Early CYP3A5 Genotype-Based Adjustment of Tacrolimus Dosage Reduces Risk of De Novo Donor-Specific HLA Antibodies and Rejection among CYP3A5-Expressing Renal Transplant Patients
Abstract
1. Introduction
2. Materials and Methods
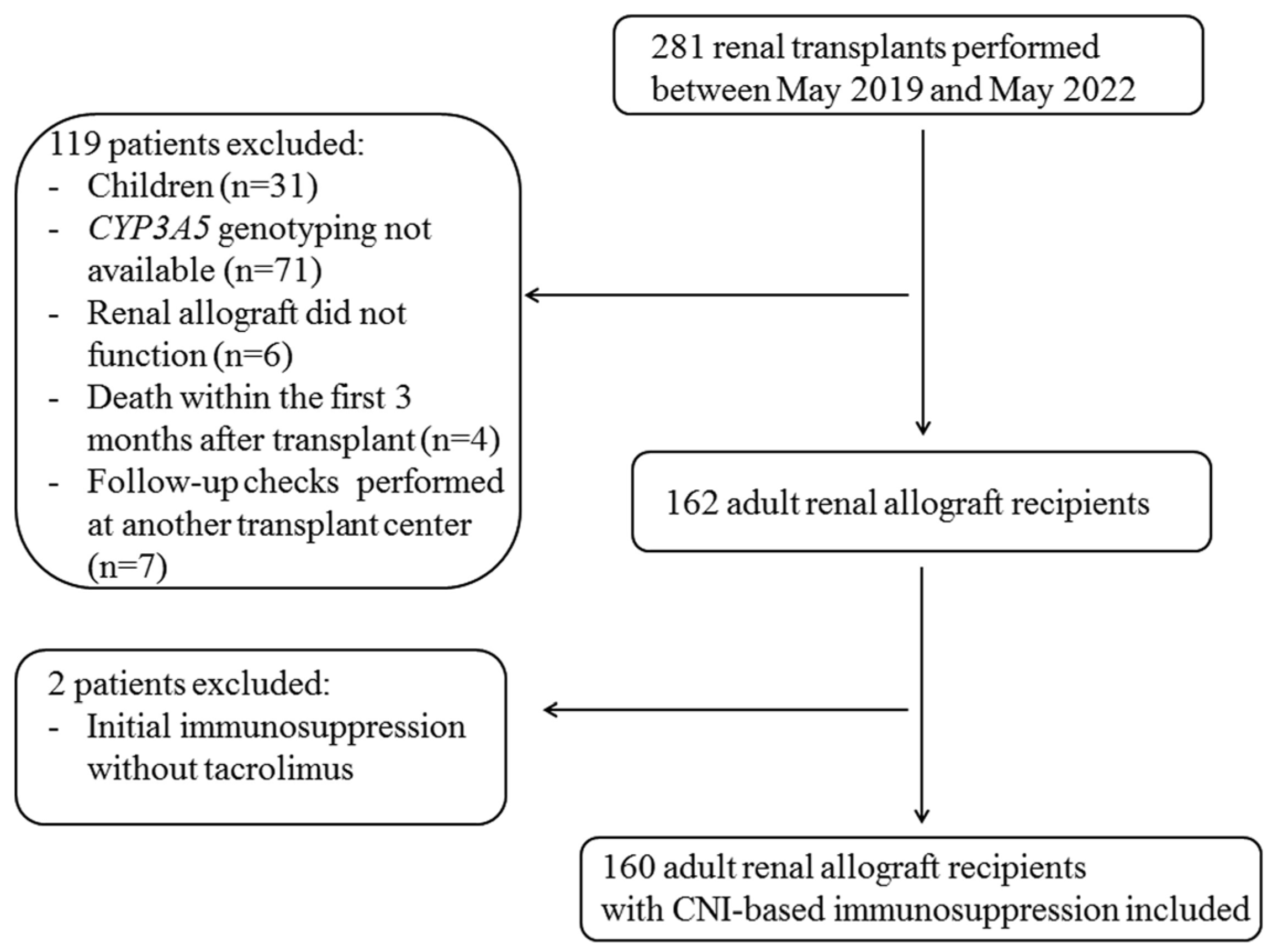
2.1. Study Population
2.2. HLA Typing of Recipients and Donors
2.3. HLA Antibody Detection and Specification
2.4. CYP3A5 Genotyping
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Genotype-Based Adjustment of Calcineurin Inhibitor Dosage Led to Comparable Tacrolimus Trough Levels among CYP3A5 Expressers and Nonexpressers, Whereas the Tacrolimus Dosage Requirement of the Expressers Was Twice as High as That for the Nonexpressers over the 2-Year Follow-Up Period
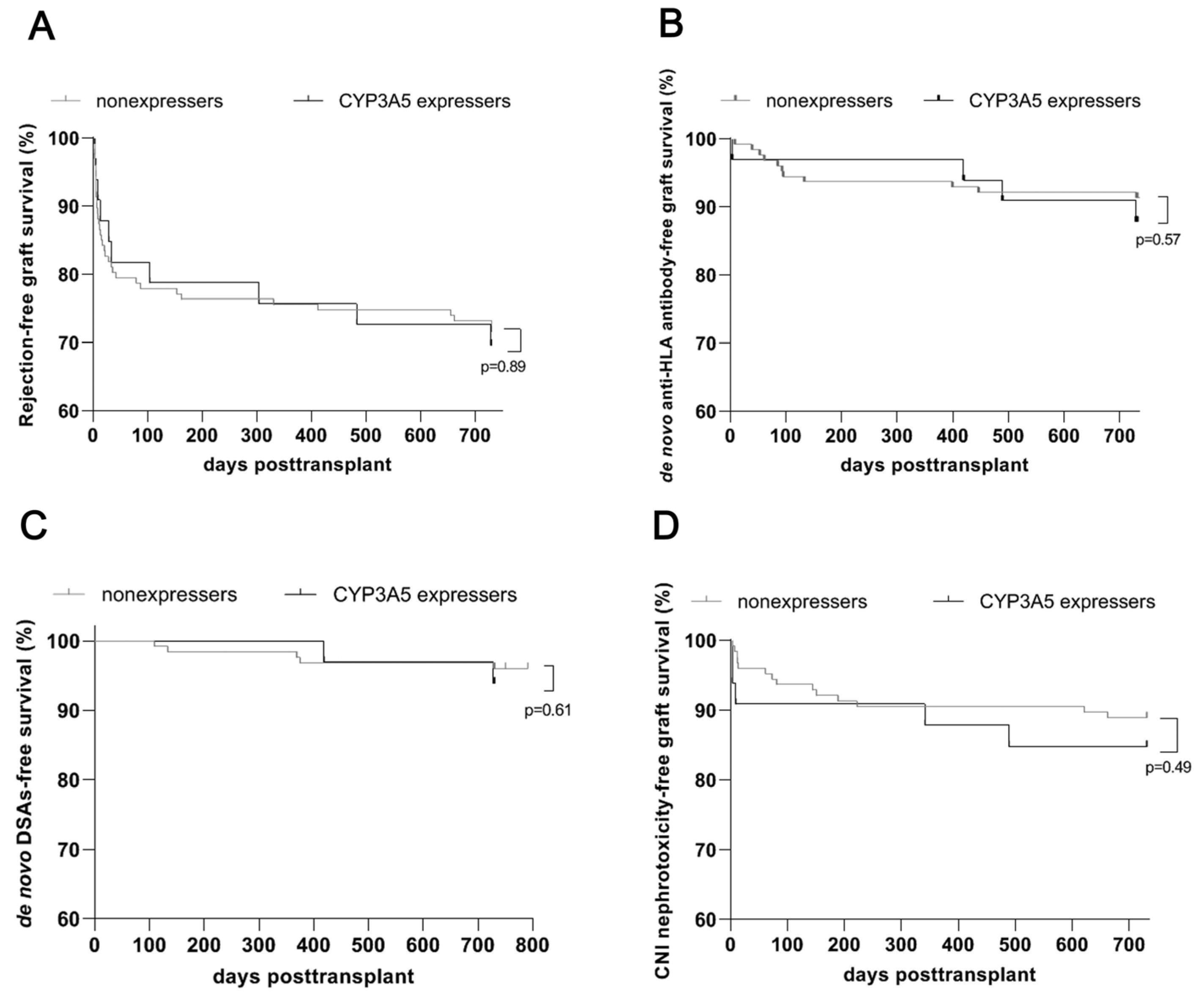
3.3. Comparable Renal Allograft Outcomes, in Particular Development of Rejection and De Novo DSAs, for CYP3A5 Expressers and Nonexpressers after an Early Genotype-Based Adjustment of Calcineurin Inhibitor Dosage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, P.M.; Hemani, R.J.; Solanki, N.; Shete, N.B.; Gang, S.; Konnur, A.M.; Srivastava, R.; Pandey, S.N. A systematic review and meta-analysis recite the efficacy of Tacrolimus treatment in renal transplant patients in association with genetic variants of CYP3A5 gene. Am. J. Clin. Exp. Urol. 2023, 11, 275–292. [Google Scholar] [PubMed]
- Brunet, M.; van Gelder, T.; Asberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Koch, I.; Weil, R.; Wolbold, R.; Brockmöller, J.; Hustert, E.; Burk, O.; Nuessler, A.; Neuhaus, P.; Eichelbaum, M.; Zanger, U.; et al. Interindividual variability and tissue-specificity in the expression of cytochrome P450 3A mRNA. Drug Metab. Dispos. 2002, 30, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Woillard, J.B.; Chouchana, L.; Picard, N.; Loriot, M.A. French Network of Pharmacogenetics (RNPGX). Pharmacogenetics of immunosuppressants: State of the art and clinical implementation—Recommendations from the French National Network of Pharmacogenetics (RNPGx). Therapie 2017, 72, 285–299. [Google Scholar] [CrossRef]
