Polycystic Ovary Syndrome Accompanied by Hyperandrogenemia or Metabolic Syndrome Triggers Glomerular Podocyte Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements of Urinary NGAL, Podocalyxin and Nephrin
2.2. Measurement of Spot Urine Albumin-to-Creatinine Ratio (uACR)
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerjaschki, D.; Sharkey, D.J.; Farquhar, M.G. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J. Cell Biol. 1984, 98, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Satchell, S.C.; Braet, F. Glomerular endothelial cell fenestrations: An integral component of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 2009, 296, F947–F956. [Google Scholar] [CrossRef] [PubMed]
- Skoberne, A.; Konieczny, A.; Schiffer, M. Glomerular epithelial cells in the urine: What has to be done to make them worthwhile? Am. J. Physiol. Renal Physiol. 2009, 296, F230–F241. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, V.; Ljungberg, P.; Wartiovaara, J.; Lenkkeri, U.; Kestilä, M.; Jalanko, H.; Holmberg, C.; Tryggvason, K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 7962–7967. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Bainton, D.F.; Sengeløv, H.; Borregaard, N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Carlson, M.; Engström, A.; Garcia, R.; Peterson, C.G.; Venge, P. Purification and characterization of a human neutrophil lipocalin (HNL) from the secondary granules of human neutrophils. Scand. J. Clin. Lab. Investig. 1994, 54, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, Z.; Larsen, S.B.; Grove, E.L.; Hvas, A.M.; Kristensen, S.D.; Magnusson, N.E. Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease. Clin. Chem. Lab. Med. 2017, 56, 5–18. [Google Scholar] [CrossRef]
- Xin, C.; Yulong, X.; Yu, C.; Changchun, C.; Feng, Z.; Xinwei, M. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren. Fail. 2008, 30, 904–913. [Google Scholar] [CrossRef]
- Bhati, M.; Prabhu, Y.D.; Renu, K.; Vellingiri, B.; Thiagarajan, P.; Panda, A.; Chakraborty, R.; Myakala, H.; Gopalakrishnan, A.V. Role of TGF-β signalling in PCOS associated focal segmental glomerulosclerosis. Clin. Chim. Acta 2020, 510, 244–251. [Google Scholar] [CrossRef]
- Zhang, R.; Liao, J.; Morse, S.; Donelon, S.; Reisin, E. Kidney disease and the metabolic syndrome. Am. J. Med. Sci. 2005, 330, 319–325. [Google Scholar] [CrossRef]
