Comparative Analysis of HPV Detection Efficiency: Evaluating Cobas 8800 Performance in Vaginal Self-Sampling versus Clinician-Collected Samples at a Regional Thai Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Cervical Cancer Screening Guidelines in Thailand
- New Sample Collection Requirement: If an HPV self-sampling test is deemed inadequate due to insufficient cellular material or poor sample quality, our protocol requires that a new self-collected sample be obtained. This sample is then sent back to the laboratory for further HPV DNA testing.
- Follow-up for Positive Non-16/18 Cases: In the event of a non-16/18 positive result, participants are requested to follow up with a clinician-obtained sample. This subdivides into a compliance group with complete LBC and Ct data and a non-compliance group with participants who did not follow up as required.
- Management of HPV Types 16 or 18: Participants with samples positive for HPV types 16 or 18, whether self-collected or clinician-collected, must undergo colposcopy to further assess and manage potential high-risk lesions.
- LBC and Ct Data Completed Group: Samples from this group are used directly from the HPV DNA test step for LBC without the need for a new collection.
- LBC and Ct Data Incomplete Group: A small subset where either LBC or Ct data, or both, are missing.
2.2. Self-Collection and Clinician Collection
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Analysis of Ct Values for HPV Detection across Various Age Groups
3.2. Analysis of Ct Values for HPV Detection across Various hrHPV Types
3.3. Comparative Analysis of HPV Detection and Cytological Outcomes in Self-Collected vs. Clinician-Collected Samples
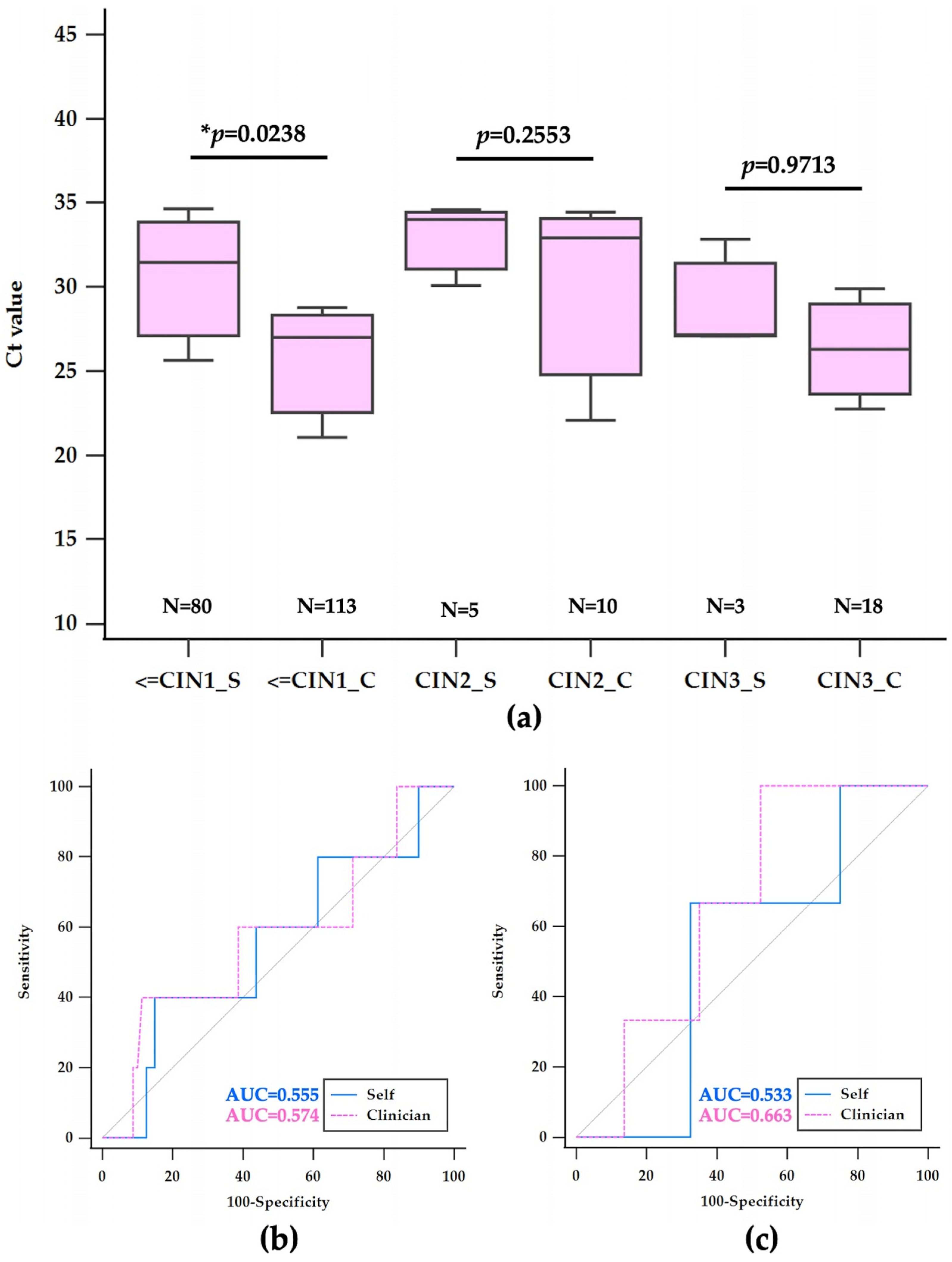