- Van Gelder, T.; Meziyerh, S.; Swen, J.J.; de Vries, A.P.J.; Moes, D.J.A.R. The Clinical Impact of the C0/D Ratio and the CYP3A5 Genotype on Outcome in Tacrolimus Treated Kidney Transplant Recipients. Front. Pharmacol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Chen, L.; Prasad, G.V.R. CYP3A5 polymorphisms in renal transplant recipients: Influence on tacrolimus treatment. Pharmgenomics Pers. Med. 2018, 11, 23–33. [Google Scholar] [CrossRef]
- Terrazzino, S.; Quaglia, M.; Stratta, P.; Canonico, P.L.; Genazzani, A.A. The effect of CYP3A5 6986A>G and ABCB1 3435C>T on tacrolimus dose-adjusted trough levels and acute rejection rates in renal transplant patients: A systematic review and meta-analysis. Pharmacogenet. Genom. 2012, 22, 642–645. [Google Scholar] [CrossRef]
- Rojas, L.; Neumann, I.; Herrero, M.J.; Bosó, V.; Reig, J.; Poveda, J.L.; Megías, J.; Bea, S.; Aliño, S.F. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: A systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2015, 15, 38–48. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; van Schaik, R.H.; Thummel, K.E.; Klein, T.E.; Caudle, K.E.; et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef]
- Khan, A.R.; Raza, A.; Firasat, S.; Abid, A. CYP3A5 gene polymorphisms and their impact on dosage and trough concentration of tacrolimus among kidney transplant patients: A systematic review and meta-analysis. Pharmacogenomics J. 2020, 20, 553–562. [Google Scholar] [CrossRef]
- Friebus-Kardash, J.; Nela, E.; Möhlendick, B.; Kribben, A.; Siffert, W.; Heinemann, F.M.; Eisenberger, U. Development of De Novo Donor-specific HLA Antibodies and AMR in Renal Transplant Patients Depends on CYP3A5 Genotype. Transplantation 2022, 106, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Mottaghi, S.; Sagheb, M.M.; Azarpira, N.; Abdizadeh, F.; Faeghi, R.; Karimzadeh, I. Association between the Three Polymorphisms of the Glucocorticoid Receptor Gene and the Early Clinical Outcome in Kidney Transplantation Patients. Iran. J. Med. Sc. 2021, 46, 444–453. [Google Scholar]
- Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 reference guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. Chronic Kidney Disease Epidemiology Collaboration. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- DIN EN ISO 15189:2014-11; Medizinische Laboratorien–Anforderungen an die Qualität und Kompetenz. German Institute for Standardization: Berlin, Germany, 2014. [CrossRef]
