Influence of Hematocrit and Storage Temperature on the Stability of Dried Blood Samples in Serological Analyses of Tetanus, Diphtheria, and Pertussis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
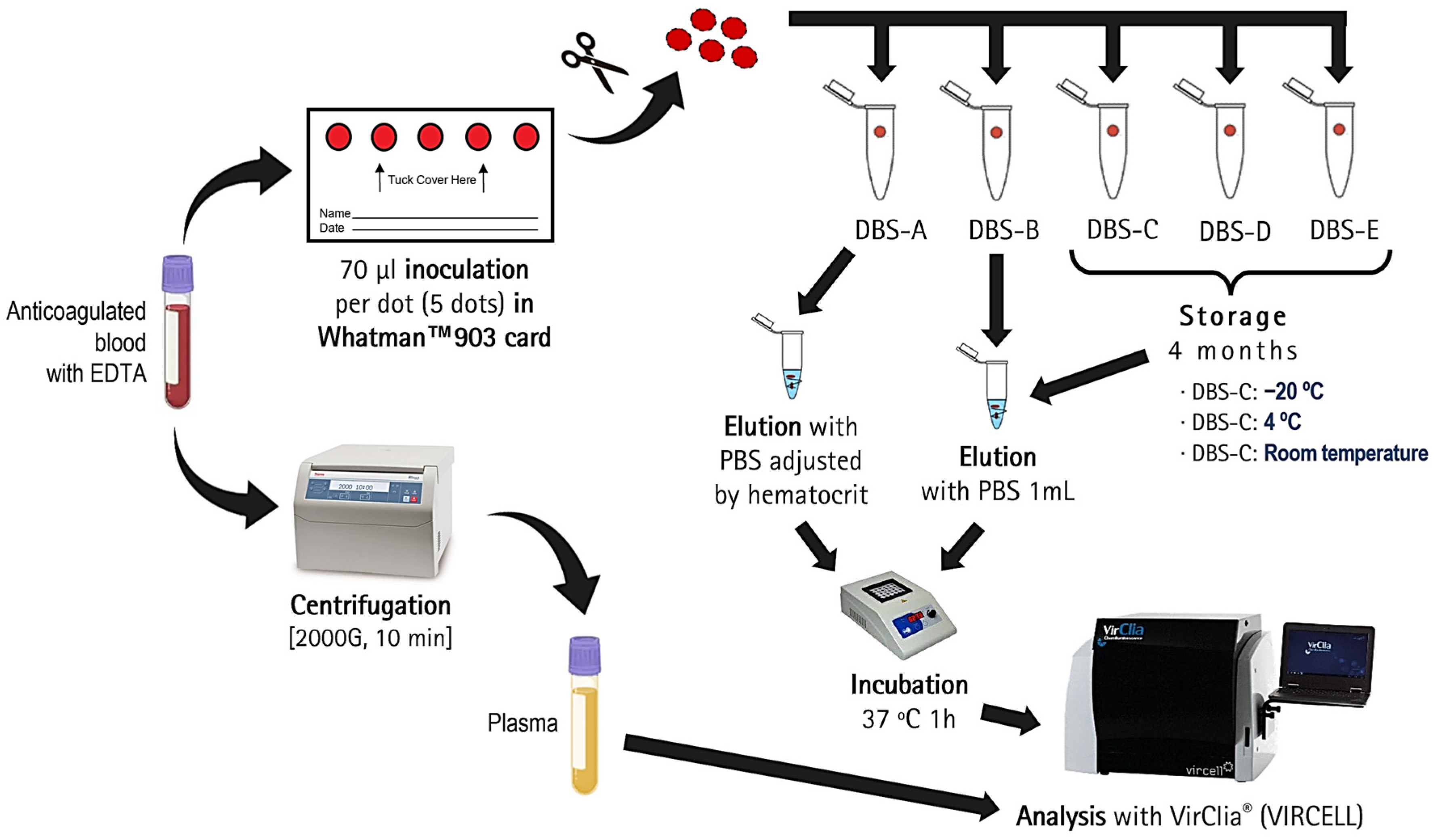
2.2. Sample Collection and Storage
2.3. DBS Elution
2.4. Serological Testing
2.5. Statistical Analysis
3. Results
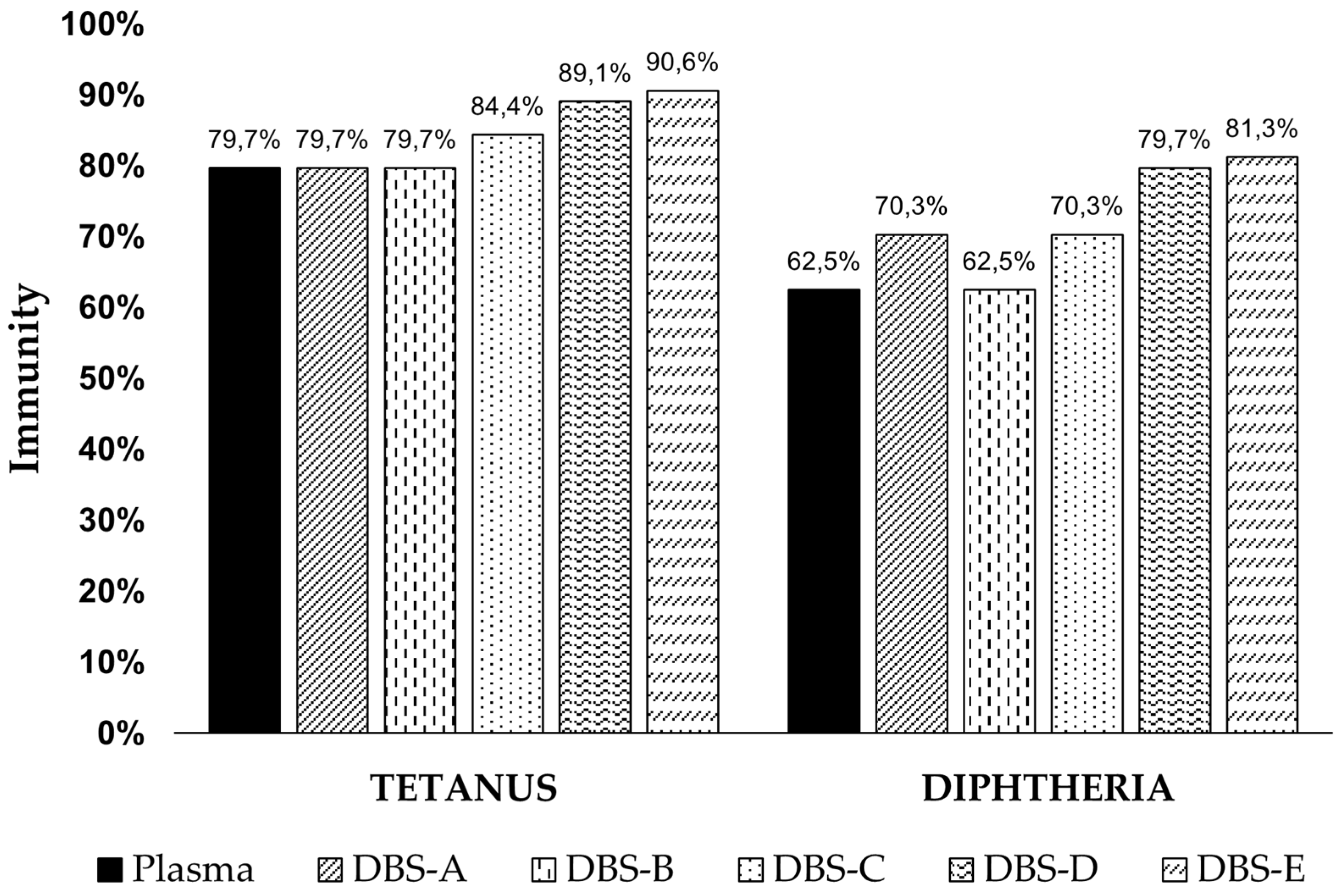
3.1. Immunization Levels in Plasma
3.2. Correlation between DBSs and Plasma
3.2.1. Effect of the Hematocrit
3.2.2. DBS Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuaillon, E.; Kania, D.; Pisoni, A.; Bollore, K.; Taieb, F.; Ontsira Ngoyi, E.N.; Schaub, R.; Plantier, J.C.; Makinson, A.; Van de Perre, P. Dried Blood Spot Tests for the Diagnosis and Therapeutic Monitoring of HIV and Viral Hepatitis B and C. Front. Microbiol. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ren, Y.; He, L.; He, X.; Xing, W.; Jiang, Y. An efficient method for simultaneously screening for HIV, syphilis, and HCV based on one dried blood spot sample. Antivir. Res. 2020, 181, 104775. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenburg, S.A.; Bruisten, S.M.; Heijman, T.; Vermeulen, W.; van Dam, A.P.; Schim van der Loeff, M.F.; de Vries, H.J.C. Use of home-based self-collected dried blood spots to test for syphilis, HIV, hepatitis C and B virus infections and measuring creatinine concentration. Sex. Transm. Dis. 2024, 51, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, B.; Taylor, R.; Dawkins, C.; Banks, T.; Watson, N.; Sundaram, A.; Ewing, D.; Danko, J.R. The use of dried blood spot cards to assess serologic responses of individuals vaccinated against measles, hepatitis A, tetanus, influenza and varicella zoster. PLoS ONE 2022, 17, e0265813. [Google Scholar] [CrossRef] [PubMed]