3.4. Histological Findings and Ct Value Analysis in hrHPV-Positive Samples
4. Discussion
4.1. Age-Related Screening Efficacy
4.2. Influence of hrHPV Type on Screening Outcomes
4.3. Comparative Analysis of HPV Detection and Cytological Outcomes
4.4. Perspective, Attitude, and Knowledge about HPV among Thai Women
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eamratsameekool, W.; Phumiressunthon, K.; Sukprasert, L.; Puldeesamai, P. Comparison of Self- to Provider-Collected Cervical Screening with HPV DNA Test at Roi Et Province, Thailand during COVID-19 Pandemic. J. Med. Assoc. Thail. 2023, 106, 8–13. [Google Scholar]
- Bogale, A.L.; Teklehaymanot, T.; Ali, J.H.; Kassie, G.M.; Medhin, G.; Baye, A.Y.; Shiferaw, A.Y. Comparison of self-collected versus clinician collected cervicovaginal specimens for detection of high-risk human papillomavirus among HIV infected women in Ethiopia. BMC Womens Health 2022, 22, 360. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.R.; Corry, E.M.A.; Malagon, T.; O’Riain, C.; Franco, E.L.; Brennan, D.J. Modeling Cervical Cancer Screening Strategies with Varying Levels of Human Papillomavirus Vaccination. JAMA Netw. Open 2021, 4, e2115321. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Recommendations on Self-Care Interventions Human Papillomavirus (HPV) Self-Sampling as Part of Cervical Cancer Screening and Treatment, 2022 Update; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Naizhaer, G.; Yuan, J.; Mijiti, P.; Aierken, K.; Abulizi, G.; Qiao, Y. Evaluation of multiple screening methods for cervical cancers in rural areas of Xinjiang, China. Medicine 2020, 99, e19135. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://secretary.dms.go.th/dataconference/12-2566/ประเด็นดำเนินการ%20มะเร็ง%207+1.pdf (accessed on 8 September 2024).