- Xie, W.; Fan, S.; Liu, R.; Yan, W.; Su, C.; Zheng, K.; Wang, X.; Wang, Z. Tacrolimus intra-patient variability measures and its associations with allograft clinical outcomes in kidney transplantation. Rev. Transpl. Rev. 2024, 38, 100842. [Google Scholar] [CrossRef]
- Heinemann, F.M. HLA genotyping and antibody characterization using the Luminex™ multiplex technology. Transfus. Med. Hemother. 2009, 36, 273–278. [Google Scholar] [CrossRef]
- Eurotransplant Manual 2020, Version 4.6. Chapter 10. Available online: https://www.eurotransplant.org/patients/eurotransplant-manual (accessed on 30 April 2021).
- Ziemann, M.; Heßler, N.; König, I.R.; Lachmann, N.; Dick, A.; Ditt, V.; Budde, K.; Reinke, P.; Eisenberger, U.; Suwelack, B.; et al. Unacceptable human leucocyte antigens for organ offers in the era of organ shortage: Influence on waiting time before kidney transplantation. Nephrol. Dial. Transplant. 2017, 32, 880–889. [Google Scholar] [CrossRef]
- Tait, B.D.; Süsal, C.; Gebel, H.M.; Nickerson, P.W.; Zachary, A.A.; Claas, F.H.; Reed, E.F.; Bray, R.A.; Campbell, P.; Chapman, J.R.; et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation 2013, 95, 19–47. [Google Scholar] [CrossRef]
- Pasari, A.S.; Balwani, M.R.; Gurjar, P.; Bawankule, C.; Bhawane, A.; Tolani, P.; Kashiv, P.; Dubey, S.; Katekhaye, V.M. CYP3A5 Polymorphism in Renal Transplantation: A Key to Personalized Immunosuppression. Transplant. Proc. 2023, 55, 1305–1309. [Google Scholar] [CrossRef]
- Thervet, E.; A Loriot, M.; Barbier, S.; Buchler, M.; Ficheux, M.; Choukroun, G.; Toupance, O.; Touchard, G.; Alberti, C.; Le Pogamp, P.; et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin. Pharmacol. Ther. 2010, 87, 721–726. [Google Scholar] [CrossRef]
- Shuker, N.; Bouamar, R.; van Schaik, R.H.N.; Groningen, M.C.C.; Damman, J.; Baan, C.C.; van de Wetering, J.; Rowshani, A.T.; Weimar, W.; van Gelder, T.; et al. A randomized controlled trial comparing the efficacy of Cyp3a5 genotype-based with body weight-based tacrolimus dosing after living donor kidney transplantation. Am. J. Transplant. 