New Insights into the Pathophysiology of Coronary Artery Aneurysms
Abstract
1. Introduction
1.1. Diagnostic
1.2. Differences between Coronary Artery Aneurysms (CAAs) and Giant Coronary Artery Aneurysms (GCAAs)
1.3. Objectives
2. Literature Search
3. Pathophysiology
4. Atherosclerosis
4.1. Takayasu Arteritis
4.2. Kawasaki Disease
4.3. CAAs and Percutaneous Coronary Interventions
4.4. Molecular Mechanisms Linked to CAAs
4.5. Disorders of the Connective Tissue and CAAs
4.6. Infections and CAAs
- (a)
- Occlusion caused by embolization: Blockage of the arterial wall’s vasa vasorum, which can occur in an artery of normal caliber or with pre-existing aneurysm—this can cause an infarction of the vessel wall.
- (b)
- Direct infiltration: Pathogens formed in a source of sepsis that is nearby and that can invade the arterial wall directly.
- (c)
- Immune complex deposition: Damage caused by the deposition of immune complexes can injure different tunics of the vessel wall, leading to rapid enlargement of the wall and aneurysm formation.
5. Discussion
6. Conclusions
- CAAs are rare but can lead to severe, potentially lethal outcomes.
- They are commonly related to CAD and are a concern due to percutaneous coronary interventions.
- Various mechanisms underlying CAA formation have been proposed, but precise processes remain elusive.
- Recent research has identified genetic polymorphisms that may increase the risk of developing CAAs, especially in conditions such as Kawasaki disease.
- Further research is needed to understand the risk factors and underlying mechanisms associated with CAAs, improving preventive and therapeutic strategies.
Funding
Conflicts of Interest
References
- Markis, J.E.; Joffe, C.D.; Cohn, P.F.; Feen, D.J.; Herman, M.V.; Gorlin, R. Clinical significance of coronary arterial ectasia. Am. J. Cardiol. 1976, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Topaz, O.; Rutherford, M.S.; Mackey-Bojack, S.; Prinz, A.W.; Katta, S.; Salter, D.; Titus, J.L. Giant aneurysms of coronary arteries and saphenous vein grafts: Angiographic findings and histopathological correlates. Cardiovasc. Pathol. 2005, 14, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Abou Sherif, S.; Ozden Tok, O.; Taşköylü, Ö.; Goktekin, O.; Kilic, I.D. Coronary Artery Aneurysms: A Review of the Epidemiology, Pathophysiology, Diagnosis, and Treatment. Front. Cardiovasc. Med. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antoniadis, A.P.; Chatzizisis, Y.S.; Giannoglou, G.D. Pathogenetic mechanisms of coronary ectasia. Int. J. Cardiol. 