Histopathological Analysis of Pseudoexfoliation Material in Ocular Surgeries: Clinical Implications
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindberg, J.G. Clinical Study of Depigmentation of the Papillary Border and Transillumination of the Iris in Cases of Senile Cataract and in Normal Eyes in Aged Persons. Arch. Ophthalmol. 1917, 46, 91–104. [Google Scholar]
- Yildirim, N.; Yasar, E.; Gursoy, H.; Colak, E. Prevalence of pseudoexfoliation syndrome and its association with ocular and systemic diseases in Eskisehir, Turkey. Int. J. Ophthalmol. 2017, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Topouzis, F.; Anastasopoulos, E. Incidence of Pseudoexfoliation Syndrome. Am. J. Ophthalmol. 2009, 148, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.P.; Ringvold, A. Epidemiology of Exfoliation Syndrome. J. Glaucoma. 2018, 27 (Suppl. S1), S4–S11. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L. The prevalence of pseudoexfoliation syndrome in Chinese people. Br. J. Ophthalmol. 2004, 88, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, S.A.; Al-Dabbagh, N.M.; Al-Shamrani, S.M.; Khan, N.M.; Arfin, M.; Tariq, M.; Al-Faleh, H.M. Prevalence of ocular pseudoexfoliation syndrome and associated complications in Riyadh, Saudi Arabia. Saudi Med. J. 2015, 36, 108–112. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Masip-Serra, R.; Martínez-Salcedo, I.; Salvat-Serra, M.; Fernández-Ballart, J.; Bautista-Pérez, Á. High Prevalence of Pseudoexfoliation Syndrome and its Complications in Tarragona in Northeast Spain. Eur. J. Ophthalmol. 2011, 21, 580–588. [Google Scholar] [CrossRef]
- Lindberg, J.G. Clinical investigations on depigmentation of the pupillary border and translucency of the iris in cases of senile cataract and in normal eyes in elderly persons. Acta Ophthalmol. Suppl. 1989, 190, 1–96. [Google Scholar]
- Shingleton, B.J.; Crandall, A.S.; Ahmed, I.I.K. Pseudoexfoliation and the cataract surgeon: Preoperative, intraoperative, and postoperative issues related to intraocular pressure, cataract, and intraocular lenses. J. Cataract. Refract. Surg. 2009, 35, 1101–1120. [Google Scholar] [CrossRef]
- Vazquez-Ferreiro, P.; Carrera-Hueso, F.J.; Jornet, J.E.P.; Fikri-Benbrahim, N.; Diaz-Rey, M.; Sanjuan-Cerveró, R. Intraoperative complications of phacoemulsification in pseudoexfoliation: Metaanalysis. J. Cataract. Refract. Surg. 2016, 42, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.Y.; Shalchi, Z. Outcomes of cataract surgery in pseudoexfoliation syndrome in England: 10-year retrospective cohort study. J. Cataract. Refract. Surg. 2021, 47, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Padhy, D. Pattern of Pseudoexfoliation Deposits on the Lens and Their Clinical Correlation- Clinical Study and Review of Literature. PLoS ONE 2014, 9, e113329. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, Z.S.; Sreenivasaiah, S.; Srinivasan, T.; Shroff, S.; Devi, S.; Rao, D.A.; Webers, C.A.; Puttaiah, N.K.; Rao, H.L. The Importance of Signal Strength Index in Optical Coherence Tomography Angiography: A Study of Eyes with Pseudoexfoliation Syndrome. Clin. Ophthalmol. 2022, 16, 3481–3489. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, D.; Machairoudia, G.; Theodossiadis, P.; Chatziralli, I. Subfoveal choroidal thickness changes in patients with pseudoexfoliation syndrome (PEX) compared to healthy controls: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2024, 47, 104095. [Google Scholar] [CrossRef] [PubMed]