- Laowjan, P.; Maichonklang, K.; Permpool, P.; Talungchit, P.; Jareemit, N. Factors Associated with Cervical Cancer Screening Overuse and Underuse, and Attitude towards Human Papillomavirus Self-sampling among Hospital Staffs. Siriraj Med. J. 2023, 75, 200–207. [Google Scholar] [CrossRef]
- Sangrajrang, S.; Pitakkarnkul, S.; Muwonge, R.; Ploysawang, P.; Pangmuang, P.; Seeda, K.; Basu, P. Agreement between Self- and Physician‚ ÄëSampling for Detection of High‚ ÄëRisk Human Papillomavirus Infections in Women Attending Cervical Screening at National Cancer Institute, Thailand. Asian Pac. J. Cancer Prev. 2023, 24, 2615–2619. [Google Scholar] [CrossRef]
- Arbyn, M.; Latsuzbaia, A.; Castle, P.E.; Sahasrabuddhe, V.V.; Broeck, D.V. HPV testing of self-samples: Influence of collection and sample handling procedures on clinical accuracy to detect cervical precancer. Lancet Reg. Health Eur. 2022, 14, 100332. [Google Scholar] [CrossRef]
- Saville, M.; Hawkes, D.; Keung, M.; Ip, E.; Silvers, J.; Sultana, F.; Malloy, M.J.; Velentzis, L.S.; Canfel, L.K.; Wrede, C.D.; et al. Analytical performance of HPV assays on vaginal self-collected vs practitioner-collected cervical samples: The SCoPE study. J. Clin. Virol. 2020, 127, 104375. [Google Scholar] [CrossRef]
- Khomphaiboonkij, U.; Sreamsukcharoenchai, N.; Pitakkarnkul, S.; Rittiluechai, K.; Tangjitgamol, S. Knowledge of Thai women in cervical cancer etiology and screening. PLoS ONE 2023, 18, e0286011. [Google Scholar] [CrossRef]
- Colonetti, T.; Uggioni, M.L.R.; Dos Santos, A.L.M.; Uggioni, N.M.; Elibio, L.U.; Balbinot, E.L.; Grande, A.J.; da Rosa, M.I. Self-sampling for HPV testing in cervical cancer screening: A scoping review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 296, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Inturrisi, F.; Aitken, C.A.; Melchers, W.J.G.; van den Brule, A.J.C.; Molijn, A.; Hinrichs, J.W.J.; Niesters, H.G.M.; Siebers, A.G.; Schuurman, R.; Heideman, D.A.M.; et al. Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: An observational study. Lancet Reg. Health Eur. 2021, 11, 100235. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, K.; Zhao, F.; Downham, L.; Baena, A.; Basu, P. Molecular triaging options for women testing HPV positive with self-collected samples. Front. Oncol. 