2008, 130, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Porcella, A.R.; Sable, C.A.; Patel, K.M.; Martin, G.R.; Singh, N. The epidemiology of Kawasaki disease in an urban hospital: Does African American race protect against coronary artery aneurysms? Pediatr. Cardiol. 2005, 26, 775–781. [Google Scholar] [CrossRef]
- Sharma, S.N.; Kaul, U.; Sharma, S.; Wasir, H.S.; Manchanda, S.C.; Bahl, V.K.; Talwar, K.K.; Rajani, M.; Bhatia, M.L. Coronary arteriographic profile in young and old Indian patients with ischemic heart disease: A comparative study. Indian. Heart J. 1990, 42, 365–369. [Google Scholar]
- Manginas, A.; Cokkinos, D.V. Coronary artery ectasias: Imaging, functional assessment and clinical implications. Eur. Heart J. 2006, 27, 1026–1031. [Google Scholar] [CrossRef]
- Mata, K.M.; Fernandes, C.R.; Floriano, E.M.; Martins, A.P.; Rossi, M.A.; Ramos, S.G. Coronary artery aneurysms: An update. In Novel Strategies in Ischemic Heart Disease; Lakshmanadoss, U., Ed.; InTech: Rijeka, Croatia, 2012; pp. 381–404. [Google Scholar]
- Pham, V.; Hemptinne, Q.; Grinda, J.M.; Duboc, D.; Varenne, O.; Picard, F. Giant coronary aneurysms, from diagnosis to treatment: A literature review. Arch. Cardiovasc. Dis. 2020, 113, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.H.C.; Hong, K.L.; Henein, M.Y.; Hanratty, C.; Boles, U. Coronary Artery Ectasia: Review of the Non-Atherosclerotic Molecular and Pathophysiologic Concepts. Int. J. Mol. Sci. 2022, 23, 5195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, C.-H.; Hao, Y.; Liu, Y.-H.; Li, X.-L.; Huang, Z.-H.; Luo, Y.; Li, R.-L. Anti-inflammatory effects of rosuvastatin treatment on coronary artery ectasia patients of different age groups. BMC Cardiovasc. Disord. 2020, 20, 330. [Google Scholar] [CrossRef]
- Printz, B.F.; Sleeper, L.A.; Newburger, J.W.; Minich, L.L.; Bradley, T.; Cohen, M.S.; Frank, D.; Li, J.S.; Margossian, R.; Shirali, G.; et al. Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory inflammatory markers in acute Kawasaki disease. J. Am. Coll. Cardiol. 2011, 57, 86–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Regar, E.; Klauss, V.; Henneke, K.H.; Werner, F.; Theisen, K.; Mudra, H. Coronary aneurysm after bailout stent implantation: Diagnosis of a false lumen with intravascular ultrasound. Cathet Cardiovasc. Diagn. 1997, 41, 407–410. