation of Inflammatory Bowel Disease: A Prospective Study on the Role of Vascular Involvement and Extraintestinal Manifestations. J. Crohn’s Colitis 2013, 7, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.R.; Parasa, S.; Navaneethan, U.; Crowell, M.D.; Olden, K. Comprehensive Study of Cardiovascular Morbidity in Hospitalized Inflammatory Bowel Disease Patients. J. Crohn’s Colitis 2011, 5, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Matta, F.; Yaekoub, A.Y.; Danescu, S.; Stein, P.D. Risk of Venous Thromboembolism with Inflammatory Bowel Disease. Clin. Appl. Thromb. Hemost. 2011, 17, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Miehsler, W.; Reinisch, W.; Valic, E.; Osterode, W.; Tillinger, W.; Feichtenschlager, T.; Grisar, J.; Machold, K.; Scholz, S.; Vogelsang, H.; et al. Is Inflammatory Bowel Disease an Independent and Disease Specific Risk Factor for Thromboembolism? Gut 2004, 53, 542–548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leeds, J.S.; Hopper, A.D.; Hadjivassiliou, M.; Tesfaye, S.; Sanders, D.S. Inflammatory Bowel Disease Is More Common in Type 1 Diabetes Mellitus. Gut 2011, 60 (Suppl. S1), A208. [Google Scholar] [CrossRef]
- Jess, T.; Jensen, B.W.; Andersson, M.; Villumsen, M.; Allin, K.H. Inflammatory Bowel Diseases Increase Risk of Type 2 Diabetes in a Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2020, 18, 881–888.e1. [Google Scholar] [CrossRef] [PubMed]
- Achebe, I.; Mbachi, C.; Palacios, P.; Wang, Y.; Asotibe, J.; Ofori-Kuragu, A.; Gandhi, S. Predictors of Venous Thromboembolism in Hospitalized Patients with Inflammatory Bowel Disease and Colon Cancer: A Retrospective Cohort Study. Thromb. Res. 2021, 199, 14–18. [Google Scholar] [CrossRef]
- Fuschillo, G.; Celentano, V.; Rottoli, M.; Sciaudone, G.; Gravina, A.G.; Pellegrino, R.; Marfella, R.; Romano, M.; Selvaggi, F.; Pellino, G. Influence of Diabetes Mellitus on Inflammatory Bowel Disease Course and Treatment Outcomes. A Systematic Review with Meta-Analysis. Dig. Liver Dis. 2023, 55, 580–586. [Google Scholar] [CrossRef]
- Kourlaba, G.; Relakis, J.; Kontodimas, S.; Holm, M.V.; Maniadakis, N. A Systematic Review and Meta-Analysis of the Epidemiology and Burden of Venous Thromboembolism Among Pregnant Women. Int. J. Gynaecol. Obstet. 2016, 132, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Pomp, E.R.; Lenselink, A.M.; Rosendaal, F.R.; Doggen, C.J.M. Pregnancy, the Postpartum Period and Prothrombotic Defects: Risk of Venous Thrombosis in the MEGA Study. J. Thromb. Haemost. 2008, 6, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Rajasekhar, A.; Middeldorp, S.; McLintock, C.; Rodger, M.A.; James, A.H.; Vazquez, S.R.; Greer, I.A.; Riva, J.J.; Bhatt, M.; et al. American Society of Hematology 2018 Guidelines for Management of Venous Thromboembolism: Venous Thromboembolism in the Context of Pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef]