2016, 16, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Etienne, I.; Buchler, M.; Bailly, E.; de Ligny, B.H.; Choukroun, G.; Colosio, C.; Thierry, A.; Vigneau, C.; Moulin, B.; et al. Long-Term Clinical Impact of Adaptation of Initial Tacrolimus Dosing to CYP3A5 Genotype. Am. J. Transplant. 2016, 16, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Anutrakulchai, S.; Pongskul, C.; Kritmetapak, K.; Limwattananon, C.; Vannaprasaht, S. Therapeutic concentration achievement and allograft survival comparing usage of conventional tacrolimus doses and CYP3A5 genotype-guided doses in renal transplantation patients. Br. J. Clin. Pharmacol. 2019, 85, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Tang, S.C.W. Personalized immunosuppression after kidney transplantation. Nephrology 2022, 27, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, J.; Deng, R.; Fu, Q.; Chen, J.; Huang, M.; Chen, X.; Wang, C. Dynamic effects of CYP3A5 polymorphism on dose requirement and trough concentration of tacrolimus in renal transplant recipients. J. Clin. Pharm. Ther. 2017, 42, 93–97. [Google Scholar] [CrossRef]
- Salvadori, M.; Tsalouchos, A. Pharmacogenetics of immunosuppressant drugs: A new aspect for individualized therapy. World J. Transplant. 2020, 10, 90–103. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Yu, X.; Hu, X.; Wang, W.; Zhang, X.; Liu, L. Clinical Impact of the Adaptation of Initial Tacrolimus Dosing to the CYP3A5 Genotype after Kidney Transplantation: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Pharmacokinet. 2021, 60, 877–885. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Buijsch, R.A.O.D.; Wong, K.M.; Chan, H.W.; Chau, K.F.; Li, C.S.; Leung, K.T.; Kwan, T.H.; E de Vrie, J.; Wijnen, P.A.; et al. Influence of different allelic variants of the CYP3A and ABCB1 genes on the tacrolimus pharmacokinetic profile of Chinese renal transplant recipients. Pharmacogenomics 2006, 7, 563–574. [Google Scholar] [CrossRef]
- Macphee, I.A. Use of pharmacogenetics to optimize immunosuppressive therapy. Ther. Drug Monit. 2010, 32, 261–264. [Google Scholar] [CrossRef]
- van Duijnhoven, E.M.; Boots, J.M.M.; Christiaans, M.H.L.; Stolk, L.M.L.; Undre, N.A.; van Hooff, J.P. Increase in tacrolimus trough levels after steroid withdrawal. Transpl. Int. 2003, 16, 721–725. [Google Scholar] [CrossRef]