2023, 13, 1243888. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schunemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Naranjo, A.R.; Duque, A.J.; Lorey, T.S.; Schapiro, J.M.; Suh-Burgmann, B.J.; Rummel, M.; Salipante, S.J.; Wentzensen, N.; Greene, D.N. Evaluation of Pre-Analytical Variables for Human Papillomavirus Primary Screening from Self-Collected Vaginal Swabs. J. Mol. Diagn. 2024, 26, 487–497. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H.; Xiao, A.; Zhang, W.; Wang, C.; Huang, X.; Qu, X.; Wang, J.; Wu, R. Verification of the association of the cycle threshold (Ct) values from HPV testing on Cobas4800 with the histologic grades of cervical lesions using data from two population-based cervical cancer screening trials. Infect. Agents Cancer 2022, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Aranda Flores, C.E.; Gomez Gutierrez, G.; Ortiz Leon, J.M.; Cruz Rodriguez, D.; Sorbye, S.W. Self-collected versus clinician-collected cervical samples for the detection of HPV infections by 14-type DNA and 7-type mRNA tests. BMC Infect. Dis. 2021, 21, 504. [Google Scholar] [CrossRef]
- Sangrajrang, S.; Laowahutanont, P.; Wongsena, M.; Muwonge, R.; Karalak, A.; Imsamran, W.; Senkomago, V.; Sankaranarayanan, R. Comparative accuracy of Pap smear and HPV screening in Ubon Ratchathani in Thailand. Papillomavirus Res. 2017, 3, 30–35. [Google Scholar] [CrossRef]
- Sherlaw-Johnson, C.; Philips, Z. An evaluation of liquid-based cytology and human papillomavirus testing within the UK cervical cancer screening programme. Br. J. Cancer 2004, 91, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Chan, K.K.L.; Wei, T.N.; Tse, K.Y.; Ngu, S.F.; Chu, M.M.Y.; Lau, L.S.K.; Cheung, A.N.Y.; Ngan, H.Y.S. Clinical performance of the Roche Cobas 4800 HPV test for primary cervical cancer screening in a Chinese population. PLoS ONE 2022, 17, e0272721. [Google Scholar] [CrossRef]
- Lam, J.U.H.; Rebolj, M.; Ejegod, D.M.; Pedersen, H.; Rygaard, C.; Lynge, E.; Harder, E.; Thomsen, L.T.; Kjaer, S.K.; Bonde, J. Prevalence of Human Papillomavirus in Self-Taken Samples from Screening Nonattenders. J. Clin. Microbiol. 2017, 55, 2913–2923. [Google Scholar] [CrossRef]