- Sarenac-Vulovic, T.S.; Janicijevic Petrovic, M.A.; Vulovic, D.D.; Pavlovic, S.M.; Simovic, S.; Zdravkovic, N.S. Systemic Manifestations of Pseudoexfoliation. Serbian J. Exp. Clin. Res. 2014, 15, 29–32. [Google Scholar] [CrossRef]
- Praveen, M.R.; Shah, S.K.; Vasavada, A.R.; Diwan, R.P.; Shah, S.M.; Zumkhawala, B.R.; Thomas, R. Pseudoexfoliation as a risk factor for peripheral vascular disease: A case-control study. Eye 2011, 25, 174–179. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Margo, C.; Harman, L. Ocular pseudoexfoliation and cardiovascular disease: A national cross-section comparison study. N. Am. J. Med. Sci. 2012, 4, 468. [Google Scholar] [CrossRef]
- Katsi, V.; Pavlidis, A.N.; Kallistratos, M.S.; Fitsios, A.; Bratsas, A.; Tousoulis, D.; Stefanadis, C.; Manolis, A.J.; Kallikazaros, I. Cardiovascular repercussions of the pseudoexfoliation syndrome. N. Am. J. Med. Sci. 2013, 5, 454. [Google Scholar] [CrossRef] [PubMed]
- Sarda, V.; Rohart, C.; Fajnkuchen, F.; Nghiem Buffet, S.; Streho, M.; Chaine, G. Syndrome pseudoexfoliatif et phakoexerèse: Étude comparative à une population témoin. J. Fr. Ophtalmol. 2010, 33, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Naumann, G.O.H. Ocular and Systemic Pseudoexfoliation Syndrome. Am. J. Ophthalmol. 2006, 141, 921–937.e2. [Google Scholar] [CrossRef]
- Ritch, R. Exfoliation syndrome. Curr. Opin. Ophthalmol. 2001, 12, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Scorolli, L.; Scorolli, L.; Campos, E.C.; Bassein, L.; Meduri, R.A. Pseudoexfoliation Syndrome: A Cohort Study on Intraoperative Complications in Cataract Surgery. Ophthalmologica 1998, 212, 278–280. [Google Scholar] [CrossRef]
- Ovodenko, B.; Rostagno, A.; Neubert, T.A.; Shetty, V.; Thomas, S.; Yang, A.; Liebmann, J.; Ghiso, J.; Ritch, R. Proteomic Analysis of Exfoliation Deposits. Investig. Opthalmology Vis. Sci. 2007, 48, 1447. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Kang, J.H.; Fan, B.; Levkovitch-Verbin, H.; Pasquale, L.R. A Role for Clusterin in Exfoliation Syndrome and Exfoliation Glaucoma? J. Glaucoma 2018, 27 (Suppl. S1), S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Streeten, B.W.; Dark, A.J.; Wallace, R.N.; Li, Z.Y.; Hoepner, J.A. Pseudoexfoliative Fibrillopathy in the Skin of Patients by Ocular Pseudoexfoliation. Am. J. Ophthalmol. 1990, 110, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Dikmetas, O.; Kapucu, Y.; Firat, A.; Sargon, M.F.; Kocabeyoglu, S. Changes in the Anterior Lens Epithelium and Basement Membrane in Pseudoexfoliation Syndrome undergoing Surgery for Senile Cataracts: A Transmission Electron Microscopic Study. J. Coll. Physicians Surg. Pak. 2021, 31, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Schlotzer-Schrehardt, U. Genetics and genomics of pseudoexfoliation syndrome/glaucoma. Middle East. Afr. J. Ophthalmol. 2011, 18, 30. [Google Scholar] [CrossRef]
- Mastronikolis, S.; Pagkalou, M.; Plotas, P.; Kagkelaris, K.; Georgakopoulos, C. Emerging roles of oxidative stress in the pathogenesis of pseudoexfoliation syndrome (Review). Exp. Ther. Med. 2022, 24, 602. [Google Scholar] [CrossRef]