- Shuker, N.; van Gelder, T.; Hesselink, D.A. Intra-patient variability in tacrolimus exposure: Causes, consequences for clinical management. Transpl. Rev. 2015, 29, 78–84. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, T. Drug interactions with tacrolimus. Drug Saf. 2002, 25, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Woillard, J.-B.; Mourad, M.; Neely, M.; Capron, A.; van Schaik, R.H.; van Gelder, T.; Lloberas, N.; Hesselink, D.A.; Marquet, P.; Haufroid, V.; et al. Tacrolimus updated guidelines through popPK modeling: How to benefit more from CYP3A pre-emptive genotyping prior to kidney transplantation. Front. Pharmacol. 2017, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Andreu, F.; Colom, H.; Elens, L.; van Gelder, T.; van Schaik, R.H.N.; Hesselink, D.A.; Bestard, O.; Torras, J.; Cruzado, J.M.; Grinyó, J.M.; et al. A new CYP3A5*3 and CYP3A4*22 cluster influencing tacrolimus target concentrations: A population approach. Clin. Pharmacokinet. 2017, 56, 963–975. [Google Scholar] [CrossRef]
- Lloberas, N.; Grinyó, J.M.; Colom, H.; Vidal-Alabró, A.; Fontova, P.; Rigo-Bonnin, R.; Padró, A.; Bestard, O.; Melilli, E.; Montero, N.; et al. A prospective controlled, randomized clinical trial of kidney transplant recipients developed personalized tacrolimus dosing using model-based Bayesian Prediction. Kidney Int. 2023, 104, 840–850. [Google Scholar] [CrossRef]
- Deininger, K.M.; Anderson, H.D.; Patrinos, G.P.; Mitropoulou, C.; Aquilante, C.L. Cost-effectiveness analysis of CYP3A5 genotype-guided tacrolimus dosing in solid organ transplantation using real-world data. Pharmacogenomics J. 2024, 24, 14. [Google Scholar] [CrossRef]
- Kuypers, D.R.; Naesens, M.; de Jonge, H.; Lerut, E.; Verbeke, K.; Vanrenterghem, Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther. Drug Monit. 2010, 32, 394–404. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, pharmacodynamics and pharmacogenetics of tacrolimus in kidney transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef]

| All Patients (n = 160) | CYP3A5 Expressers (n = 33) | Nonexpressers (n = 127) | RR (95%CI) | p Value | |