- de Aguirre, P.M.; Carlos, S.; Pina-Sánchez, M.; Mbikayi, S.; Burgueño, E.; Tendobi, C.; Chiva, L.; Holguín, Á.; Reina, G. High pre-Delta and early-Omicron SARS-CoV-2 seroprevalence detected in dried blood samples from Kinshasa (D.R. Congo). J. Med. Virol. 2024, 96, e29529. [Google Scholar] [CrossRef]
- Rodríguez-Mateos, M.; Jaso, J.; Martínez de Aguirre, P.; Carlos, S.; Fernández-Ciriza, L.; Holguín, Á.; Reina, G. Effect of the Hematocrit and Storage Temperature of Dried Blood Samples in the Serological Study of Mumps, Measles and Rubella. Diagnostics 2023, 13, 349. [Google Scholar] [CrossRef]
- Hannon, W.H.; Therell, B.L. Overview of the history and applications of dried blood spot samples. In Dried Blood Spots: Applications and Techniques; Li, W., Lee, M.S., Eds.; Wiley: Hoboken, NJ, USA; East Hanover, NJ, USA, 2014; Volume 3, pp. 3–15. [Google Scholar]
- Su, X.; Carlson, B.F.; Wang, X.; Li, X.; Zhang, Y.; Montgomery, J.P.; Ding, Y.; Wagner, A.L.; Gillespie, B.; Boulton, M.L. Dried blood spots: An evaluation of utility in the field. J. Infect. Public Health 2018, 11, 373–376. [Google Scholar] [CrossRef]
- Barquín, D.; Ndarabu, A.; Carlos, S.; Fernández-Alonso, M.; Rubio-Garrido, M.; Makonda, B.; Holguín, Á.; Reina, G. HIV-1 diagnosis using dried blood spots from patients in Kinshasa, DRC: A tool to detect misdiagnosis and achieve World Health Organization 2030 targets. Int. J. Infect. Dis. 2021, 111, 253–260. [Google Scholar] [CrossRef]