- Elhawy, E.; Kamthan, G.; Dong, C.Q.; Danias, J. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Hum. Genomics 2012, 6, 22. [Google Scholar] [CrossRef]
- Zenkel, M.; Kruse, F.E.; Ju¨nemann, A.G.; Naumann, G.O.H.; Schlötzer-Schrehardt, U. Clusterin Deficiency in Eyes with Pseudoexfoliation Syndrome May Be Implicated in the Aggregation and Deposition of Pseudoexfoliative Material. Investig. Opthalmology Vis. Sci. 2006, 47, 1982. [Google Scholar] [CrossRef] [PubMed]
- Zenkel, M.; Lewczuk, P.; Jünemann, A.; Kruse, F.E.; Naumann, G.O.H.; Schlötzer-Schrehardt, U. Proinflammatory Cytokines Are Involved in the Initiation of the Abnormal Matrix Process in Pseudoexfoliation Syndrome/Glaucoma. Am. J. Pathol. 2010, 176, 2868–2879. [Google Scholar] [CrossRef]
- Ho, S.L. Elevated aqueous humour tissue inhibitor of matrix metalloproteinase-1 and connective tissue growth factor in pseudoexfoliation syndrome. Br. J. Ophthalmol. 2005, 89, 169–173. [Google Scholar] [CrossRef]
- Rönkkö, S.; Rekonen, P.; Kaarniranta, K.; Puustjärvi, T.; Teräsvirta, M.; Uusitalo, H. Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 697. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk-Socha, M.; Tomczak, W.; Winkler-Lach, W.; Turno-Kręcicka, A. Pseudoexfoliation Syndrome—Clinical Characteristics of Most Common Cause of Secondary Glaucoma. J. Clin. Med. 2023, 12, 3580. [Google Scholar] [CrossRef]
- Ritch, R. Why is glaucoma associated with exfoliation syndrome? Prog. Retin. Eye Res. 2003, 22, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Hao, Y. Determination of the density of human nuclear cataract lenses. Mol. Med. Rep. 2013, 8, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Wanzeler, A.C.; Barbosa, I.A.; Duarte, B.; Borges, D.; Barbosa, E.B.; Kamiji, D.; Huarachi, D.R.; Melo, M.B.; Alves, M. Mechanisms and biomarker candidates in pterygium development. Arq. Bras. Oftalmol. 2019, 82, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Bashour, M. Lower Lid Ectropion Blepharoplasty Workup. Medscape. 22 December 2015. Available online: https://emedicine.medscape.com/article/1281565-workup (accessed on 26 August 2024).
- Skorin, L., Jr.; Norberg, S.; Erickson, J.A. Entropion: Etiology, Classification, Diagnosis, and Treatment. Consultant. December 2018. Available online: https://www.consultant360.com/article/consultant360/ophthalmology/entropion-etiology-classification-diagnosis-and-treatment (accessed on 26 August 2024).
- Aviv, U.; Ben Ner, D.; Sharif, N.; Gur, Z.; Achiron, A. Pseudoexfoliation: An Ocular Finding with Possible Systemic Implications. Isr. Med. Assoc. J. 2017, 19, 49–54. Available online: https://pubmed.ncbi.nlm.nih.gov/28457115/ (accessed on 13 December 2023).
- Tătaru, C.I.; Tătaru, C.P.; Costache, A.; Boruga, O.; Zemba, M.; Ciuluvică, R.C.; Sima, G. Congenital cataract—Clinical and morphological aspects. Rom. J. Morphol. Embryol. 2020, 61, 105–112. [Google Scholar] [CrossRef]
- Jawad, M.; Nadeem, A.-u.R.; Khan, A.u.H.; Aftab, M. Complications of cataract surgery in patients with pseudoexfoliation syndrome. J. Ayub Med. Coll. Abbottabad 2009, 21, 33–36. Available online: https://ayubmed.edu.pk/JAMC/PAST/21-2/Jawad.pdf (accessed on 3 September 2024).