- Martinelli, I.; De Stefano, V.; Taioli, E.; Paciaroni, K.; Rossi, E.; Mannucci, P.M. Inherited Thrombophilia and First Venous Thromboembolism During Pregnancy and Puerperium. Thromb. Haemost. 2002, 87, 791–795. [Google Scholar]
- Ginsberg, J.S.; Brill-Edwards, P.; Burrows, R.F.; Bona, R.; Prandoni, P.; Büller, H.R.; Lensing, A. Venous Thrombosis During Pregnancy: Leg and Trimester of Presentation. Thromb. Haemost. 1992, 67, 519–520. [Google Scholar] [CrossRef]
- James, A.H.; Jamison, M.G.; Brancazio, L.R.; Myers, E.R. Venous Thromboembolism During Pregnancy and the Postpartum Period: Incidence, Risk Factors, and Mortality. Am. J. Obstet. Gynecol. 2006, 194, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- James, A.H.; Tapson, V.F.; Goldhaber, S.Z. Thrombosis During Pregnancy and the Postpartum Period. Am. J. Obstet. Gynecol. 2005, 193, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Maughan, B.C.M.; Maughan, B.C.M.; Marin, M.B.; Marin, M.B.; Han, J.B.; Han, J.B.; Gibbins, K.J.M.; Gibbins, K.J.M.; Brixey, A.G.; Brixey, A.G.; et al. Venous Thromboembolism During Pregnancy and the Postpartum Period: Risk Factors, Diagnostic Testing, and Treatment. Obstet. Gynecol. Surv. 2022, 77, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Ficheur, G.; Caron, A.; Beuscart, J.B.; Ferret, L.; Jung, Y.J.; Garabedian, C.; Beuscart, R.; Chazard, E. Case-Crossover Study to Examine the Change in Postpartum Risk of Pulmonary Embolism Over Time. BMC Pregnancy Childbirth 2017, 17, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tepper, N.K.; Boulet, S.L.; Whiteman, M.K.; Monsour, M.; Marchbanks, P.A.; Hooper, W.C.; Curtis, K.M. Postpartum Venous Thromboembolism: Incidence and Risk Factors. Obstet. Gynecol. 2014, 123, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Boudreau, H.; Harris, M.L.; Maxwell, C.V. Outcomes of Obstetric Hospitalizations among Women with Inflammatory Bowel Disease in the United States. Clin. Gastroenterol. Hepatol. 2009, 7, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Bollen, L.; Vande Casteele, N.; Ballet, V.; van Assche, G.; Ferrante, M.; Vermeire, S.; Gils, A. Thromboembolism as an Important Complication of Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Andrews, J.M.; Kariyawasam, V.; Moran, N.; Gounder, P.; Collins, G.; Walsh, A.J.; Connor, S.; Lee, T.W.; Koh, C.E.; et al. Review Article: Acute Severe Ulcerative Colitis—Evidence-Based Consensus Statements. Aliment. Pharmacol. Ther. 2016, 44, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Novacek, G.; Weltermann, A.; Sobala, A.; Tilg, H.; Petritsch, W.; Reinisch, W.; Mayer, A.; Haas, T.; Kaser, A.; Feichtenschlager, T.; et al. Inflammatory Bowel Disease Is a Risk Factor for Recurrent Venous Thromboembolism. Gastroenterology 2010, 139, 779–787. [Google Scholar] [CrossRef]
- Thrombosis and Embolism during Pregnancy and the Puerperium: Acute Management (Green-Top Guideline No. 37B). RCOG. Available online: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/thrombosis-and-embolism-during-pregnancy-and-the-puerperium-acute-management-green-top-guideline-no-37b/ (accessed on 29 May 2024).