- Ma, Y.; Di, J.; Bi, H.; Zhao, Q.; Qin, T.; Xu, W.; Liu, Z.; Yi, N.; Zhao, J.; Zhou, D.; et al. Comparison of the detection rate of cervical lesion with TruScreen, LBC test and HPV test: A Real-world study based on population screening of cervical cancer in rural areas of China. PLoS ONE 2020, 15, e0233986. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Saidu, R.; Boa, R.; Tergas, A.; Moodley, J.; Persing, D.; Campbell, S.; Tsai, W.Y.; Wright, T.C.; Denny, L. Clinical evaluation of modifications to a human papillomavirus assay to optimise its utility for cervical cancer screening in low-resource settings: A diagnostic accuracy study. Lancet Glob. Health 2020, 8, e296–e304. [Google Scholar] [CrossRef] [PubMed]
- Avian, A.; Clemente, N.; Mauro, E.; Isidoro, E.; Di Napoli, M.; Dudine, S.; Del Fabro, A.; Morini, S.; Perin, T.; Giudici, F.; et al. Clinical validation of full HR-HPV genotyping HPV Selfy assay according to the international guidelines for HPV test requirements for cervical cancer screening on clinician-collected and self-collected samples. J. Transl. Med. 2022, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Du, H.; Qu, X.; Chen, Y.; Wang, J.; Wu, R. A comparative analysis of cycle threshold (Ct) values from Cobas4800 and AmpFire HPV assay for triage of women with positive hrHPV results. BMC Infect. Dis. 2023, 23, 783. [Google Scholar] [CrossRef]
- Ibanez, R.; Mareque, M.; Granados, R.; Andia, D.; Garcia-Rojo, M.; Quilez, J.C.; Oyaguez, I. Comparative cost analysis of cervical cancer screening programme based on molecular detection of HPV in Spain. BMC Womens Health 2021, 21, 178. [Google Scholar] [CrossRef]
- Duan, L.; Du, H.; Wang, C.; Huang, X.; Qu, X.; Shi, B.; Liu, Y.; Zhang, W.; Duan, X.; Wei, L.; et al. The effectiveness of HPV viral load, reflected by Cobas 4800 HPV-Ct values for the triage of HPV-positive women in primary cervical cancer screening: Direct endocervical samples. PLoS ONE 2020, 15, e0232107. [Google Scholar] [CrossRef]
- Aarnio, R.; Isacson, I.; Sanner, K.; Gustavsson, I.; Gyllensten, U.; Olovsson, M. Comparison of vaginal self-sampling and cervical sampling by medical professionals for the detection of HPV and CIN2+: A randomized study. Int. J. Cancer 2021, 148, 3051–3059. [Google Scholar] [CrossRef]