|---|---|---|---|---|---|
| Recipient | |||||
| Age, median (range) | 52 (19–76) | 49 (23–76) | 52 (19–77) | 0.79 | |
| Sex, women, n (%) | 56 (35) | 9 (27) | 47 (37) | 0.74 (0.4–1.3) | 0.3 |
| Sex, men, n (%) | 104 (65) | 24 (73) | 80 (63) | 1.16 (0.9–1.4) | 0.3 |
| Previous transplants, n (%) | 26 (16) | 8 (24) | 18 (14) | 1.71 (0.8–3.4) | 0.16 |
| PRA, n (%) | 8 (5) | 1 (3) | 7 (6) | 0.55 (0.1–3.2) | 0.56 |
| Preformed anti-HLA antibodies, n (%) | 67 (42) | 12 (36) | 55 (43) | 0.84 (0.5–1.3) | 0.47 |
| Class I, n (%) | 45 (28) | 9 (27) | 36 (28) | 0.96 (0.5–1.7) | 0.9 |
| Class II, n (%) | 43 (27) | 6 (18) | 37 (29) | 0.62 (0.3–1.3) | 0.21 |
| Class I and II, n (%) | 21 (13) | 3 (9) | 18 (14) | 0.64 (0.2–1.8) | 0.44 |
| Cold ischemia time in min, median (range) | 593 (85–10,404) | 566 (103–1094) | 598 (85–1404) | 0.74 | |
| Warm ischemia time in min, median (range) | 25 (11–48) | 26 (11–46) | 25 (11–48) | 0.81 | |
| Donor | |||||
| Deceased donors, n (%) | 128 (80) | 26 (79) | 102 (80) | 0.98 (0.8–1.2) | 0.85 |
| Age, median (range) | 56 (16–82) | 58 (30–72) | 55 (16–82) | 0.29 | |
| Sex, women, n (%) | 71 (44) | 18 (55) | 53 (42) | 1.31 (0.9–1.8) | 0.19 |
| Sex, men, n (%) | 89 (56) | 15 (45) | 74 (58) | 0.78 (0.5–1.1) | 0.19 |
| ABO-incompatible transplant, n (%) | 19 (12) | 4 (12) | 15 (12) | 1. 03 (0.4–2.7) | 0.96 |
| Immunosuppression at transplant | |||||
| Interleukin-2 receptor antagonist, n (%) | 144 (90) | 29 (88) | 115 (91) | 0.97 (0.8–1.1) | 0.65 |
| ATG, n (%) | 16 (10) | 3 (9) | 13 (10) | 0.89 (0.3–2.6) | 0.85 |
| Tacrolimus extended-release formulation, n (%) | 1 (1) | 0 (0) | 1 (1) | 0 (0–11.4) | 0.61 |
| MMF/MPA, n (%) | 160 (100) | 33 (100) | 127 (100) | ||
| Steroids, n (%) | 160 (100) | 33 (100) | 127 (100) | ||
| HLA mismatches | |||||
| MM A/B, n (%) | 133 (83) | 31 (94) | 102 (80) | 1.17 (1.0–1.3) | 0.06 |
| HLA class I MM A/B: 1–2, n (%) | 82 (51) | 22 (67) | 60 (47) | 1.41 (1.0–1.9) | 0.05 |
| HLA class I MM A/B: 3–4, n (%) | 52 (33) | 10 (30) | 42 (33) | 0.92 (0.5–1.6) | 0.76 |
| MM DR, n (%) | 108 (68) | 26 (79) | 82 (65) | 1.22 (0.9–1.5) | 0.12 |
| HLA class II MM DR: 1, n (%) | 71 (44) | 16 (48) | 55 (43) | 1.12 (0.7–1.6) | 0.59 |