- Selinger, C.; Carey, N.; Cassere, S.; Nelson-Piercy, C.; Fraser, A.; Hall, V.; Harding, K.; Limdi, J.; Smith, L.; Smith, M.; et al. Standards for the Provision of Antenatal Care for Patients with Inflammatory Bowel Disease: Guidance Endorsed by the British Society of Gastroenterology and the British Maternal and Fetal Medicine Society. Frontline Gastroenterol. 2020, 12, 182–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, H.; Burisch, J.; Ellul, P.; Karmiris, K.; Katsanos, K.; Allocca, M.; Bamias, G.; Barreiro-de Acosta, M.; Braithwaite, T.; Greuter, T.; et al. ECCO Guidelines on Extraintestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2024, 18, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.R.; Coupland, B.; Mytton, J.; De Silva, S.; Trudgill, N.J. Venous Thromboembolism Following Discharge from Hospital in Patients Admitted for Inflammatory Bowel Disease. J. Crohn’s Colitis 2023, 17, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Faye, A.S.; Wen, T.; Ananthakrishnan, A.N.; Lichtiger, S.; Kaplan, G.G.; Friedman, A.M.; Lawlor, G.; Wright, J.D.; Attenello, F.J.; Mack, W.J.; et al. Acute Venous Thromboembolism Risk Highest Within 60 Days after Discharge from the Hospital in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1133–1141.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, T.P.C.; Grainge, M.J.; Card, T.R. The Risk of Venous Thromboembolism during and after Hospitalisation in Patients with Inflammatory Bowel Disease Activity. Aliment. Pharmacol. Ther. 2018, 48, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, T.; Wang, J.; Springer, J.E.; Gross, P.L.; Forbes, S.; Eskicioglu, C. Extended Thromboprophylaxis Following Colorectal Surgery in Patients with Inflammatory Bowel Disease: A Comprehensive Systematic Clinical Review. Color. Dis. 2020, 22, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Ore, A.S.; Vigna, C.; Fabrizio, A.; Cataldo, T.E.; Messaris, E.; Crowell, K. Are IBD Patients Underscored When Determining Postoperative VTE Risk? J. Gastrointest. Surg. 2023, 27, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.A.; Zuily, S.; Kotze, P.G.; Regnault, V.; Al Awadhi, S.; Bossuyt, P.; Gearry, R.B.; Ghosh, S.; Kobayashi, T.; Lacolley, P.; et al. International Consensus on the Prevention of Venous and Arterial Thrombotic Events in Patients with Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 857–873. [Google Scholar] [CrossRef]
- Girolami, A.; Cosi, E.; Tasinato, V.; Santarossa, C.; Ferrari, S.; Girolami, B. Drug-Induced Thrombophilic or Prothrombotic States: An Underestimated Clinical Problem That Involves Both Legal and Illegal Compounds. Clin. Appl. Thromb. Hemost. 2017, 23, 481–491. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Munck, L.K. Drug Insight: Aminosalicylates for the Treatment of IBD. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Carty, E.; MacEwen, M.; Rampton, D.S. Inhibition of Platelet Activation by 5-Aminosalicylic Acid in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2000, 14, 1169–1179. [Google Scholar] [CrossRef]
- Dunn, A.J. Cytokine Activation of the HPA Axis. Ann. N. Y. Acad. Sci. 2000, 917, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Majoor, C.J.; Sneeboer, M.M.; de Kievit, A.; Meijers, J.C.; van der Poll, T.; Lutter, R.; Bel, E.H.; Kamphuisen, P.W. The Influence of Corticosteroids on Hemostasis in Healthy Subjects. J. Thromb. Haemost. 2016, 14, 716–723. [Google Scholar] [CrossRef]