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakazawa, G.; Joner, M.; Kolodgie, F.D.; Mont, E.K.; Gold, H.K.; Virmani, R. Vascular responses to drug eluting stents: Importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.M.; Hong, B.K.; Kim, J.Y.; Min, P.K.; Yoon, Y.W.; Lee, B.K.; Kwon, H.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; et al. Incidence and natural history of coronary artery aneurysm developing after drug-eluting stent implantation. Am. Heart J. 2010, 160, 987–994. [Google Scholar] [CrossRef]
- Togni, M.; Windecker, S.; Cocchia, R.; Wenaweser, P.; Cook, S.; Billinger, M.; Meier, B.; Hess, O.M. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol. 2005, 46, 231–236. [Google Scholar] [CrossRef]
- Hofma, S.H.; van der Giessen, W.J.; van Dalen, B.M.; Lemos, P.A.; McFadden, E.P.; Sianos, G.; Ligthart, J.M.; van Essen, D.; de Feyter, P.J.; Serruys, P.W. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur. Heart J. 2006, 27, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.; Cheng, J. Coronary artery aneurysm after implantation of a bioresorbable vascular scaffold: Case report and literature review. Catheter. Cardiovasc. Interv. 2017, 90, E41–E45. [Google Scholar] [CrossRef] [PubMed]
- Serban, R.; Scridon, A.; Dobreanu, D.; Elkaholut, A. Coronary artery aneurysm formation within everolimus-eluting bioresorbable stent. Int. J. Cardiol. 2014, 177, e4–e5. [Google Scholar] [CrossRef]
- Bararu Bojan Bararu, I.; Pleșoianu, C.E.; Badulescu, O.V.; Vladeanu, M.C.; Badescu, M.C.; Iliescu, D.; Bojan, A.; Ciocoiu, M. Molecular and Cellular Mechanisms Involved in Aortic Wall Aneurysm Development. Diagnostics 2023, 13, 253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olson, S.L.; Wijesinha, M.A.; Panthofer, A.M.; Blackwelder, W.C.; Upchurch, G.R., Jr.; Terrin, M.L.; Curci, J.A.; Baxter, B.T.; Matsumura, J.S. Evaluating Growth Patterns of Abdominal Aortic Aneurysm Diameter with Serial Computed Tomography Surveillance. JAMA Surg. 2021, 156, 363–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burgner, D.; Davila, S.; Breunis, W.B.; Ng, S.B.; Li, Y.; Bonnard, C.; Ling, L.; Wright, W.J.; Thalamuthu, A.; Odam, M.; et al. International Kawasaki Disease Genetics Consortium: A genome-wide association study identifies novel and functionally related susceptibility loci for Kawasaki disease. PLoS Genet. 2009, 5, e1000319. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.G.; Yaacoub, N.; Nader, V.; Moussallem, N.; Carrie, D.; Roncalli, J. Coronary artery aneurysm: A review. World J. Cardiol. 