- Ekström, C.; Botling Taube, A. Pseudoexfoliation and cataract surgery: A population-based 30-year follow-up study. Acta Ophthalmol. 2015, 93, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Sangal, N.; Chen, T.C. Cataract Surgery in Pseudoexfoliation Syndrome. Semin. Ophthalmol. 2014, 29, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Coassin, M.; Iovieno, A.; Moramarco, A.; Cimino, L. Cataract surgery in patients with pseudoexfoliation syndrome: Current updates. Clin. Ophthalmol. 2017, 11, 1377–1383. [Google Scholar] [CrossRef]
- Shingleton, B.J.; Marvin, A.C.; Heier, J.S.; O’Donoghue, M.W.; Laul, A.; Wolff, B.; Rowland, A. Pseudoexfoliation: High risk factors for zonule weakness and concurrent vitrectomy during phacoemulsification. J. Cataract. Refract. Surg. 2010, 36, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Ferreiro, P.; Carrera-Hueso, F.J.; Fikri-Benbrahim, N.; Barreiro-Rodriguez, L.; Diaz-Rey, M.; Ramón Barrios, M.A. Intraocular lens dislocation in pseudoexfoliation: A systematic review and meta-analysis. Acta Ophthalmol. 2017, 95, e164–e169. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Odayappan, A.; Tripathy, K.; Krishnamurthy, P.; Nachiappan, S. Predicting zonular strength based on maximum pupillary mydriasis in patients with pseudoexfoliation syndrome. Med. Hypotheses 2021, 146, 110402. [Google Scholar] [CrossRef] [PubMed]