- Sarlos, P.; Szemes, K.; Hegyi, P.; Garami, A.; Szabo, I.; Illes, A.; Solymar, M.; Petervari, E.; Vincze, A.; Par, G.; et al. Steroid but Not Biological Therapy Elevates the Risk of Venous Thromboembolic Events in Inflammatory Bowel Disease: A Meta-Analysis. J. Crohn’s Colitis 2018, 12, 489–498. [Google Scholar] [CrossRef]
- Gargallo-Puyuelo, C.J.; Laredo, V.; Gomollón, F. Thiopurines and the Risk of Venous Thromboembolism in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 358–359. [Google Scholar] [CrossRef]
- Peter, M.I.; Irving, P.M.; Marion, J.; Macey, M.; Lee, W.; Louise, L.; Rampton, D.S. Formation of Platelet-Leukocyte Aggregates in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 361–372. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, Y.S.; Kim, H.; Lee, J.K.; Kim, H.M.; Park, H.J.; Kim, H.; Kim, J.; Kang, D.R. Venous Thromboembolism Risk in Asian Patients with Inflammatory Bowel Disease: A Population-Based Nationwide Inception Cohort Study. Gut Liver 2022, 16, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.; Cronstein, B. Methotrexate Mechanism in Treatment of Rheumatoid Arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef]
- Papa, A.; De Stefano, V.; Danese, S.; Chiusolo, P.; Persichilli, S.; Casorelli, I.; Zappacosta, B.; Giardina, B.; Gasbarrini, A.; Leone, G.; et al. Hyperhomocysteinemia and Prevalence of Polymorphisms of Homocysteine Metabolism-Related Enzymes in Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2001, 96, 2677–2682. [Google Scholar] [CrossRef]
- Kim, S.C.; Solomon, D.H.; Liu, J.; Franklin, J.M.; Glynn, R.J.; Schneeweiss, S. Risk of Venous Thromboembolism in Patients with Rheumatoid Arthritis: Initiating Disease-Modifying Antirheumatic Drugs. Am. J. Med. 2015, 128, 539.e7–539.e17. [Google Scholar] [CrossRef] [PubMed]
- Seinen, M.L.; Ponsioen, C.Y.; de Boer, N.K.; Oldenburg, B.; Bouma, G.; Mulder, C.J.; van Bodegraven, A.A. Sustained Clinical Benefit and Tolerability of Methotrexate Monotherapy after Thiopurine Therapy in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Fumery, M.; Hedin, C.R.H. Does Cardiovascular Risk Matter in IBD Patients? J. Intern. Med. 2023, 294, 708–720. [Google Scholar] [CrossRef]
- Detrez, I.; Thomas, D.; Van Steen, K.; Ballet, V.; Peeters, M.; Hoylaerts, M.F.; Van Assche, G.; Vermeire, S.; Ferrante, M.; Gils, A. Successful Infliximab Treatment is Associated with Reversal of Clotting Abnormalities in Inflammatory Bowel Disease Patients. J. Clin. Gastroenterol. 2020, 54, 819–825. [Google Scholar] [CrossRef]
- Bollen, L.; Vande Casteele, N.; Peeters, M.; Bessonov, K.; Van Steen, K.; Rutgeerts, P.; Ferrante, M.; Hoylaerts, M.F.; Vermeire, S.; Gils, A. Short-Term Effect of Infliximab is Reflected in the Clot Lysis Profile of Patients with Inflammatory Bowel Disease: A Prospective Study. Inflamm. Bowel Dis. 2015, 21, 570–578. [Google Scholar] [CrossRef]
- Higgins, P.D.; Skup, M.; Mulani, P.M.; Lin, J.; Chao, J. Increased Risk of Venous Thromboembolic Events with Corticosteroid vs Biologic Therapy for Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 316–321. [Google Scholar] [CrossRef]
- de Fonseka, A.M.; Tuskey, A.; Conaway, M.R.; Behm, B.W. Antitumor Necrosis Factor-α Therapy Is Associated with Reduced Risk of Thromboembolic Events in Hospitalized Patients with Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2016, 50, 578–583. [Google Scholar] [CrossRef]