- Øverbø, J.; Aziz, A.; Zaman, K.; Julin, C.H.; Qadri, F.; Stene-Johansen, K.; Biswas, R.; Islam, S.; Bhuiyan, T.R.; Haque, W.; et al. Stability and Feasibility of Dried Blood Spots for Hepatitis E Virus Serology in a Rural Setting. Viruses 2022, 14, 2525. [Google Scholar] [CrossRef]
- Ryan, P.; Valencia, J.; Cuevas, G.; Troya, J.; Torres-Macho, J.; Muñoz-Gómez, M.J.; Muñoz-Rivas, N.; Canorea, I.; Vázquez-Morón, S.; Resino, S. HIV screening and retention in care in people who use drugs in Madrid, Spain: A prospective study. Infect. Dis. Poverty 2021, 10, 111. [Google Scholar] [CrossRef]
- Bajis, S.; Grebely, J.; Hajarizadeh, B.; Applegate, T.; Marshall, A.D.; Ellen Harrod, M.; Byrne, J.; Bath, N.; Read, P.; Edwards, M.; et al. Hepatitis C virus testing, liver disease assessment and treatment uptake among people who inject drugs pre- and post-universal access to direct-acting antiviral treatment in Australia: The LiveRLife study. J. Viral Hepat. 2020, 27, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.A.; Ancel, P.Y.; Simeon, T.; Marchand-Martin, L.; Zaros, C.; Dufourg, M.N.; Benhamou, V.; Blanché, H.; Deleuze, J.F.; Rahib, D.; et al. SARS-CoV-2 seroprevalence in French 9-year-old children and their parents after the first lockdown in 2020. Front. Pediatr. 2023, 11, 1274113. [Google Scholar] [CrossRef] [PubMed]
- International Air Transport Association (IATA). Dangerous Goods Regulations, 62nd ed.; International Air Transport Association: Montreal, Canada, 2021. [Google Scholar]
- WHO. WHO Manual for HIV Drug Resistance Testing Using Dried Blood Spot Specimens; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240009424 (accessed on 20 March 2024).