- Detorakis, E.; Bontzos, G.; Drakonaki, E.; Spandidos, D. Changes in peri-ocular anatomy and physiology in pseudoexfoliation syndrome (Review). Exp. Ther. Med. 2021, 21, 650. [Google Scholar] [CrossRef] [PubMed]
- Škegro, I.; Suić, S.P.; Kordić, R.; Jandroković, S.; Petriček, I.; Kuzman, T.; Kalauz, M.; Perić, S.; Masnec, S. Ocular surface disease in pseudoexfoliation syndrome. Coll. Antropol. 2015, 39, 43–45. [Google Scholar]
- Noori, S.; Sati, A.; Moulick, P.S.; Kaushik, J.; Shankar, S.; Bose, R. Tear film abnormalities in pseudoexfoliation syndrome and normal healthy participants: A comparative analysis. Med. J. Armed Forces India 2020, 76, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Streeten, B.W.; Bookman, L.; Ritch, R.; Prince, A.M.; Dark, A.J. Pseudoexfoliative Fibrillopathy in the Conjunctiva. Ophthalmology 1987, 94, 1439–1449. [Google Scholar] [CrossRef]
- Rao, A.; Das, G.; Sarangi, S.; Padhy, D. Conjunctival changes in different clinical variants of early pseudoexfoliation. Int. Ophthalmol. 2018, 38, 2477–2485. [Google Scholar] [CrossRef]
- Sollosy, M.; Preda, M.; Simionescu, C. Modificări conjunctivale in sindromul exfoliativ [Conjunctival changes in pseudoexfoliative syndrome]. Oftalmologia 2006, 50, 103–107. [Google Scholar]