- Saville, M.; Sultana, F.; Malloy, M.J.; Velentzis, L.S.; Caruana, M.; Ip, E.L.O.; Keung, M.H.T.; Canfell, K.; Brotherton, J.M.L.; Hawkes, D. Clinical Validation of the cobas HPV Test on the cobas 6800 System for the Purpose of Cervical Screening. J. Clin. Microbiol. 2019. [Google Scholar] [CrossRef]
- Clifford, G.M.; Baussano, I.; Heideman, D.A.M.; Tshering, S.; Choden, T.; Lazzarato, F.; Tenet, V.; Franceschi, S.; Darragh, T.M.; Tobgay, T.; et al. Human papillomavirus testing on self-collected samples to detect high-grade cervical lesions in rural Bhutan: The REACH-Bhutan study. Cancer Med. 2023, 12, 11828–11837. [Google Scholar] [CrossRef] [PubMed]
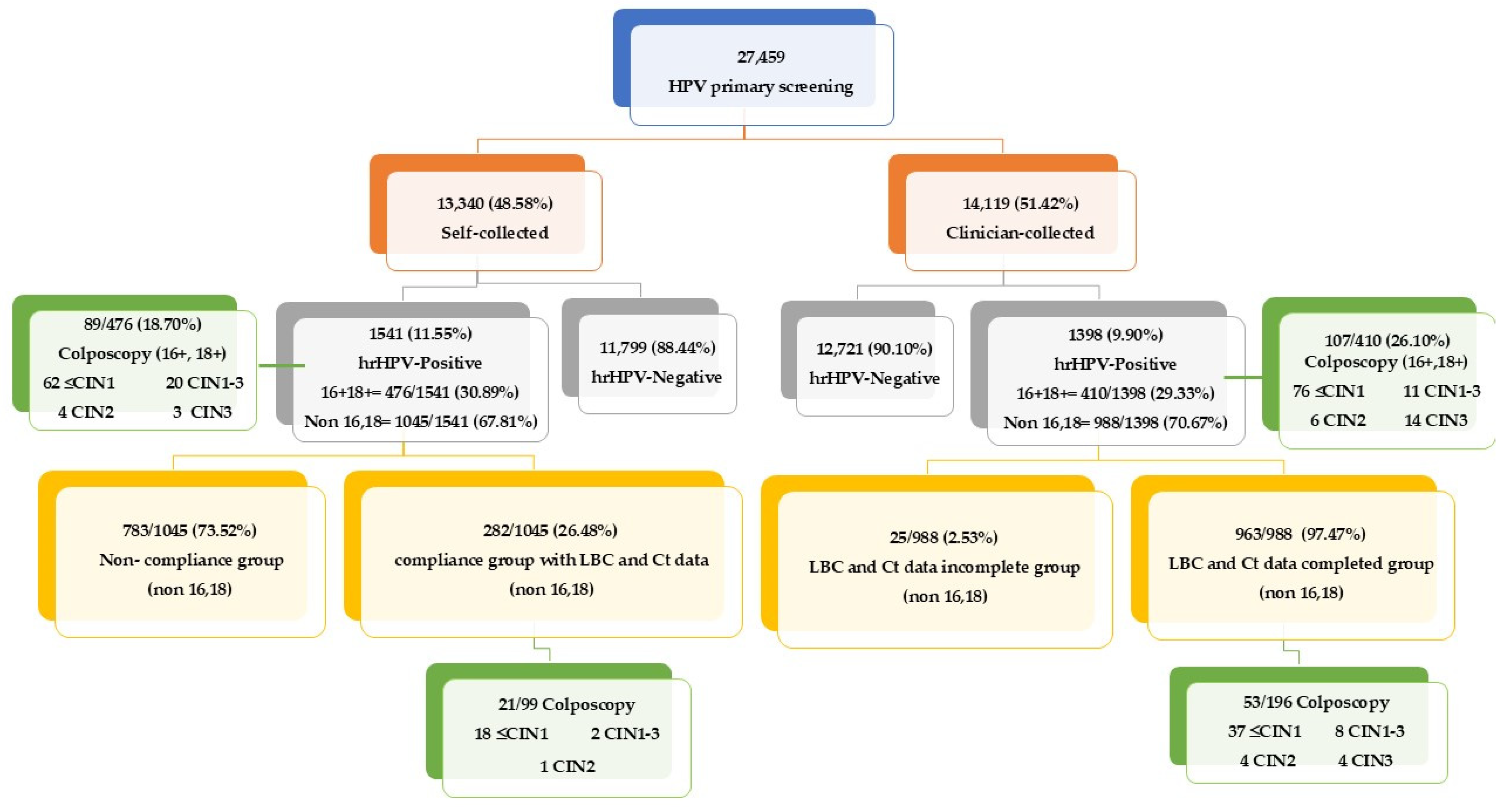
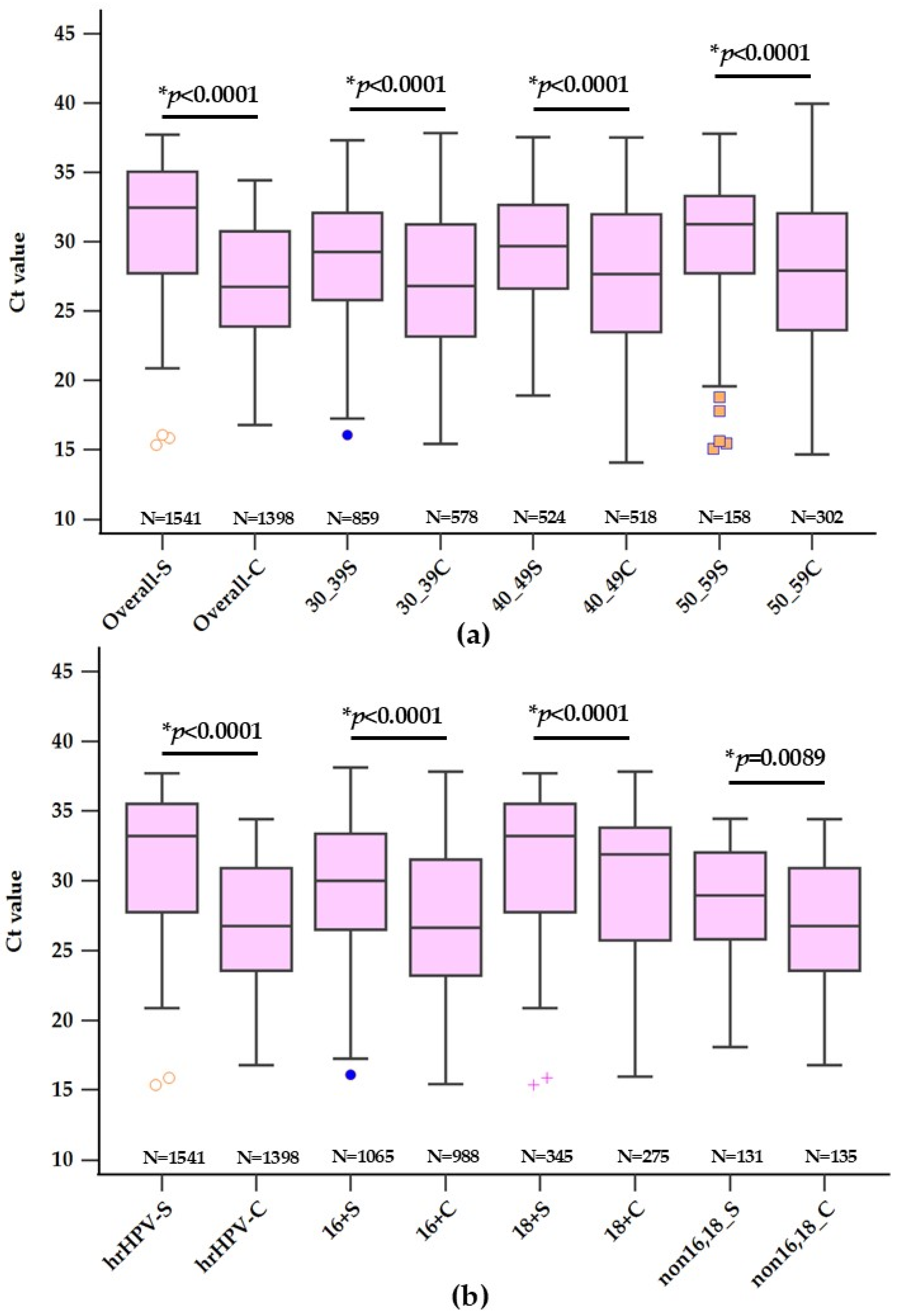

| Self-Collected | Clinician-Collected | Mean Difference | p-Value | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | (95% CI) | ||
| Total | 1541 | 29.22 ± 4.54 | 1398 | 27.69 ± 4.96 | 1.53 (1.18–1.87) | p < 0.0001 |
| Age (years) | ||||||
| 30–39 | 859 (55.75%) | 29.15 ± 4.43 | 578 (41.35%) | 27.48 ± 4.81 | 1.67 (1.18–2.15) | p < 0.0001 |
| 40–49 | 524 (34.00%) | 29.09 ± 4.66 | 518 (37.05%) | 27.71 ± 5.03 | 1.38 (0.79–1.96) | p < 0.0001 |
| 50–59 | 158 (10.25%) | 30.00 ± 4.67 | 302 (21.60%) | 28.06 ± 5.10 | 1.94 (0.99–2.90) | p < 0.0001 |
| HPV-DNA | ||||||
| 16 | 345 (22.39%) | 30.36 ± 4.47 | 275 (19.67%) | 28.13 ± 5.25 | 2.23 (1.46–3.00) | p < 0.0001 |
| 18 | 131(8.50%) | 31.58 ± 4.93 | 135 (9.66%) | 29.93 ± 5.24 | 1.65 (0.41–2.88) | p = 0.0089 |
| non 16,18 | 1065 (69.11%) | 28.56 ± 4.33 | 988 (70.67%) | 27.26 ± 4.75 | 1.30 (0.90–1.69) | p < 0.0001 |
| Cytology | 282 | 963 | ||||
| Negative | 183 (64.89%) | 29.44 ± 3.78 | 767 (79.65%) | 28.37 ± 4.17 | 1.07 (0.41–1.73) | p = 0.0016 |
| Positive | 99 (35.11%) | 196 (20.35%) | ||||
| LSIL | 86 (30.49%) | 26.88 ± 4.56 | 170 (17.65%) | 23.02 ± 4.66 | 3.86 (2.41–4.88) | p < 0.0001 |
| HSIL | 13 (4.62%) | 26.80 ± 4.29 | 26 (2.70%) | 22.39 ± 3.13 | 4.41 (1.96–6.85) | p < 0.0008 |
| Histology | 110 | 160 | ||||
| ≤CIN1 | 80 (72.72%) | 29.04 ± 5.06 | 113 (70.64%) | 27.22 ± 5.76 | 1.82 (0.24–3.40) | p = 0.0238 |
| CIN1–3* | 22 (20.00%) | 32.32 ± 4.88 | 19 (11.86%) | 27.45 ± 5.08 | 4.87 (1.71–8.01) | p = 0.0034 |