- Amini, F.; Auma, E.; Hsia, Y.; Bilton, S.; Hall, T.; Ramkhelawon, L.; Heath, P.T.; Le Doare, K. Reliability of dried blood spot (DBS) cards in antibody measurement: A systematic review. PLoS ONE 2021, 16, e0248218. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Tetanus—Annual Epidemiological Report for 2021; European Centre for Disease Prevention and Control (ECDC): Solna, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/tetanus-annual-epidemiological-report-2021 (accessed on 22 March 2024).
- European Centre for Disease Prevention and Control (ECDC). Diphtheria—Annual Epidemiological Report for 2021; European Centre for Disease Prevention and Control (ECDC): Solna, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/diphtheria-annual-epidemiological-report-2021 (accessed on 22 March 2024).
- European Centre for Disease Prevention and Control (ECDC). Pertussis—Annual Epidemiological Report for 2018; European Centre for Disease Prevention and Control (ECDC): Solna, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/pertussis-annual-epidemiological-report-2018 (accessed on 22 March 2024).
- Clarke, K.E.N.; MacNeil, A.; Hadler, S.; Scott, C.; Tiwari, T.S.P.; Cherian, T. Global Epidemiology of Diphtheria, 2000–2017. Emerg. Infect. Dis. 2019, 25, 1834–1842. [Google Scholar] [CrossRef]
- Nicholson, L.; Adkins, E.; Karyanti, M.R.; Ong-Lim, A.; Shenoy, B.; Huoi, C.; Vargas-Zambrano, J.C. What is the true burden of diphtheria, tetanus, pertussis and poliovirus in children aged 3–18 years in Asia? A systematic literature review. Int. J. Infect. Dis. 2022, 117, 116–129. [Google Scholar] [CrossRef]
- Rodríguez-Galet, A.; Rubio-Garrido, M.; Valadés-Alcaraz, A.; Rodríguez-Domínguez, M.; Galán, J.C.; Ndarabu, A.; Reina, G.; Holguín, A. Immune surveillance for six vaccinable pathogens using paired plasma and dried blood spots in HIV infected and uninfected children in Kinshasa. Sci. Rep. 2022, 12, 7920. [Google Scholar] [CrossRef]
- Eick, G.; Urlacher, S.S.; McDade, T.W.; Kowal, P.; Snodgrass, J.J. Validation of an Optimized ELISA for Quantitative Assessment of Epstein-Barr Virus Antibodies from Dried Blood Spots. Biodemography Soc. Biol. 2016, 62, 222–233. [Google Scholar] [CrossRef]
- Sultana, R.; Bhuiyan, T.R.; Sathi, A.S.; Sharmin, S.; Yeasmin, S.; Uddin, M.I.; Bhuiyan, S.; Mannoor, K.; Karim, M.M.; Zaman, K.; et al. Developing and validating a modified enzyme linked immunosorbent assay method for detecting HEV IgG antibody from dried blood spot (DBS) samples in endemic settings. Microbes Infect. 2022, 24, 104890. [Google Scholar] [CrossRef]
- Magalhaes, T.; Portilho, M.M.; Moreira, P.S.S.; Marinho, M.L.; Dias, W.P.; Gonçalves, N.M.; Rodrigues, O.A.S.; Montes, J.; Reis, L.; Jesus, D.F.; et al. Validation of the use of dried blood spots in a chikungunya virus IgG serological assay. J. Immunol. Methods 2023, 522, 113571. [Google Scholar] [CrossRef]
- Torres, C.; Muldrow, R.A.; Naranjo, A.R.; Cotton, S.W.; Pierre, C.C.; Greene, D.N. Development and validation of an LC-MSMS method to quantify creatinine from dried blood spots. J. Mass Spectrom. Adv. Clin. Lab 2024, 32, 50–59. [Google Scholar] [CrossRef]