| HLA class II MM DR: 2, n (%) | 37 (23) | 10 (30) | 27 (21) | 1.43 (0.8–2.5) | 0.27 |
| Causes of renal failure | |||||
| 1. Diabetic glomerulosclerosis, n (%) | 16 (10) | 5 (15) | 11 (9) | 1.75 (0.7–4.4) | 0.27 |
| 2. Chronic glomerulonephritis, n (%) | 8 (5) | 2 (6) | 6 (5) | 1.28 (0.3–5.2) | 0.75 |
| 3. Nephrosclerosis, n (%) | 26 (16) | 5 (15) | 21 (17) | 0.92 (0.4–2.1) | 0.85 |
| 4. Polycystic kidney disease, n (%) | 28 (18) | 4 (12) | 24 (19) | 0.64 (0.2–1.6) | 0.36 |
| 5. Tubulointerstitial nephritis, n (%) | 32 (20) | 7 (21) | 25 (20) | 1.08 (0.5–2.2) | 0.85 |
| 6. Congenital anomalies, n (%) | 16 (10) | 3 (9) | 13 (10) | 0.89 (0.3–2.6) | 0.85 |
| 7. Autoimmune diseases, n (%) | 10 (6) | 2 (6) | 8 (6) | 0.96 (0.2–3.7) | 0.96 |
| 8. Reflux nephropathy/recurrent pyelonephritis, n (%) | 8 (5) | 0 (0) | 8 (6) | 0 (0–1.7) | 0.14 |
| 9. TMA, n (%) | 3 (2) | 1 (3) | 2 (2) | 1.92 (0.3.11.1) | 0.58 |
| 10. Other, n (%) | 15 (9) | 4 (12) | 11 (9) | 1.4 (0.5–3.8) | 0.54 |
| All Patients (n = 160) | CYP3A5 Expressers (n = 33) | Nonexpressers (n = 127) | RR (95%CI) | p Value | |
|---|---|---|---|---|---|
| Delayed graft function, n (%) | 33 (21) | 6 (18) | 27 (21) | 0.86 (0.4–1.8) | 0.7 |
| Biopsy, n (%) | 73 (46) | 19 (58) | 54 (43) | 1.35 (0.9–1.9) | 0.12 |
| >1 biopsy, n (%) | 33 (21) | 9 (27) | 24 (19) | 1.44 (0.7–2.7) | 0.29 |
| Rejection, Banff categories 2, 3, and 4, n (%) | 46 (29) | 10 (30) | 36 (28) | 1.07 (0.6–1.8) | 0.82 |
| Rejection Banff categories 2 and 4, n (%) | 29 (18) | 8 (24) | 21 (17)) | 1.47 (0.7–2.9) | 0.31 |
| ABMR Banff category 2, n (%) | 3 (2) | 2 (6) | 1 (1) | 7.7 (1.0–57.4) | 0.05 |
| TCMR Banff categories 3 and 4, n (%) | 44 (28) | 9 (27) | 35 (28) | 0.99 (0.5–1.8) | 0.97 |
| TCMR Banff category 4, n (%) | 21 (13) | 4 (12) | 17 (13) | 0.91 (0.3–2.3) | 0.85 |
| Transplant failure, n (%) | 5 (3) | 3 (9) | 2 (2) | 5.77 (1.2–27.8) | 0.03 |
| eGFR CKD-EPI mL/min/1.73 m2 at 2 years after Tx, median (range) | 54 (12–119) | 58 (12–99) | 54 (112–119) | 0.36 | |
| eGFR < 30 mL/min/1.73 m2 at 2 years after Tx, median (range) | 21 (13) | 4 (12) | 17 (13) | 0.91 (0.3–2.3) | 0.85 |
| Death, n (%) | 1 (1) | 0 (0) | 1 (1) | 0 (0.14.4) | 0.61 |
| De novo anti-HLA antibodies, n (%) | 17 (11) | 4 (12) | 13 (10) | 1.18 (0.4–3.1) | 0.75 |