2021, 13, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Maehara, A.; Mintz, G.S.; Ahmed, J.M.; Fuchs, S.; Castagna, M.T.; Pichard, A.D.; Satler, L.F.; Waksman, R.; Suddath, W.O.; Kent, K.M.; et al. An intravascular ultrasound classification of angiographic coronary artery aneurysms. Am. J. Cardiol. 2001, 88, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Nichols, L.; Lagana, S.; Parwani, A. Coronary artery aneurysm: A review and hypothesis regarding etiology. Arch. Pathol. Lab. Med. 2008, 132, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Swaye, P.S.; Fisher, L.D.; Litwin, P.; Vignola, P.A.; Judkins, M.P.; Kemp, H.G.; Mudd, J.G.; Gosselin, A.J. Aneurysmal coronary artery disease. Circulation 1983, 67, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, C.; Zhang, L.; Xie, X.; Gu, X.; Sang, C.; Xu, M.; Xu, W.; Jia, H. Molecular basis of coronary artery dilation and aneurysms in patients with Kawasaki disease based on differential protein expression. Mol. Med. Rep. 2018, 17, 2402–2414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greco, A.; De Virgilio, A.; Rizzo, M.I.; Tombolini, M.; Gallo, A.; Fusconi, M.; Ruoppolo, G.; Pagliuca, G.; Martellucci, S.; de Vincentiis, M. Kawasaki disease: An evolving paradigm. Autoimmun. Rev. 2015, 14, 703–709. [Google Scholar] [CrossRef]
- Burns, J.C.; Herzog, L.; Fabri, O.; Tremoulet, A.H.; Rodó, X.; Uehara, R.; Burgner, D.; Bainto, E.; Pierce, D.; Tyree, M.; et al. Seasonality of Kawasaki disease: A globalperspective. PLoS ONE 2013, 8, e74529. [Google Scholar] [CrossRef]
- Iba, T.; Thachil, J. Is antithrombin III for sepsis-associated disseminated intravascular coagulation really ineffective? Intensive Care Med. 2016, 42, 1193–1194. [Google Scholar] [CrossRef]
- Simioni, P.; Tormene, D.; Spiezia, L.; Tognin, G.; Rossetto, V.; Radu, C.; Prandoni, P. Inherited thrombophilia and venous thromboembolism. Semin. Thromb. Hemost. 2006, 32, 700–708. [Google Scholar] [CrossRef]
- Cooper, P.C.; Coath, F.; Daly, M.E.; Makris, M. The phenotypic and genetic assessment of antithrombin deficiency. Int. J. Lab. Hematol. 2011, 33, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, H.; Okada, T.; Nozu, K.; Takaoka, Y.; Shono, A.; Asanuma, K.; Zhang, L.; Nakanishi, K.; Taniguchi-Ikeda, M.; Kaito, H.; et al. Identification of mutations in FN1 leading to glomerulopathy with fibronectin deposits. Pediatr. Nephrol. 