- Calderon-Margalit, R.; Siscovick, D.; Merkin, S.S.; Wang, E.; Daviglus, M.L.; Schreiner, P.J.; Sternfeld, B.; Williams, O.D.; Lewis, C.E.; Azziz, R.; et al. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-media thickness: The Coronary Artery Risk Development in Young Adults Women’s study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Yanes, L.L.; Romero, D.G.; Moulana, M.; Lima, R.; Davis, D.D.; Zhang, H.; Lockhart, R.; Racusen, L.C.; Reckelhoff, J.F. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend. Med. 2011, 8, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.N.; Racusen, L.C.; Reckelhoff, J.F. Consequences of advanced aging on renal function in chronic hyperandrogenemic female rat model: Implications for aging women with polycystic ovary syndrome. Physiol. Rep. 2017, 5, e13461. [Google Scholar] [CrossRef]
- Kravets, I.; Mallipattu, S.K. The Role of Podocytes and Podocyte-Associated Biomarkers in Diagnosis and Treatment of Diabetic Kidney Disease. J. Endocr. Soc. 2020, 4, bvaa029. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Loyd, S.; Groome, L.J. Increased urinary excretion of nephrin, podocalyxin, and βig-h3 in women with preeclampsia. Am. J. Physiol. Renal Physiol. 2012, 302, F1084–F1089. [Google Scholar] [CrossRef] [PubMed]
- Kamrul-Hasan, A.B.; Aalpona, F.Z.; Chanda, P.K.; Ananya, K.F.; Kobra, T.; Miah, O.F.; Kazal, R.K.; Selim, S. Frequency and Correlates of Albuminuria in Adult Bangladeshi Women with Polycystic Ovary Syndrome. Mymensingh Med. J. 2020, 29, 234–240. [Google Scholar]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Eknoyan, G.; Hostetter, T.; Bakris, G.L.; Hebert, L.; Levey, A.S.; Parving, H.-H.; Steffes, M.W.; Toto, R. Proteinuria and other markers of chronic kidney disease: A position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am. J. Kidney Dis. 2003, 42, 617–622. [Google Scholar] [CrossRef]
- Crowe, E.; Halpin, D.; Stevens, P.; Guideline, D.G. Early identification and management of chronic kidney disease: Summary of NICE guidance. BMJ 2008, 337, a1530. [Google Scholar] [CrossRef]
- Olivoto, T.; Lucio, A.D.C. Metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Gomez, J.M.D.; VanHise, K.; Stachenfeld, N.; Chan, J.L.; Merz, N.B.; Shufelt, C. Subclinical cardiovascular disease and polycystic ovary syndrome. Fertil. Steril. 2022, 117, 912–923. [Google Scholar] [CrossRef]
- Cussons, A.J.; Watts, G.F.; Burke, V.; Shaw, J.E.; Zimmet, P.Z.; Stuckey, B.G. Cardiometabolic risk in polycystic ovary syndrome: A comparison of different approaches to defining the metabolic syndrome. Hum. Reprod. 2008, 23, 2352–2358. [Google Scholar] [CrossRef]