| Class I, n (%) | 8 (5) | 2 (6) | 6 (5) | 1.28 (0.3–5.2) | 0.75 |
| Class II, n (%) | 11 (7) | 3 (9) | 8 (6) | 1.44 (0.4–4.6) | 0.57 |
| De novo anti-HLA DSAs, n (%) | 7 (4) | 2 (6) | 5 (4) | 1.54 (0.4–6.5) | 0.6 |
| Class I, n (%) | 3 (2) | 1 (3) | 2 (2) | 1.92 (0.3–14.1) | 0.58 |
| Class II, n (%) | 6 (4) | 2 (6) | 4 (3) | 1.92 (0.4–8.5) | 0.43 |
| CNI nephrotoxicity, n (%) | 19 (12) | 5 (15) | 14 (11) | 1.37 (0.5–3.3) | 0.51 |
| Follow-up time in years, median (range) | 2 (0.3–2) | 2 (0.3–2) | 2 (0.5–2) | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönfelder, K.; Möhlendick, B.; Eisenberger, U.; Kribben, A.; Siffert, W.; Heinemann, F.M.; Gäckler, A.; Wilde, B.; Friebus-Kardash, J. Early CYP3A5 Genotype-Based Adjustment of Tacrolimus Dosage Reduces Risk of De Novo Donor-Specific HLA Antibodies and Rejection among CYP3A5-Expressing Renal Transplant Patients. Diagnostics 2024, 14, 2202. https://doi.org/10.3390/diagnostics14192202
Schönfelder K, Möhlendick B, Eisenberger U, Kribben A, Siffert W, Heinemann FM, Gäckler A, Wilde B, Friebus-Kardash J. Early CYP3A5 Genotype-Based Adjustment of Tacrolimus Dosage Reduces Risk of De Novo Donor-Specific HLA Antibodies and Rejection among CYP3A5-Expressing Renal Transplant Patients. Diagnostics. 2024; 14(19):2202. https://doi.org/10.3390/diagnostics14192202
Chicago/Turabian StyleSchönfelder, Kristina, Birte Möhlendick, Ute Eisenberger, Andreas Kribben, Winfried Siffert, Falko M. Heinemann, Anja Gäckler, Benjamin Wilde, and Justa Friebus-Kardash. 2024. "Early CYP3A5 Genotype-Based Adjustment of Tacrolimus Dosage Reduces Risk of De Novo Donor-Specific HLA Antibodies and Rejection among CYP3A5-Expressing Renal Transplant Patients" Diagnostics 14, no. 19: 2202. https://doi.org/10.3390/diagnostics14192202
APA StyleSchönfelder, K., Möhlendick, B., Eisenberger, U., Kribben, A., Siffert, W., Heinemann, F. M., Gäckler, A., Wilde, B., & Friebus-Kardash, J. (2024). Early CYP3A5 Genotype-Based Adjustment of Tacrolimus Dosage Reduces Risk of De Novo Donor-Specific HLA Antibodies and Rejection among CYP3A5-Expressing Renal Transplant Patients. Diagnostics, 14(19), 2202. https://doi.org/10.3390/diagnostics14192202