2016, 31, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Mata, K.M.; Prudente, P.S.; Rocha, F.S.; Prado, C.M.; Floriano, E.M.; Elias, J., Jr.; Rizzi, E.; Gerlach, R.F.; Rossi, M.A.; Ramos, S.G. Combining two potential causes, of metalloproteinase secretion causes abdominal aortic aneurysms in rats: A new experimental model. Int. J. Exp. Pathol. 2011, 92, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Pahlavan, P.S.; Niroomand, F. Coronary artery aneurysm: A review. Clin. Cardiol. 2006, 29, 439–443. [Google Scholar] [CrossRef]
- Lin, Y.; Wan, L.; Wu, J.; Sheu, J.; Lin, C.; Lan, Y.; Lai, C.H.; Hung, C.H.; Tsai, Y.; Tsai, C.H.; et al. HLA-E gene polymorphism associated with susceptibility to Kawasaki disease and formation of coronary artery aneurysms. Arthritis Rheum. 2009, 60, 604–610. [Google Scholar] [CrossRef]
- Matsuyama, A.; Sakai, N.; Ishigami, M.; Hiraoka, H.; Kashine, S.; Hirata, A.; Nakamura, T.; Yamashita, S.; Matsuzawa, Y. Matrix metalloproteinases as novel disease markers in Takayasu arteritis. Circulation 2003, 108, 1469–1473. [Google Scholar] [CrossRef]
- Vladeanu, M.C.; Bojan, I.B.; Bojan, A.; Iliescu, D.; Badescu, M.C.; Badulescu, O.V.; Badescu, M.; Georgescu, C.A.; Ciocoiu, M. Angiotensin-converting enzyme gene D-allele and the severity of coronary artery disease. Exp. Ther. Med. 2020, 20, 3407–3411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evrengul, H.; Kilic, D.I.; Zungur, M.; Alihanoglu, Y.I.; Tanriverdi, H. Myocardial infarction in a 17-year-old patient due to neurofibromatosis-associated coronary aneurysm. Cardiol. Young 2013, 23, 454–456. [Google Scholar] [CrossRef]
- Ford, S.R.; Rao, A.; Kochilas, L. Giant coronary artery aneurysm formation following meningococcal septicaemia. Pediatr. Cardiol. 2007, 28, 300–302. [Google Scholar] [CrossRef]
- Singh, H.; Singh, C.; Aggarwal, N.; Dugal, J.S.; Kumar, A.; Luthra, M. Mycotic aneurysm of left anterior descending artery after sirolimus-eluting stent implantation: A case report. Catheter. Cardiovasc. Interv. 2005, 65, 282–285. [Google Scholar] [CrossRef]
- Kukkar, V.; Kapoor, H.; Aggarwal, A. Mycotic and non-mycotic coronary artery aneurysms-A review of the rarity. J. Clin. Imaging Sci. 2022, 12, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borgi, J.F.; Natrajan, K.M.; Sun, J.C. Mycotic aneurysm of the right coronary artery presenting as infected pericardial effusion. Circulation 2014, 130, e7–e8. [Google Scholar] [CrossRef] [PubMed]