- Honap, S.; Agorogianni, A.; Colwill, M.J.; Mehta, S.K.; Donovan, F.; Pollok, R.; Poullis, A.; Patel, K. JAK Inhibitors for Inflammatory Bowel Disease: Recent Advances. Frontline Gastroenterol. 2023, 15, 59–69. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Gong, M.; Gu, Y.; Zhang, H.; Dong, B.; Guo, Q.; Pang, X.; Xiang, Q.; He, X.; et al. Risk of Venous Thromboembolism with Janus Kinase Inhibitors in Inflammatory Immune Diseases: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1189389. [Google Scholar] [CrossRef]
- Yates, M.; Mootoo, A.; Adas, M.; Bechman, K.; Rampes, S.; Patel, V.; Qureshi, S.; Cope, A.P.; Norton, S.; Galloway, J.B. Venous Thromboembolism Risk with JAK Inhibitors: A Meta-Analysis. Arthritis Rheumatol. 2021, 73, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-L.; Lee, L.-L.; Huang, H.-K.; Chen, L.-Y.; Loh, C.-H.; Chi, C.-C. Association of Risk of Incident Venous Thromboembolism with Atopic Dermatitis and Treatment with Janus Kinase Inhibitors: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2022, 158, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Taxonera, C.; Olivares, D.; Alba, C. Real-World Effectiveness and Safety of Tofacitinib in Patients with Ulcerative Colitis: Systematic Review with Meta-Analysis. Inflamm. Bowel Dis. 2021, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Panés, J.; Sands, B.E.; Reinisch, W.; Su, C.; Lawendy, N.; Koram, N.; Fan, H.; Jones, T.V.; Modesto, I. Venous Thromboembolic Events in the Tofacitinib Ulcerative Colitis Clinical Development Programme. Aliment. Pharmacol. Ther. 2019, 50, 1068–1076. [Google Scholar] [CrossRef]
- Loftus, E.V.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’hAens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Schreiber, S.; D’Haens, G.; Tanida, S.; Siffledeen, J.; Enejosa, J.; Zhou, W.; Othman, A.A.; et al. Efficacy of Upadacitinib in a Long-Term Extension Study in Ulcerative Colitis. Gastroenterology 2021, 161, 457–471. [Google Scholar] [CrossRef]
- Sands, B.E.; Schreiber, S.; Blumenstein, I.; Chiorean, M.V.; Ungaro, R.C.; Rubin, D.T. Clinician’s Guide to Using Ozanimod for the Treatment of Ulcerative Colitis. J. Crohn’s Colitis 2023, 17, 2012–2025. [Google Scholar] [CrossRef]
- Armuzzi, A.; Cross, R.K.; Lichtenstein, G.R.; Hou, J.; Deepak, P.; Regueiro, M.; Wolf, D.C.; Akukwe, L.; Ahmad, H.A.; Jain, A.; et al. Cardiovascular Safety of Ozanimod in Patients with Ulcerative Colitis: True North and Open-Label Extension Analyses. Clin. Gastroenterol. Hepatol. 2024, 22, 1067–1076.e3. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results from the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohn’s Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef]
- Park, S.C.; Jeen, Y.T. Anti-Integrin Therapy for Inflammatory Bowel Disease. World J. Gastroenterol. 2018, 24, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V., Jr.; Sankoh, S.; Fox, I.; et al. The Safety of Vedolizumab for Ulcerative Colitis and Crohn’s Disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Peerani, F.; Meserve, J.; Kochhar, G.; Chaudrey, K.; Hartke, J.; Chilukuri, P.; Koliani-Pace, J.; Winters, A.; Katta, L.; et al. Vedolizumab for Ulcerative Colitis: Treatment Outcomes from the VICTORY Consortium. Am. J. Gastroenterol. 2018, 113, 1345. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Mocci, G.; Faggiani, R.; Allegretta, L.; Valle, N.D.; Medici, A.; Forti, G.; Franceschi, M.; Ferronato, A.; Gallina, S.; et al. Vedolizumab is Effective and Safe in Real-Life Treatment of Inflammatory Bowel Diseases Outpatients: A Multicenter, Observational Study in Primary Inflammatory Bowel Disease Centers. Eur. J. Intern. Med. 2019, 66, 85–91. [Google Scholar] [CrossRef]