- Desai, R.; Savkovic, S.; Handelsman, D.J. Dried blood spot sampling of testosterone microdosing in healthy females. J. Steroid Biochem. Mol. Biol. 2024, 240, 106496. [Google Scholar] [CrossRef] [PubMed]
- Bahmany, S.; Hassanzai, M.; Flint, R.B.; van Onzenoort, H.A.W.; de Winter, B.C.M.; Koch, B.C.P. Dried blood spot analysis for the quantification of vancomycin and creatinine using liquid chromatography—Tandem mass spectrometry: Method development and validation. Clin. Chim. Acta 2024, 553, 117689. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, A.L.; Patel, E.U.; Packman, Z.R.; Fernandez, R.E.; Piwowar-Manning, E.; Hamilton, E.L.; MacPhail, C.; Hughes, J.; Pettifor, A.; Kallas, E.G.; et al. Short Communication: Dried Blood Spots Stored at Room Temperature Should Not Be Used for HIV Incidence Testing. AIDS Res. Hum. Retroviruses 2018, 34, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.; Ward, G. Interferences in immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar] [PubMed]
- Mei, J.V.; Alexander, J.R.; Adam, B.W.; Hannon, W.H. Use of filter paper for the collection and analysis of human whole blood specimens. J. Nutr. 2001, 131, 1631S–1636S. [Google Scholar] [CrossRef]
- Vlasak, J.; Ionescu, R. Fragmentation of monoclonal antibodies. mAbs 2011, 3, 253–263. [Google Scholar] [CrossRef]
- Interterritorial Council of the National Health System. Vaccination Schedule throughout Life; Ministerio de Sanidad: Madrid, Spain, 2024; Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/calendario/docs/CalendarioVacunacion_Todalavida.pdf (accessed on 30 August 2024).
- Ministerio de Sanidad. Ministry of Health’s Vaccination Information System (SIVAMIN); Ministerio de Sanidad: Madrid, Spain, 2017; Available online: https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/S/sivamin (accessed on 19 March 2024).
- Limia Sánchez, A.; Olmedo Lucerón, C. 2nd Seroprevalence Study in Spain, 2017–2018. Rev. Española Salud Pública 2021, 95, e202103059. [Google Scholar]
- Wendelboe, A.M.; Van Rie, A.; Salmaso, S.; Englund, J.A. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 2005, 24, S58–S61. [Google Scholar] [CrossRef]
- McGirr, A.; Fisman, D.N. Duration of pertussis immunity after DTaP immunization: A meta-analysis. Pediatrics 2015, 135, 331–343. [Google Scholar] [CrossRef]
- Burdin, N.; Handy, L.K.; Plotkin, S.A. What Is Wrong with Pertussis Vaccine Immunity? The Problem of Waning Effectiveness of Pertussis Vaccines. Cold Spring Harb. Perspect. Biol. 2017, 9, a029454. [Google Scholar] [CrossRef]
- Tan, T.; Trindade, E.; Skowronski, D. Epidemiology of pertussis. Pediatr. Infect. Dis. J. 2005, 24, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- de Melker, H.E.; Versteegh, F.G.; Conyn-Van Spaendonck, M.A.; Elvers, L.H.; Berbers, G.A.; van Der Zee, A.; Schellekens, J.F. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 2000, 38, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.L.; Klein, S.L. Sex and gender impact immune responses to vaccines among the elderly. Physiology 2015, 30, 408–416. [Google Scholar] [CrossRef]
- Klein, S.; Flanagan, K. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]

| Male | Female | Total | |
|---|---|---|---|
| n (%) | 31 (48%) | 33 (52%) | 64 |
| Median age at sampling [IQR] | 42.8 [37.6–58.3] | 41.8 [31.6–56.7] | 42.6 [36.5–58.1] |