- Buono, A.; Maloberti, A.; Bossi, I.M.; Piccaluga, E.; Piccalo, G.; Oreglia, J.A. Mycotic coronary aneurysms. J. Cardiovasc. Med. 2019, 20, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.W.; Whitehead, N.J.; Barlow, M. Mycotic coronary aneurysms. Heart Lung Circ. 2020, 29, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Nakaoka, H.; Sumitsuji, S.; Nakatsuji, H.; Ihara, M.; Nojima, Y.; Shimomura, I.; Nagai, Y. Successful surgical treatment of an infected right coronary artery aneurysm-to-right ventricle fistula after sirolimus-eluting stent implantation. Intern. Med. 2007, 46, 865–871. [Google Scholar] [CrossRef]
- Hartnell, G.G.; Parnell, B.M.; Pridie, R.B. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br. Heart J. 1985, 54, 392–395. [Google Scholar] [CrossRef]
- Li, D.; Wu, Q.; Sun, L.; Song, Y.; Wang, W.; Pan, S.; Luo, G.; Liu, Y.; Qi, Z.; Tao, T.; et al. Surgical treatment of giant coronary artery aneurysm. J. Thorac. Cardiovasc. Surg. 2005, 130, 817–821. [Google Scholar] [CrossRef]

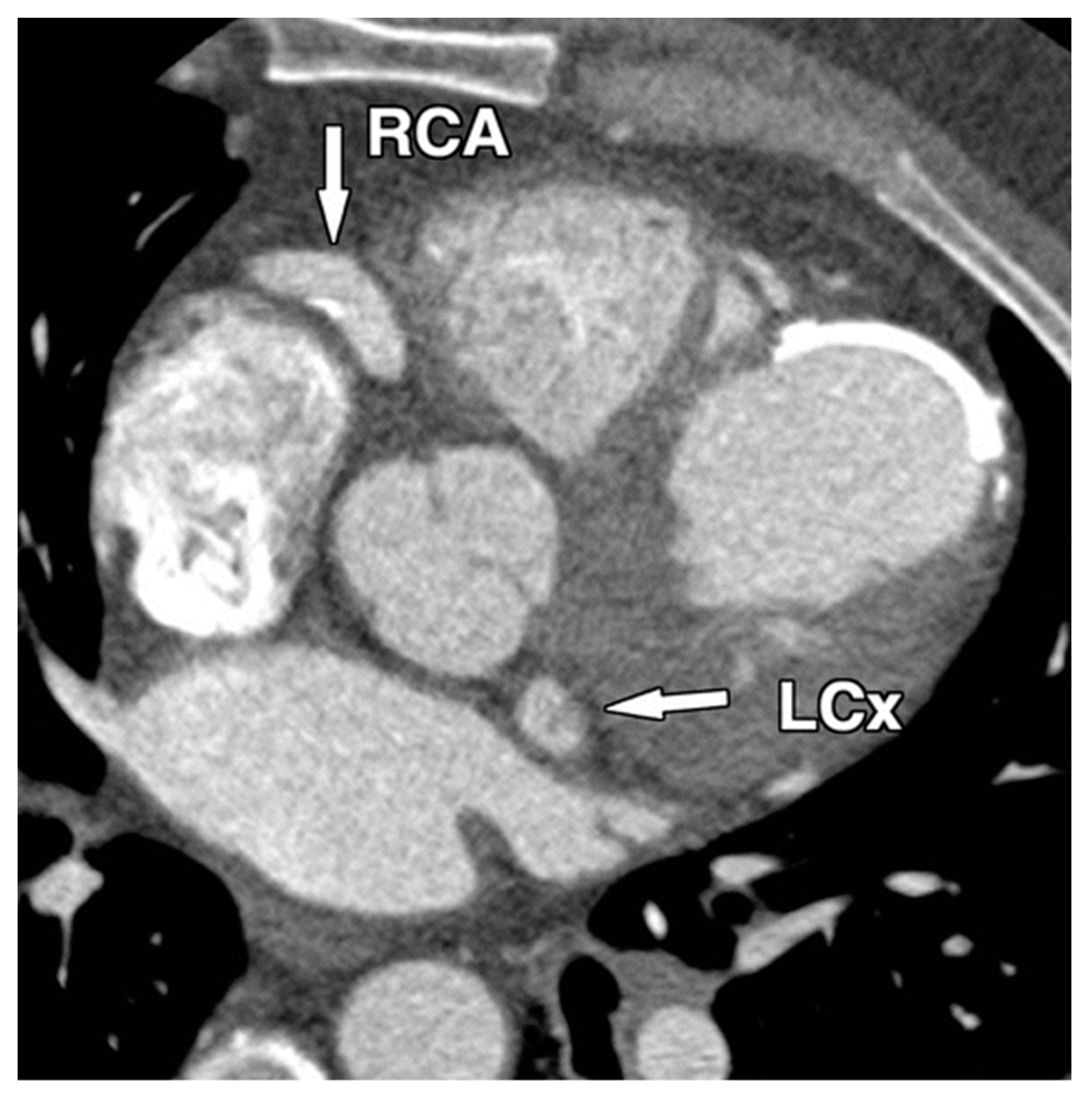
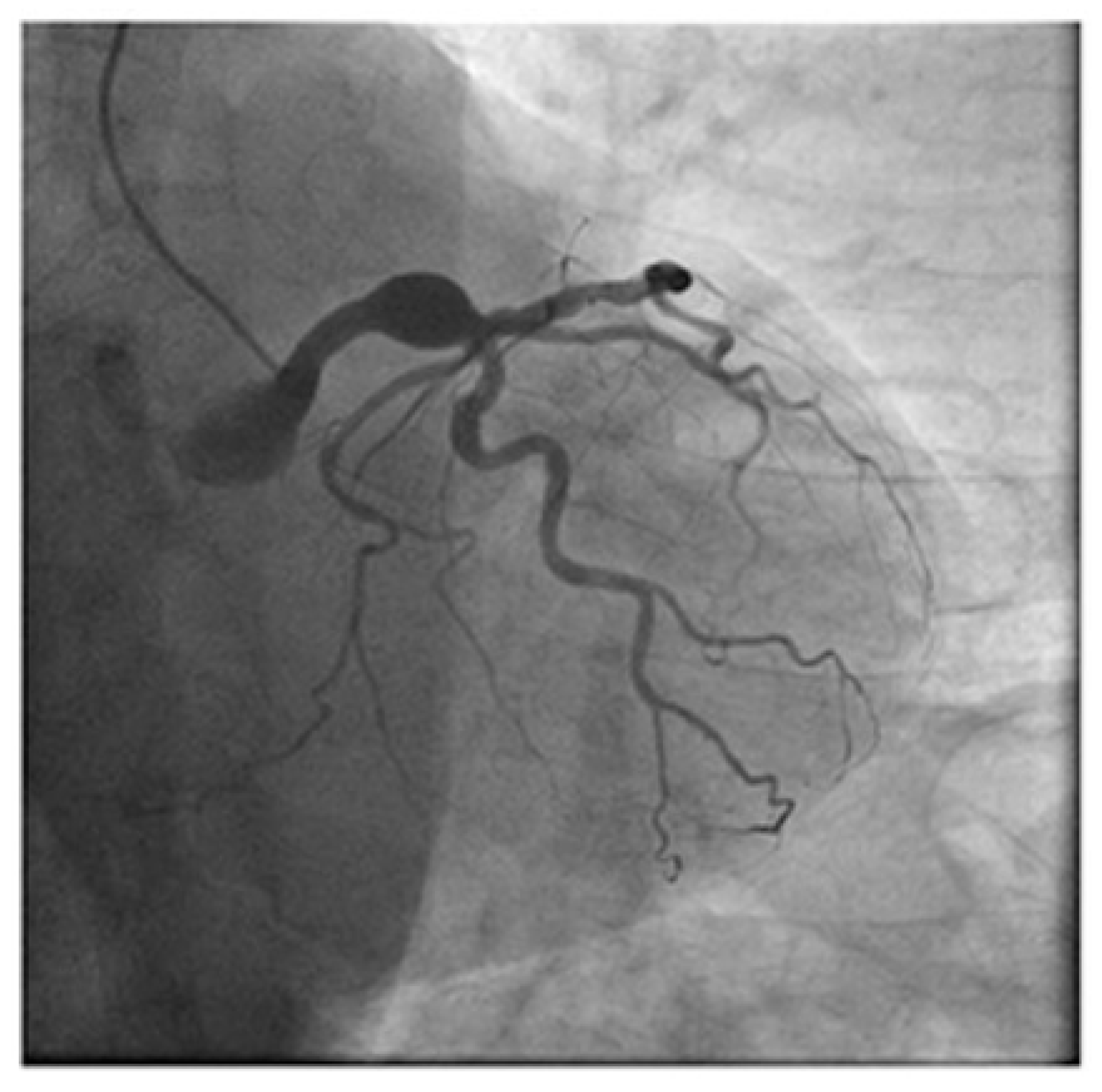
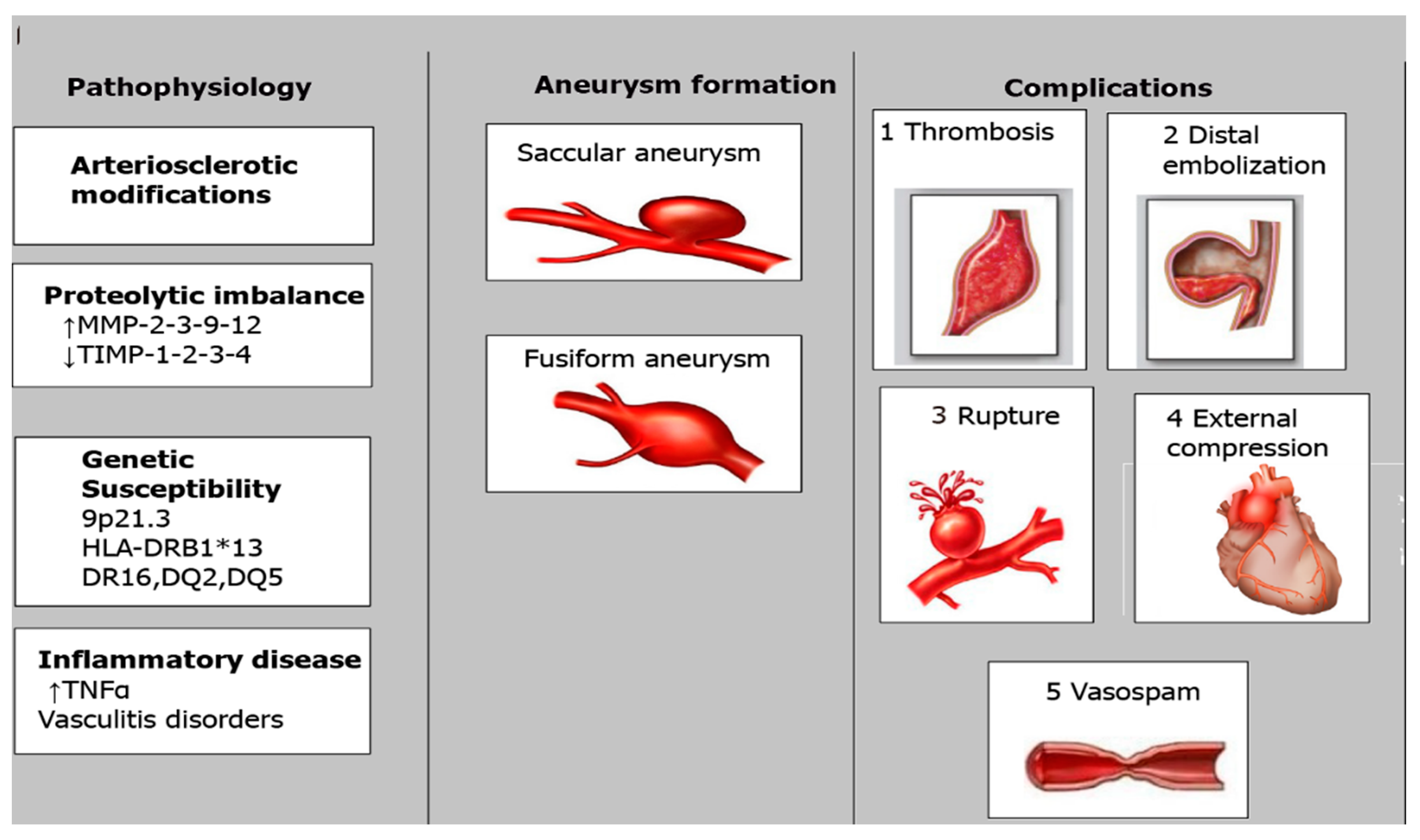
| Cause/Condition | Mechanism/Pathophysiology | Risk Factors |
|---|---|---|
| Atherosclerosis | Reduced tolerance to intraluminal pressure, leading to artery dilation; possible genetic predisposition (MMP3-5A allele) [8,9] | Chronic inflammation, high cholesterol, hypertension, smoking, diabetes |
| Congenital GCAAs | Developmental anomalies in coronary arteries leading to localized structural defects [9] | Genetic predisposition |
| Kawasaki Disease | Immune-mediated inflammation targeting arterial walls [10] | Genetic and environmental factors |
| Takayasu Arteritis | Systemic inflammatory process affecting large arteries [10] | Autoimmune conditions |
| Connective Tissue Diseases | (Marfan syndrome, Ehlers–Danlos syndrome, fibromuscular dysplasia, neurofibromatosis) [11] | Genetic disorders leading to weakened arterial walls |
| Other Vasculitis | (Lupus, polyarteritis nodosa, Behçet’s disease, rheumatoid arthritis, ankylosing spondylitis, scleroderma) [11,12] | Immune-mediated inflammation |
| Infections | (HIV, bacterial, mycobacterial, syphilis, Lyme disease, mycotic aneurysm, septic emboli) [12] | Infectious agents causing direct damage to arterial walls |
| Drug Use | (Cocaine, amphetamines, protease inhibitors) [10,11] | Direct toxic effects on arterial walls |
| Chest Trauma | Direct physical injury to coronary artery [10,11] | Blunt chest trauma |