- Christensen, B.; Colman, R.J.; Micic, D.; Gibson, P.R.; Goeppinger, S.R.; Yarur, A.; Weber, C.R.; Cohen, R.D.; Rubin, D.T. Vedolizumab as Induction and Maintenance for Inflammatory Bowel Disease: 12-Month Effectiveness and Safety. Inflamm. Bowel Dis. 2018, 24, 849–860. [Google Scholar] [CrossRef]
- Facey, M.; Khurana, A.; Adil, S.; Nadeem, A.; Martin, S.; Sinh, P.; Katz, J.; Nguyen, V.; Cooper, G.; Regueiro, M.; et al. S1022 Long Term Risk of Venous Thromboembolism in Patients with Crohn’s Disease Treated With Biologics or Immunomodulators. Am. J. Gastroenterol. 2023, 118, S775–S776. [Google Scholar] [CrossRef]
- Mannon, P.J.; Fuss, I.J.; Mayer, L.; Elson, C.O.; Sandborn, W.J.; Present, D.; Dolin, B.; Goodman, N.; Groden, C.; Hornung, R.L.; et al. Anti-Interleukin-12 Antibody for Active Crohn’s Disease. N. Engl. J. Med. 2004, 351, 2069–2079. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Maida, M.; Ventimiglia, M.; Cottone, M.; Orlando, A. Effectiveness and Safety of Ustekinumab for the Treatment of Crohn’s Disease in Real-Life Experiences: A Meta-Analysis of Observational Studies. Expert Opin. Biol. Ther. 2020, 20, 193–203. [Google Scholar] [CrossRef]
- Janssen. Tremfya® (Guselkumab) Studies Underscore Its Potential to Be the Only IL-23 Inhibitor to Offer Both Subcutaneous and Intravenous Dosing. Available online: https://www.janssen.com/tremfyar-guselkumab-studies-underscore-its-potential-be-only-il-23-inhibitor-offer-both-subcutaneous (accessed on 21 July 2024).
- Gravina, A.G.; Pellegrino, R.; Durante, T.; Palladino, G.; D’oNofrio, R.; Mammone, S.; Arboretto, G.; Auletta, S.; Imperio, G.; Ventura, A.; et al. Inflammatory Bowel Diseases Patients Suffer from Significant Low Levels and Barriers to Physical Activity: The “BE-FIT-IBD” Study. World J. Gastroenterol. 2023, 29, 5668–5682. [Google Scholar] [CrossRef]
| Drug Type/Name | Perceived Thrombotic Effect in IBD Populations |
|---|---|
| 5-ASAs | Uncertain—no additional risk observed |
| Corticosteroids | Increased |
| Thiopurines | Uncertain—no additional risk observed |
| Methotrexate | Uncertain—no additional risk observed |
| TNF-a Inhibitors | Reduced |
| JAK inhibitors | Uncertain—drug and dose-dependent |
| S1P receptor modulators | Neutral |
| Anti-integrin | Uncertain—no additional risk observed |
| Anti-IL-12 and IL-23 | Uncertain—no additional risk observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Tewatia, P.; Harvey, P.R.; Kumar, A. Controversies in Venous Thromboembolism Risk Assessment in Inflammatory Bowel Disease: A Narrative Review. Diagnostics 2024, 14, 2112. https://doi.org/10.3390/diagnostics14192112
Sharma N, Tewatia P, Harvey PR, Kumar A. Controversies in Venous Thromboembolism Risk Assessment in Inflammatory Bowel Disease: A Narrative Review. Diagnostics. 2024; 14(19):2112. https://doi.org/10.3390/diagnostics14192112
Chicago/Turabian StyleSharma, Nikhil, Pavit Tewatia, Philip R. Harvey, and Aditi Kumar. 2024. "Controversies in Venous Thromboembolism Risk Assessment in Inflammatory Bowel Disease: A Narrative Review" Diagnostics 14, no. 19: 2112. https://doi.org/10.3390/diagnostics14192112