| Age Groups | |||
| 18–30 | 5 (16%) | 7 (21%) | 12 (18.8%) |
| 30–45 | 12 (39%) | 12 (36%) | 24 (37.5%) |
| 45–60 | 7 (23%) | 8 (24%) | 15 (23.4%) |
| >60 | 7 (23%) | 6 (18%) | 13 (20.3%) |
| Median hematocrit by age group | |||
| 18–30 | 42.7 | 40.5 | 41.9 |
| 30–45 | 44.8 | 41.0 | 42.1 |
| 45–60 | 43.2 | 38.6 | 41.0 |
| >60 | 38.2 | 38.1 | 38.2 |
| Total [IQR] | 42.7 [38.9–45.7] | 39.8 [37.2–41.7] | 41.0 [37.8–43.6] |
| Pathogen immunity (plasma), by age group | |||
| Diphtheria | |||
| 18–30 | 100% | 100% | 100% |
| 30–45 | 83.3% | 75.0% | 79.2% |
| 45–60 | 42.9% | 25.0% | 33.3% |
| >60 | 0% * | 66.7% * | 30.8% |
| Total | 58.1% | 66.7% | 62.5% |
| Tetanus | |||
| 18–30 | 100% | 100% | 100% |
| 30–45 | 91.7% | 100% | 95.8% |
| 45–60 | 71.4% | 87.5% | 80.0% |
| >60 | 14.3% * | 50.0% * | 30.8% |
| Total | 71.0% * | 87.9% * | 79.7% |
| Pertussis | 0% | 0% | 0% |
| DBS-A (T0) | DBS-B (T0) | DBS-C (T4m, −20 °C) | DBS-D (T4m, 4 °C) | DBS-E (T4m, RT) | |
|---|---|---|---|---|---|
| DIPHTHERIA | 0.961 | 0.966 | 0.921 (−4.45%) | 0.922 (−4.39%) | 0.855 (−8.10%) |
| TETANUS | 0.983 | 0.971 | 0.978 (−0.35%) | 0.968 (−0.56%) | 0.960 (−1.47%) |
| PERTUSSIS | 0.849 | 0.753 | 0.591 (−21.53%) | 0.351 (−53.44%) | 0.162 (−78.50%) |
| Diphtheria * | Tetanus ** | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S | Sp | PPV | NPV | S | Sp | PPV | NPV | ||
| DBS-A (T0) | Plasma cut-off | 100% | 4.2% | 63.5% | 100% | 100% | 69.2% | 92.7% | 100% |
| Optimized DBS cut-off | 97.5% | 75.0% | 86.7% | 94.7% | 98.0% | 92.3% | 98.0% | 92.3% | |
| DBS-B (T0) | Plasma cut-off | 100% | 4.2% | 63.5% | 100% | 100% | 53.8% | 89.5% | 100% |
| Optimized DBS cut-off | 95.0% | 91.7% | 95.0% | 91.7% | 98.0% | 92.3% | 98.0% | 92.3% | |
| DBS-C (T4m, −20 °C) | Plasma cut-off | 100% | 4.2% | 63.5% | 100% | 100% | 23.1% | 83.6% | 100% |
| Optimized DBS cut-off | 92.5% | 66.7% | 82.2% | 84.2% | 100% | 76.9% | 94.4% | 100% | |
| DBS-D (T4m, 4 °C) | Plasma cut-off | 100% | 4.2% | 63.5% | 100% | 100% | 23.1% | 83.6% | 100% |
| Optimized DBS cut-off | 95.0% | 45.8% | 74.5% | 84.6% | 100% | 53.8% | 89.5% | 100% | |
| DBS-E (T4m, RT) | Plasma cut-off | 100% | 4.2% | 63.5% | 100% | 100% | 7.7% | 81.0% | 100% |
| Optimized DBS cut-off | 95.0% | 41.7% | 73.1% | 83.3% | 100% | 46.2% | 87.9% | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Mateos, M.; Carlos, S.; Jaso, J.; Holguín, Á.; Reina, G. Influence of Hematocrit and Storage Temperature on the Stability of Dried Blood Samples in Serological Analyses of Tetanus, Diphtheria, and Pertussis. Diagnostics 2024, 14, 2195. https://doi.org/10.3390/diagnostics14192195
Rodríguez-Mateos M, Carlos S, Jaso J, Holguín Á, Reina G. Influence of Hematocrit and Storage Temperature on the Stability of Dried Blood Samples in Serological Analyses of Tetanus, Diphtheria, and Pertussis. Diagnostics. 2024; 14(19):2195. https://doi.org/10.3390/diagnostics14192195
Chicago/Turabian StyleRodríguez-Mateos, Mariano, Silvia Carlos, Javier Jaso, África Holguín, and Gabriel Reina. 2024. "Influence of Hematocrit and Storage Temperature on the Stability of Dried Blood Samples in Serological Analyses of Tetanus, Diphtheria, and Pertussis" Diagnostics 14, no. 19: 2195. https://doi.org/10.3390/diagnostics14192195