- de Mik, S.M.; Hoogduijn, M.J.; de Bruin, R.W.; Dor, F.J. Pathophysiology and treatment of focal segmental glomerulosclerosis: The role of animal models. BMC Nephrol. 2013, 14, 74. [Google Scholar] [CrossRef]
- Vinai, M.; Waber, P.; Seikaly, M.G. Recurrence of focal segmental glomerulosclerosis in renal allograft: An in-depth review. Pediatr. Transplant. 2010, 14, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Quinkler, M.; Meyer, B.; Bumke-Vogt, C.; Grossmann, C.; Gruber, U.; Oelkers, W.; Diederich, S.; Bahr, V. Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur. J. Endocrinol. 2002, 146, 789–799. [Google Scholar] [CrossRef]
- Quan, A.; Chakravarty, S.; Chen, J.-C.; Loleh, S.; Saini, N.; Harris, R.C.; Capdevila, J.; Quigley, R. Androgens augment proximal tubule transport. Am. J. Physiol. Renal Physiol. 2004, 287, F452–F459. [Google Scholar] [CrossRef]
- Dalmasso, C.; Maranon, R.; Patil, C.; Bui, E.; Moulana, M.; Zhang, H.; Smith, A.; Cardozo, L.L.Y.; Reckelhoff, J.F. Cardiometabolic Effects of Chronic Hyperandrogenemia in a New Model of Postmenopausal Polycystic Ovary Syndrome. Endocrinology 2016, 157, 2920–2927. [Google Scholar] [CrossRef]
- Gozukara, I.O.; Gozukara, K.H.; Kucur, S.K.; Karakılıc, E.U.; Keskin, H.; Akdeniz, D.; Aksoy, A.N.; Carlıoglu, A. Association of Glomerular Filtration Rate with Inflammation in Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2015, 9, 176–182. [Google Scholar]
- Behboudi-Gandevani, S.; Amiri, M.; Yarandi, R.B.; Noroozzadeh, M.; Farahmand, M.; Dovom, M.R.; Tehrani, F.R. The risk of metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Clin. Endocrinol. 2018, 88, 169–184. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, Y.; Zhu, Q.; Wang, Y.; Lindheim, S.R.; Qi, J.; Li, X.; Ding, Y.; Shi, Y.; Wei, D.; et al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am. J. Obstet. Gynecol. 2019, 221, 138.e1–138.e12. [Google Scholar] [CrossRef]
- Carmina, E. Metabolic syndrome in polycystic ovary syndrome. Minerva Ginecol. 2006, 58, 109–114. [Google Scholar]
- Pinola, P.; Puukka, K.; Piltonen, T.T.; Puurunen, J.; Vanky, E.; Sundström-Poromaa, I.; Stener-Victorin, E.; Hirschberg, A.L.; Ravn, P.; Andersen, M.S.; et al. Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil. Steril. 2017, 107, 788–795.e2. [Google Scholar] [CrossRef]