| Tumors/Cardiac Lymphoma | Invasion and damage to coronary arteries [10,11] | Malignant growths |
| Percutaneous Coronary Intervention | Localized damage to arterial wall during procedure [13,14] | Chronic total occlusion, lesion length > 33 mm, lesion on the left anterior descending artery, implantation in infarct-related artery |
| Drug-coated Balloons | Localized arterial wall damage [15,16,17] | Interventional procedures |
| Bioresorbable Vascular Scaffolds | Remodeling processes post-implantation [18,19,20] | Interventional procedures |
| Rotablation | Mechanical damage to arterial wall [14,19,20] | Interventional procedures |
| Intervention | Contributing Factors | Aneurysm Occurrence Rate | Key Findings |
|---|---|---|---|
| Coronary Angioplasty | Persistent dissection, arterial barotrauma from high-pressure balloon inflation, and the use of oversized balloons [23] | 3.9% | Aneurysm formation linked to high-pressure balloon inflation and dissection. |
| Atherectomy | Arterial barotrauma, atherectomy techniques [24,25] | 10% | Higher rate of aneurysm formation compared to other interventions. |
| Stenting | Nonhealing dissection, stent malapposition, oversized stents [26] | 3.5% to 5% | Incidence of CAAs in stenting ranges between 3.5% and 5%. |
| Drug-Eluting Stents (DESs) | Delayed reendothelialization, inflammatory changes, hypersensitivity reactions, reduced neointimal growth [13] | 1.25% to 3.9% | DESs reduce restenosis but can increase CAA risk due to delayed healing and inflammatory responses. Reported CAA incidence is between 1.25% and 3.9%. |
| Bioabsorbable Vascular Scaffolds (BVSs) | Lesion preparation, scaffold degradation, strut discontinuity, outward scaffold displacement [18,19,20] | Not well defined; further research needed | Factors influencing CAA formation include thorough lesion preparation and scaffold degradation. Further research required to measure incidence and understand mechanisms. |
| Aspect | Coronary Artery Aneurysm (CAA) |
|---|---|
| Protein Expression | Higher levels of proteins related to wounding response and cholesterol components compared to controls [27]. |
| Signaling Network | Same pathways as CAD, with a focus on infection, inflammation, and metabolism [28,29]. |
| CFH (Complement Factor H) | Plays a role in complement regulation. Reduced levels indicate dysfunction in the complement system [28,29]. |
| MBL2 (Mannose-Binding Lectin 2) | Participates in complement activation. Decreased levels in KD with CAAs suggest dysfunction in the complement system [29]. |