- Akdemir, M.O.; Kirgiz, A.; Ayar, O.; Kaldirim, H.; Mert, M.; Cabuk, K.S.; Taskapili, M. The Effect of Pseudoexfoliation and Pseudoexfoliation Induced Dry Eye on Central Corneal Thickness. Curr. Eye Res. 2015, 41, 305–310. [Google Scholar] [CrossRef] [PubMed]



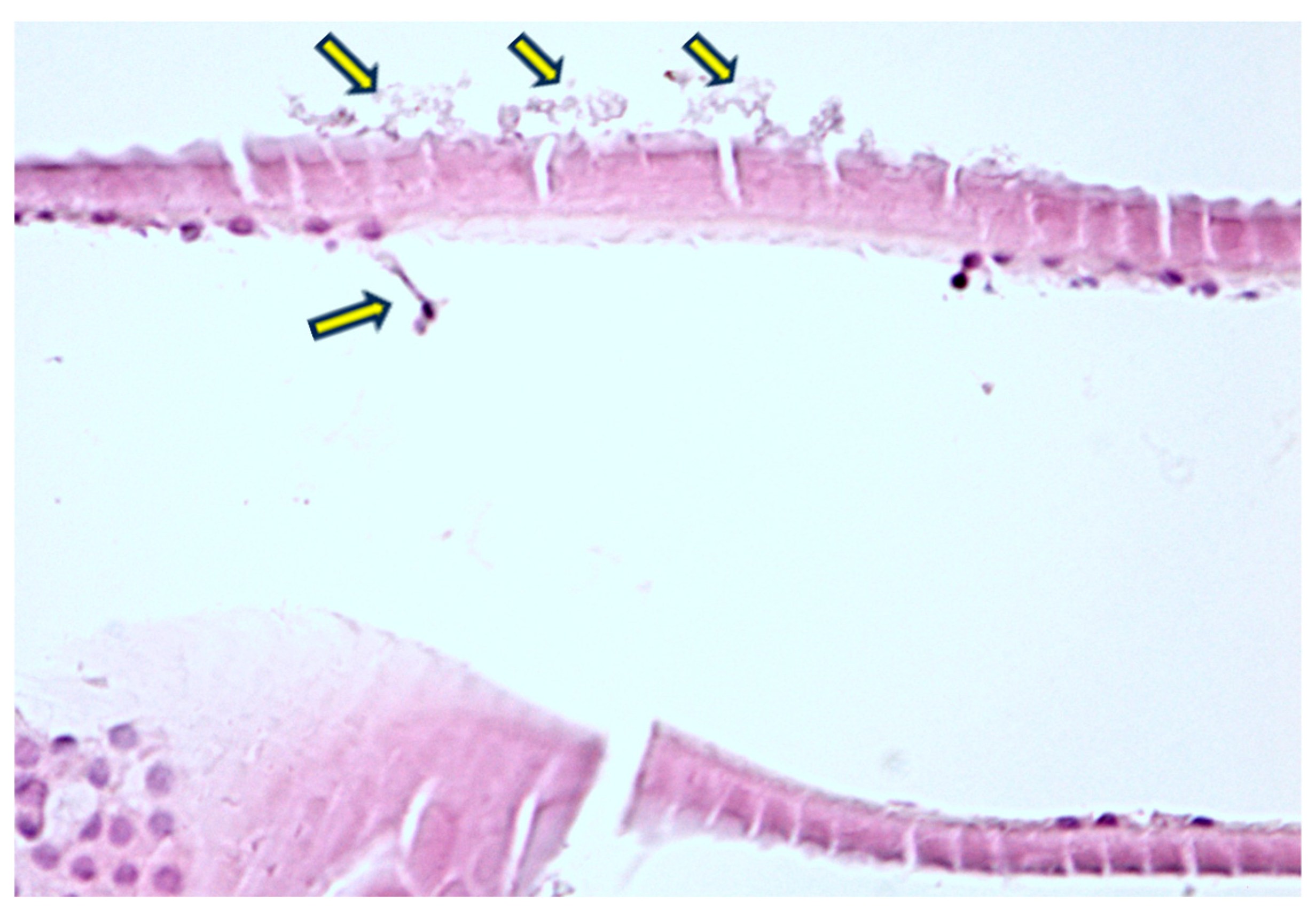
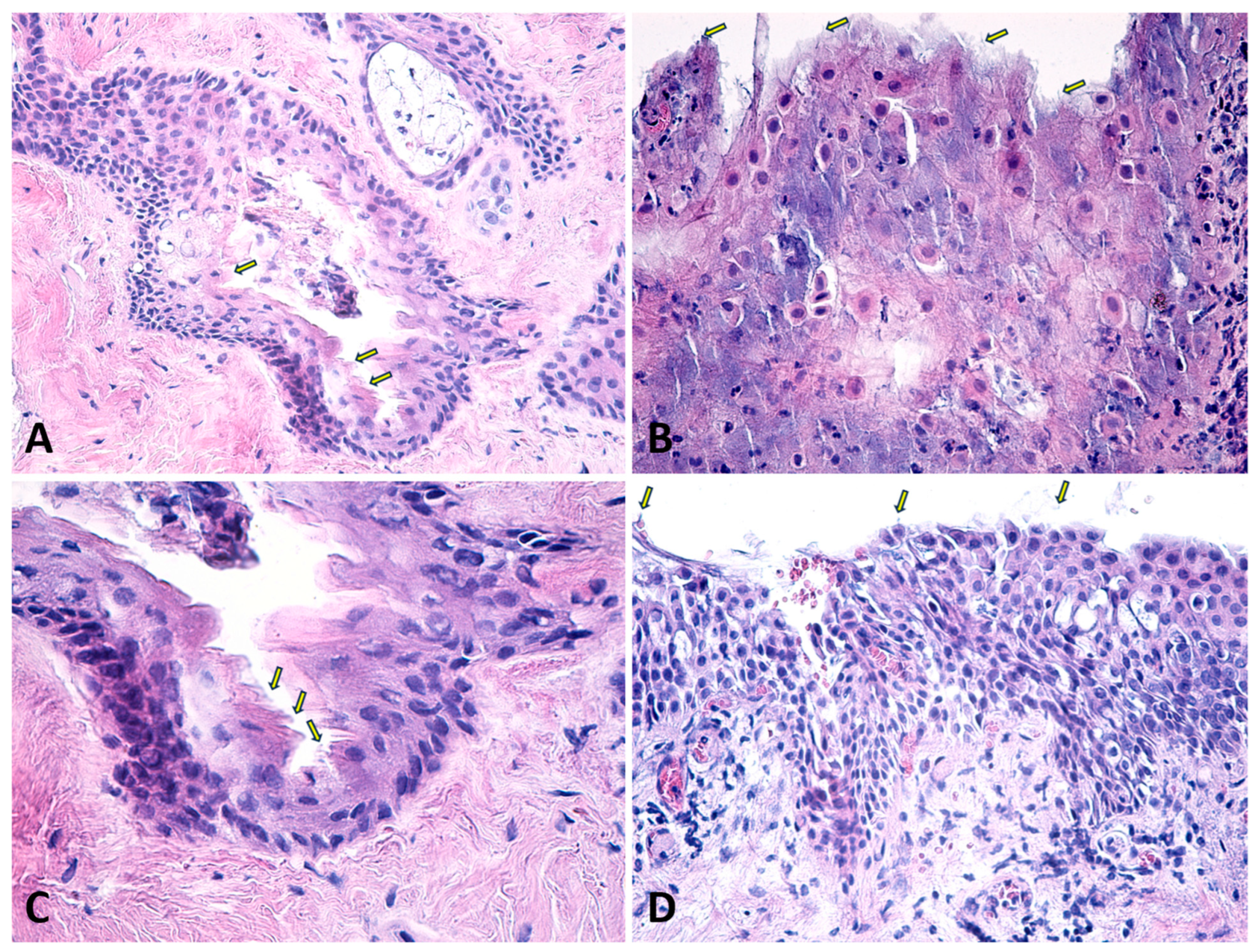
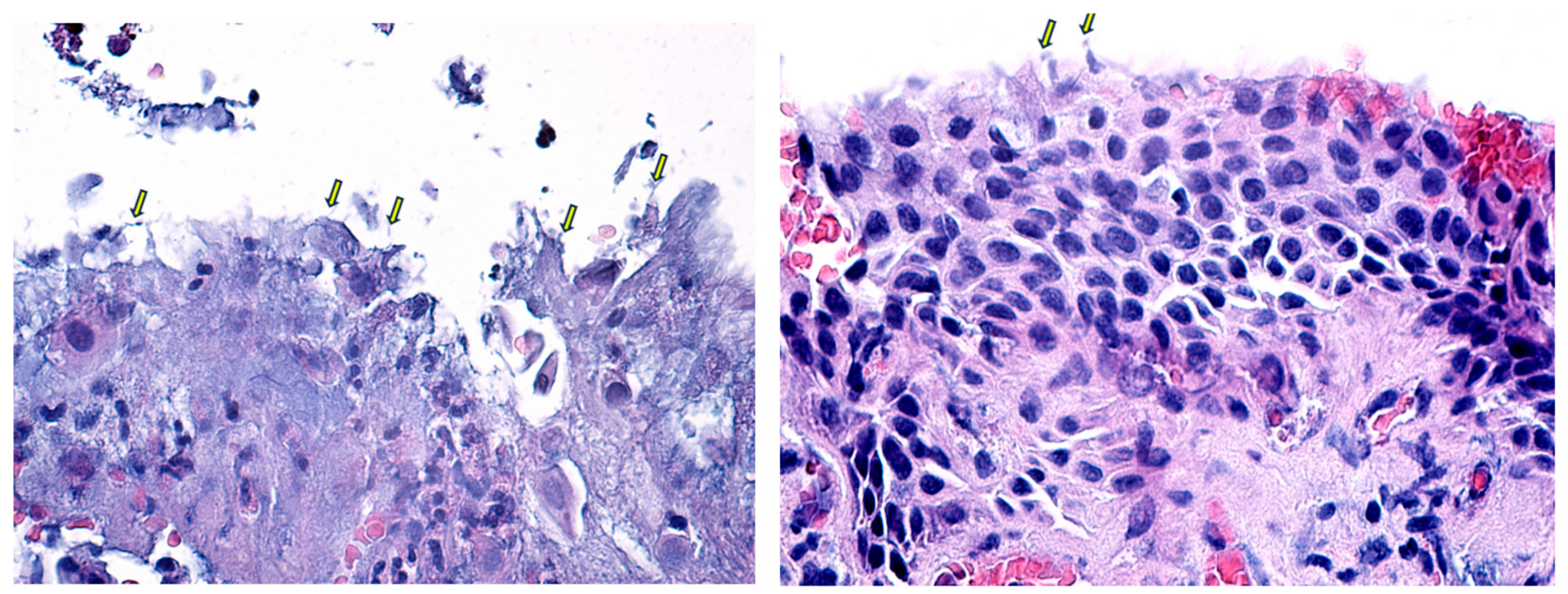

| Biometry Data | Mean | SD | 95% CI |
|---|---|---|---|
| Corneal curvature (D) | 44.21 | ±0.308 | 43.59–44.83 |
| ACA (grade) | 28.80 | ±1.07 | 26.65–31.00 |
| ACD (mm) | 3.017 | ±0.07 | 2.86–3.17 |
| CCT (μm) | 541 | ±4.5 | 532.23–550.41 |
| Endothelial cell count | 2361 | ±52.73 | 2255.04–2467.96 |
| % Hexagonal cells | 63.76% | ± 1.08 | 61.59–65.93 |
| Characteristic | Percentage | |
|---|---|---|
| Cornea | Normal | 83.05% |
| Endothelial fibrillar material | 3.75% | |
| Endothelial decompensation | 1.6% | |
| Endothelial pigment | 10% | |
| Corneal dystrophy | 1.6% | |
| Iris | Normal | 67.6% |
| Pupillary ruff loss | 20% | |
| Radial atrophy | 10.8% | |
| Pigment dispersion in AC | 1.6% | |
| Iridodonesis | 0% | |
| Lens | Lens in normal position | 100% |
| TM morphology | Normal | 60% |
| TM Hyperpigmentation | 30% | |
| Sampaolesi line | 10% | |
| Angle | Open | 88.75% |
| Narrow | 11.25% | |
| Closed | 0 | |
| Glaucoma | HTIO | 7.2% |
| OAG | 9.6% | |
| CAG | 0% | |