- Yu, H.F.; Chen, H.S.; Rao, D.P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4863. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Laville, M. Conséquences rénales de l’obésité [Renal consequences of obesity]. Nephrol. Ther. 2011, 7, 80–85. [Google Scholar] [CrossRef]
- Nashar, K.; Egan, B.M. Relationship between chronic kidney disease and metabolic syndrome: Current perspectives. Diabetes Metab. Syndr. Obes. 2014, 7, 421–435. [Google Scholar] [CrossRef]
- Kambham, N.; Markowitz, G.S.; Valeri, A.M.; Lin, J.; D’Agati, V.D. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001, 59, 1498–1509. [Google Scholar] [CrossRef] [PubMed]


| PCOS (n = 50) | Control (n = 50) | p Values | |
|---|---|---|---|
| Age (year) | 23.5 (22–25) | 24 (22–26) | 0.989 |
| BMI (kg/m2) | 23.2 (22.1–24.0) | 22,5 (21.15–24.75) | 0.117 |
| Waist circumference (cm) | 85.22 ± 8.65 | 82.56 ± 6.49 | 0.085 |
| SBP (mm/Hg) | 112.70 ± 12.87 | 107.60 ± 10.89 | 0.035 |
| DBP (mm/Hg) | 75.00 (70.00–80.00) | 70.00 (67.00–80.00) | 0.132 |
| FSH (U/L) | 5.5 (5.0–6.0) | 5.8 (5.10–6.25) | 0.139 |
| LH (U/L) | 8.94 ± 1.60 | 6.54 ± 0.98 | <0.001 |
| T. testosterone (ng/dL) | 37.66 ± 7.71 | 28.72 ± 5.05 | <0.001 |
| HOMA-IR | 2.47 ± 0.48 | 1.59 ± 0.31 | <0.001 |
| HDL-C (mg/dL) | 40.96 ± 6.42 | 46.28 ± 6.58 | <0.001 |
| Triglyceride (mg/dL) | 121.50 (102.00–154.25) | 114.00 (102.75–130.00) | 0.072 |
| FBG (mg/dL) | 90.96 ± 16.07 | 83.16 ± 11.36 | 0.006 |
| Creatinine (mg/dL) | 0.82 ± 0.76 | 0.68 ± 0.19 | 0.183 |
| Microalbuminuria, n (%) | 12 (24%) | 2 (4%) | 0.008 |
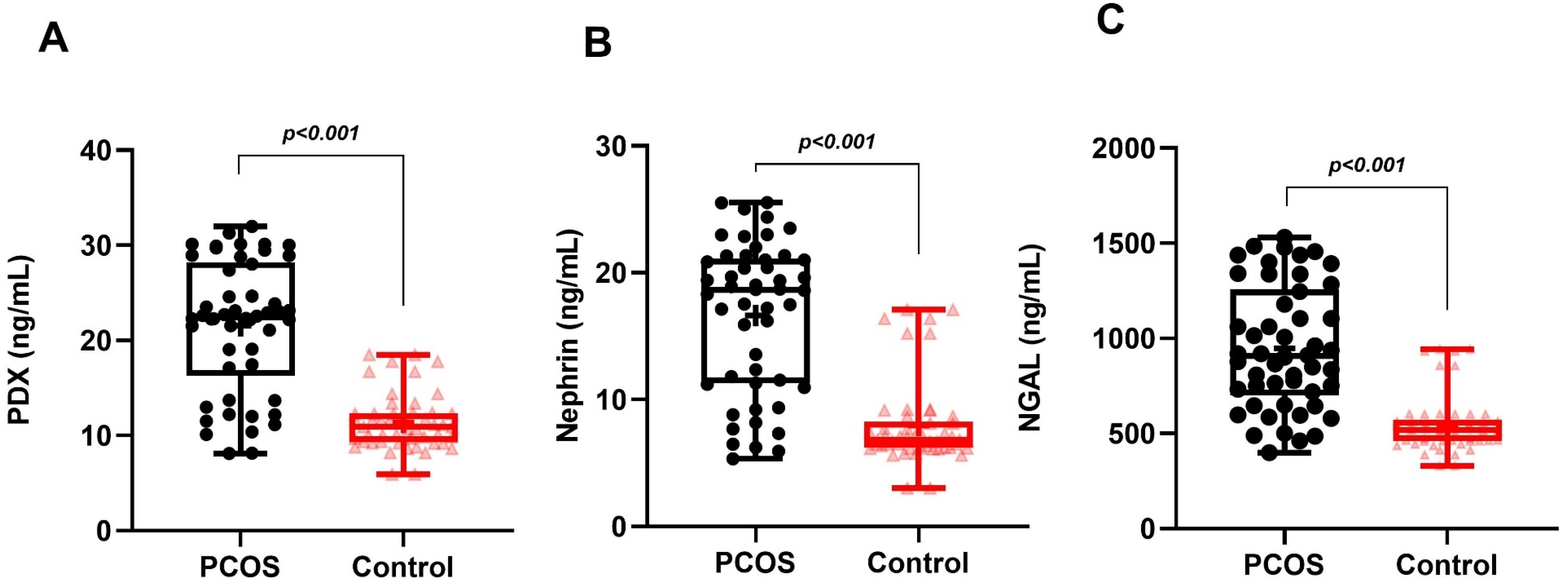
| Nephrin (ng/mL) | 16.63 ± 5.93 | 7.95 ± 3.33 | <0.001 |
| PDX (ng/mL) | 21.56 ± 6.86 | 11.37 ± 2.94 | <0.001 |
| NGAL (ng/mL) | 906.51 (701.24–1255.12) | 518.16 (460.30–572.04) | <0.001 |
| uACR (mg/gCr) | 6.00 (4.00–26.5) | 3.00 (3.00–9.25) | <0.001 |
| Phenotype A | Phenotype B | Phenotype C | Phenotype D | |
|---|---|---|---|---|
| Variables | HA+OD+PCOM | HA+OD | HA+PCOM | OD+PCOM |
| N, (%) | 19 (38%) | 15 (30%) | 9 (18%) | 7 (14%) |
| MetS | 6 (31.5%) | 4 (26.6%) | 2 (22.2%) | - |
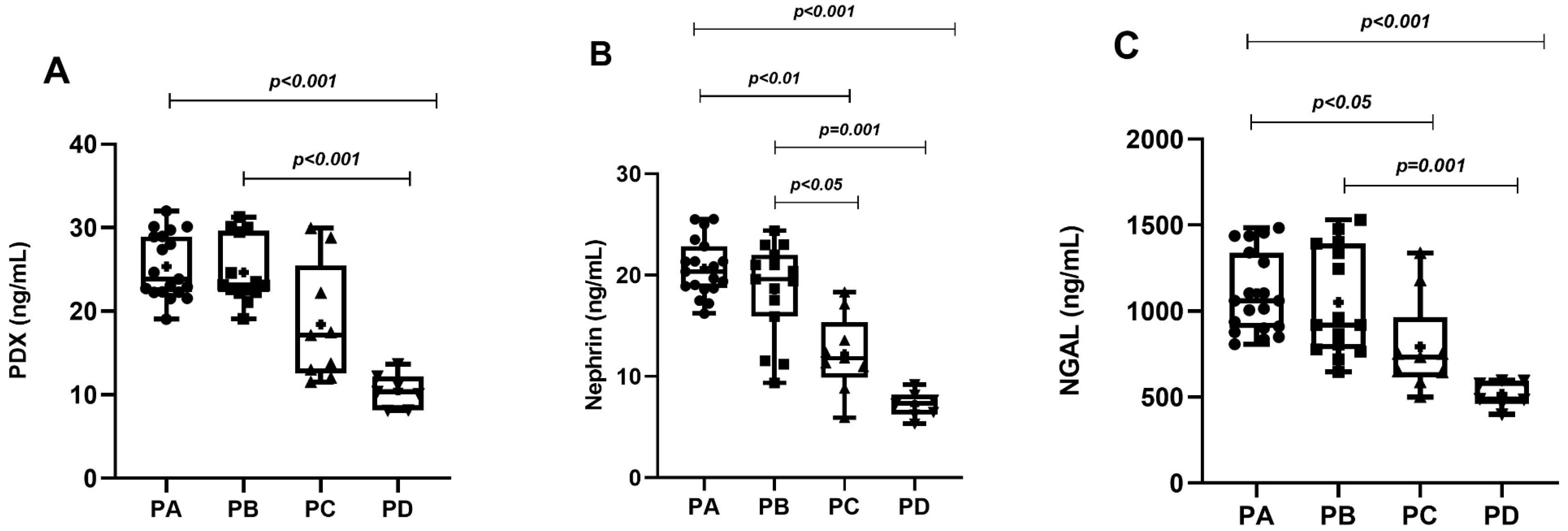
| PDX (ng/mL) | 23.86 (22.27–28.96) * | 23.08 (22.19–29.49) * | 13.72 (12.50–23.11) | 10.38 (8.14–12.17) |
| Nephrin (ng/mL) | 20.35 (18.71–22.86) *,‡ | 19.62 (15.90–22.02) *,‡ | 11.81 (9.89–15.35) | 7.34 (6.24–8.18) |
| N-GAL (ng/mL) | 1061.32 (901.51–1340.98) *,‡ | 918.63 (777.32–1391.88) * | 732.600 (615.00–965.39) | 488.95 (461.35–595.69) |
| Normoalbuminuria (uACR ≤ 30 mg/gCr) | 12 (63.2%) | 11 (73.3%) | 8 (88.8%) | 7 (100%) |