| KNG1 (Kininogen 1) | Downregulated in KD with CAA, possibly contributing to increased thrombosis [30]. |
| SERPINC1 (Antithrombin III) | Downregulated in KD with CAA, which might increase thrombosis risk [30]. |
| FN1 (Fibronectin 1) | Reduced in both CAD and CAA, indicating endothelial dysfunction and stress responses in coronary artery lesions [31]. |
| Histopathological Findings | Destruction of coronary artery walls and diffuse vasculitis suggest involvement of matrix metalloproteinases (MMPs) [31]. |
| MMPs (Matrix Metalloproteinases) | Increased levels of MMP-2, MMP-3, MMP-9, and MMP-12, along with decreased TIMPs. This imbalance leads to vessel wall degradation and CAA formation [32]. |
| Tissue-Specific Inhibitors (TIMPs) | Decreased TIMP-1, TIMP-2, TIMP-3, and TIMP-4 levels in aneurysmal vessels, contributing to proteolytic imbalance [33]. |
| Genetic Factors | MMP-9 gene polymorphisms linked to aneurysm formation in KD. Other associated factors include HLA-E, MMP-3 gene disruptions, ACE DD genotype, SRC-1, and GRIN3A genes [33]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bararu-Bojan, I.; Badulescu, O.-V.; Badescu, M.C.; Vladeanu, M.C.; Plesoianu, C.E.; Bojan, A.; Iliescu-Halitchi, D.; Tudor, R.; Huzum, B.; Frasinariua, O.E.; et al. New Insights into the Pathophysiology of Coronary Artery Aneurysms. Diagnostics 2024, 14, 2167. https://doi.org/10.3390/diagnostics14192167
Bararu-Bojan I, Badulescu O-V, Badescu MC, Vladeanu MC, Plesoianu CE, Bojan A, Iliescu-Halitchi D, Tudor R, Huzum B, Frasinariua OE, et al. New Insights into the Pathophysiology of Coronary Artery Aneurysms. Diagnostics. 2024; 14(19):2167. https://doi.org/10.3390/diagnostics14192167
Chicago/Turabian StyleBararu-Bojan, Iris, Oana-Viola Badulescu, Minerva Codruta Badescu, Maria Cristina Vladeanu, Carmen Elena Plesoianu, Andrei Bojan, Dan Iliescu-Halitchi, Razvan Tudor, Bogdan Huzum, Otilia Elena Frasinariua, and et al. 2024. "New Insights into the Pathophysiology of Coronary Artery Aneurysms" Diagnostics 14, no. 19: 2167. https://doi.org/10.3390/diagnostics14192167
APA StyleBararu-Bojan, I., Badulescu, O.-V., Badescu, M. C., Vladeanu, M. C., Plesoianu, C. E., Bojan, A., Iliescu-Halitchi, D., Tudor, R., Huzum, B., Frasinariua, O. E., & Ciocoiu, M. (2024). New Insights into the Pathophysiology of Coronary Artery Aneurysms. Diagnostics, 14(19), 2167. https://doi.org/10.3390/diagnostics14192167