| CIN2 | 5 (4.55%) | 29.75 ± 5.06 | 10 (6.25%) | 26.65 ± 4.61 | 3.1 (2.53–8.72) | p = 0.2553 |
| CIN3 | 3 (2.73%) | 29.02 ± 3.30 | 18 (11.25%) | 28.90 ± 5.55 | 0.12 (6.87–7.12) | p = 0.9713 |
| Ct (Cut-Off) | Sensitivity (95% CI) | Specificity (95% CI) | AUC | |
|---|---|---|---|---|
| Cytology | ||||
| Self-LSIL | ≤26.07 | 48.84 (37.9–59.9) | 82.51 (76.2–87.7) | 0.668 |
| Clinician-LSIL | ≤24.95 | 69.41(61.9–76.2) | 77.84 (74.7–80.7) | 0.793 |
| Self-HSIL | ≤24.46 | 46.15 (19.2–74.9) | 86.63 (84.3–93.6) | 0.680 |
| Clinician-HSIL | ≤25.47 | 88.46 (69.8–97.6) | 73.01 (69.7–76.1) | 0.866 |
| Colposcopy | ||||
| Self-CIN2 | >33.68 | 40 (5.3–85.3) | 85.00 (75.3–92.0) | 0.555 |
| Clinician-CIN2 | ≤29.65 | 80 (44.4–97.5) | 38.05 (29.1–47.7) | 0.539 |
| Self-CIN3 | ≤27.16 | 66.67 (9.4–99.2) | 67.50 (56.1–77.6) | 0.533 |
| Clinician-CIN3 | >22.31 | 100 (81.5–100.0) | 21.24 (14.1–29.9) | 0.566 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruttanamora, U.; Thongsalak, P.; Sammor, A.; Chomean, S.; Kaset, C. Comparative Analysis of HPV Detection Efficiency: Evaluating Cobas 8800 Performance in Vaginal Self-Sampling versus Clinician-Collected Samples at a Regional Thai Hospital. Diagnostics 2024, 14, 2177. https://doi.org/10.3390/diagnostics14192177
Ruttanamora U, Thongsalak P, Sammor A, Chomean S, Kaset C. Comparative Analysis of HPV Detection Efficiency: Evaluating Cobas 8800 Performance in Vaginal Self-Sampling versus Clinician-Collected Samples at a Regional Thai Hospital. Diagnostics. 2024; 14(19):2177. https://doi.org/10.3390/diagnostics14192177
Chicago/Turabian StyleRuttanamora, Umaporn, Pinsawitar Thongsalak, Araya Sammor, Sirinart Chomean, and Chollanot Kaset. 2024. "Comparative Analysis of HPV Detection Efficiency: Evaluating Cobas 8800 Performance in Vaginal Self-Sampling versus Clinician-Collected Samples at a Regional Thai Hospital" Diagnostics 14, no. 19: 2177. https://doi.org/10.3390/diagnostics14192177
APA StyleRuttanamora, U., Thongsalak, P., Sammor, A., Chomean, S., & Kaset, C. (2024). Comparative Analysis of HPV Detection Efficiency: Evaluating Cobas 8800 Performance in Vaginal Self-Sampling versus Clinician-Collected Samples at a Regional Thai Hospital. Diagnostics, 14(19), 2177. https://doi.org/10.3390/diagnostics14192177