| Microalbuminuria (uACR > 30–300 mg/g Cr) | 7 (36.8%) α | 4 (26.7%) α | 1 (11.1%) | - |
| PCOS with MetS (n = 12) | PCOS without MetS (n = 38) | p | |
|---|---|---|---|
| Nephrin (ng/mL) | 22.44 (18.99–24.86) | 16.72 (9.32–19.63) | <0.001 |
| Podocalyxin (ng/mL) | 29.97 (28.82–30.11) | 21.86 (12.79–22.95) | <0.001 |
| NGAL (ng/mL) | 1339.55 (1195.10–1428.10) | 806.03 (632.13–943.29) | <0.001 |
| Microalbuminuria (uACR > 30–300 mg/gCr) | 8 (66.7%) | 4 (10.5%) | <0.001 |
| Unadjusted | Adjusted (1) | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| PDX (ng/mL) | 1.653 (1.243–2.199) | <0.001 | 1.849 (1.184–2.889) | <0.01 |
| Nephrin (ng/mL) | 1.609 (1.153–2.244) | <0.01 | 1.435 (1.029–2.001) | <0.05 |
| NGAL (ng/mL) | 1.005 (1.002–1.007) | <0.01 | 1.004 (1.001–1.009) | <0.05 |
| PCOS increases urinary levels of acute (NGAL) and chronic podocyte damage markers (PDX and nephrin). The increase in podocyte breakdown products is more pronounced in the presence of hyperandrogenemia or metabolic syndrome. Podocyturia is accompanied by microalbuminuria, but no increase in creatinine. Podocyturia is more pronounced in classical phenotypes than in ovulatory and normoandrogenic phenotypes. Urinary podocyte breakdown products are an independent risk factor for microalbuminuria, independent of age and BMI. BMI, systolic blood pressure, testosterone, glucose, HOMA-IR and uACR stimulate podocyte breakdown. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gungor, K.; Gungor, N.D.; Celik, O.; Ersahin, A.; Celik, N.; Yardim, M.; Yurci, A.; Kobaner, M.; Ilkov Maslarski, I. Polycystic Ovary Syndrome Accompanied by Hyperandrogenemia or Metabolic Syndrome Triggers Glomerular Podocyte Injury. Diagnostics 2024, 14, 2197. https://doi.org/10.3390/diagnostics14192197
Gungor K, Gungor ND, Celik O, Ersahin A, Celik N, Yardim M, Yurci A, Kobaner M, Ilkov Maslarski I. Polycystic Ovary Syndrome Accompanied by Hyperandrogenemia or Metabolic Syndrome Triggers Glomerular Podocyte Injury. Diagnostics. 2024; 14(19):2197. https://doi.org/10.3390/diagnostics14192197
Chicago/Turabian StyleGungor, Kagan, Nur D. Gungor, Onder Celik, Aynur Ersahin, Nilufer Celik, Meltem Yardim, Arzu Yurci, Murat Kobaner, and Ivan Ilkov Maslarski. 2024. "Polycystic Ovary Syndrome Accompanied by Hyperandrogenemia or Metabolic Syndrome Triggers Glomerular Podocyte Injury" Diagnostics 14, no. 19: 2197. https://doi.org/10.3390/diagnostics14192197