| Without glaucoma | 83.2% |
| Mean | SD | 95% CI | |
|---|---|---|---|
| BCVA baseline | 1.315 | ±0.747 | 1.108–1.522 |
| BCVA 7 days | 0.164 | ±0.156 | 0.121–0.207 |
| BCVA 1 month | 0.08 | ±0.088 | 0.055–0.104 |
| BCVA 3 months | 0.07 | ±0.083 | 0.047–0.093 |
| Mean | SD | 95% CI | |
|---|---|---|---|
| IOP baseline | 16.42 | ±4.20 | 15.25–17.58 |
| IOP 7 days | 14.80 | ±3.42 | 13.85–15.75 |
| IOP 1 month | 14.20 | ±2.56 | 13.49–14.91 |
| IOP 3 months | 14.08 | ±2.63 | 13.35–14.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stejar, L.D.; Istrate-Ofițeru, A.-M.; Tofolean, I.T.; Preoteasa, D.; Baltă, F. Histopathological Analysis of Pseudoexfoliation Material in Ocular Surgeries: Clinical Implications. Diagnostics 2024, 14, 2187. https://doi.org/10.3390/diagnostics14192187
Stejar LD, Istrate-Ofițeru A-M, Tofolean IT, Preoteasa D, Baltă F. Histopathological Analysis of Pseudoexfoliation Material in Ocular Surgeries: Clinical Implications. Diagnostics. 2024; 14(19):2187. https://doi.org/10.3390/diagnostics14192187
Chicago/Turabian StyleStejar, Laura Denisa, Anca-Maria Istrate-Ofițeru, Ioana Teodora Tofolean, Dana Preoteasa, and Florian Baltă. 2024. "Histopathological Analysis of Pseudoexfoliation Material in Ocular Surgeries: Clinical Implications" Diagnostics 14, no. 19: 2187. https://doi.org/10.3390/diagnostics14192187






