Comprehensive Literature Review on Melanoma of Unknown Primary Site Triggered by an Intriguing Case Report
Abstract
1. Introduction
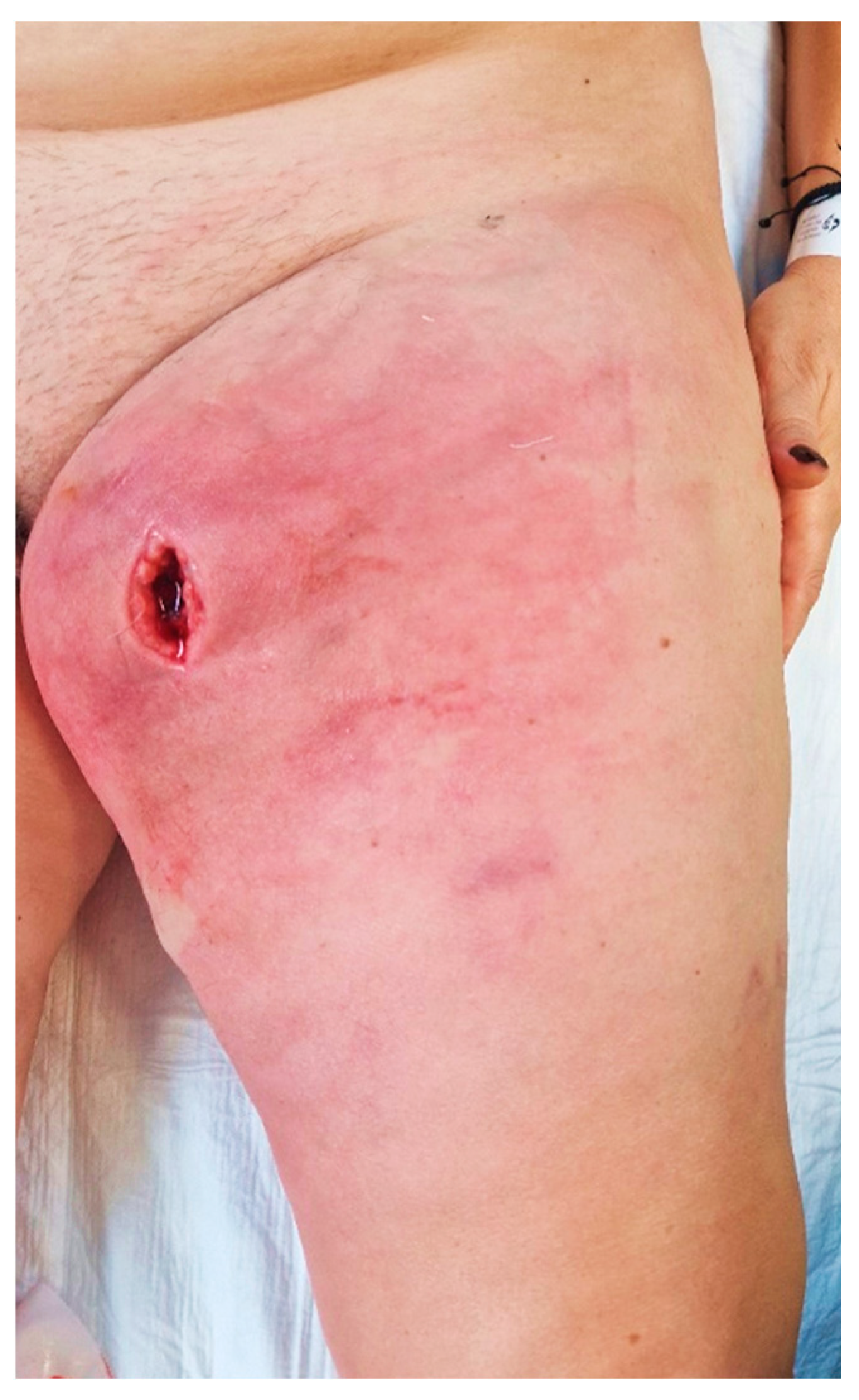
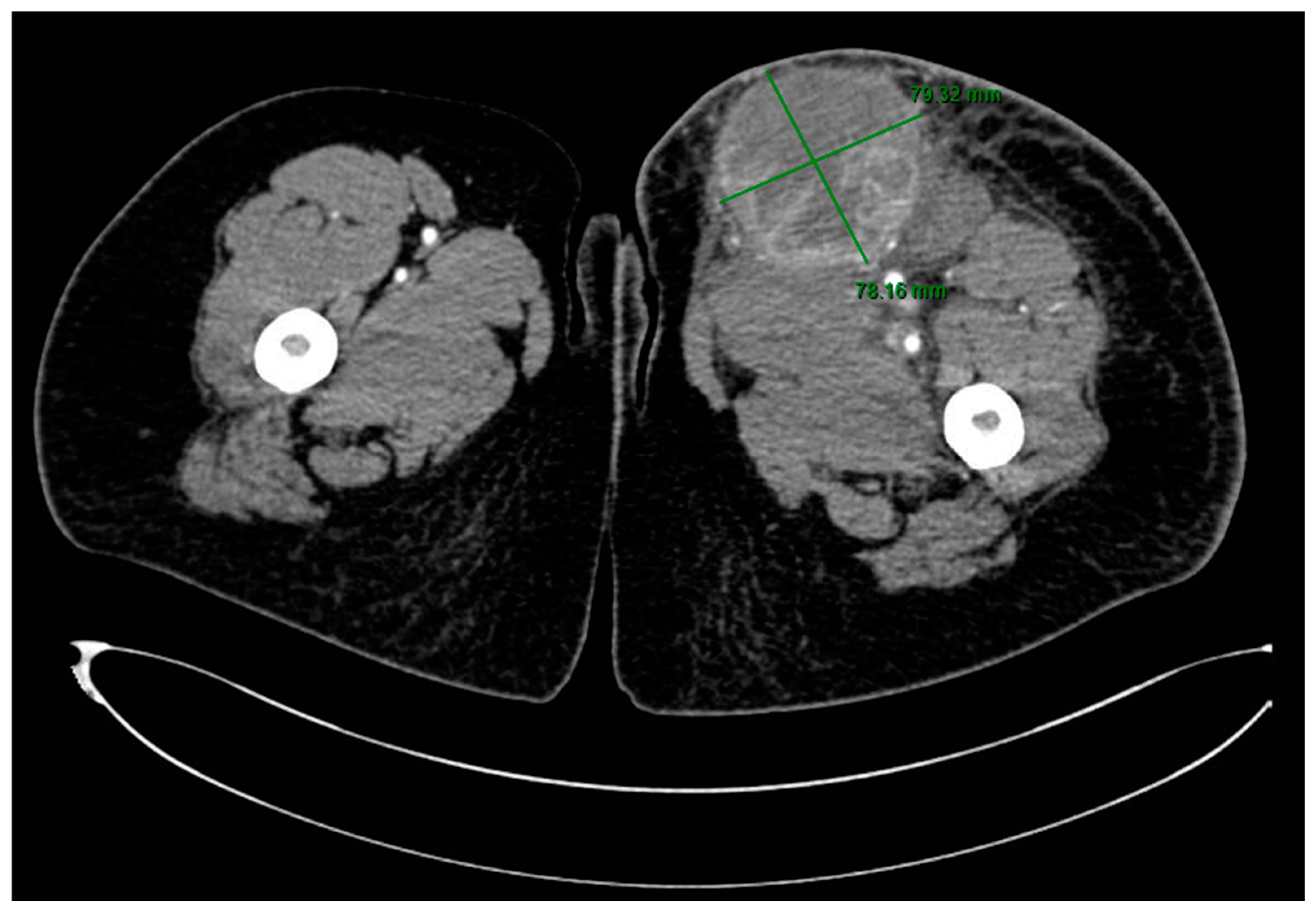
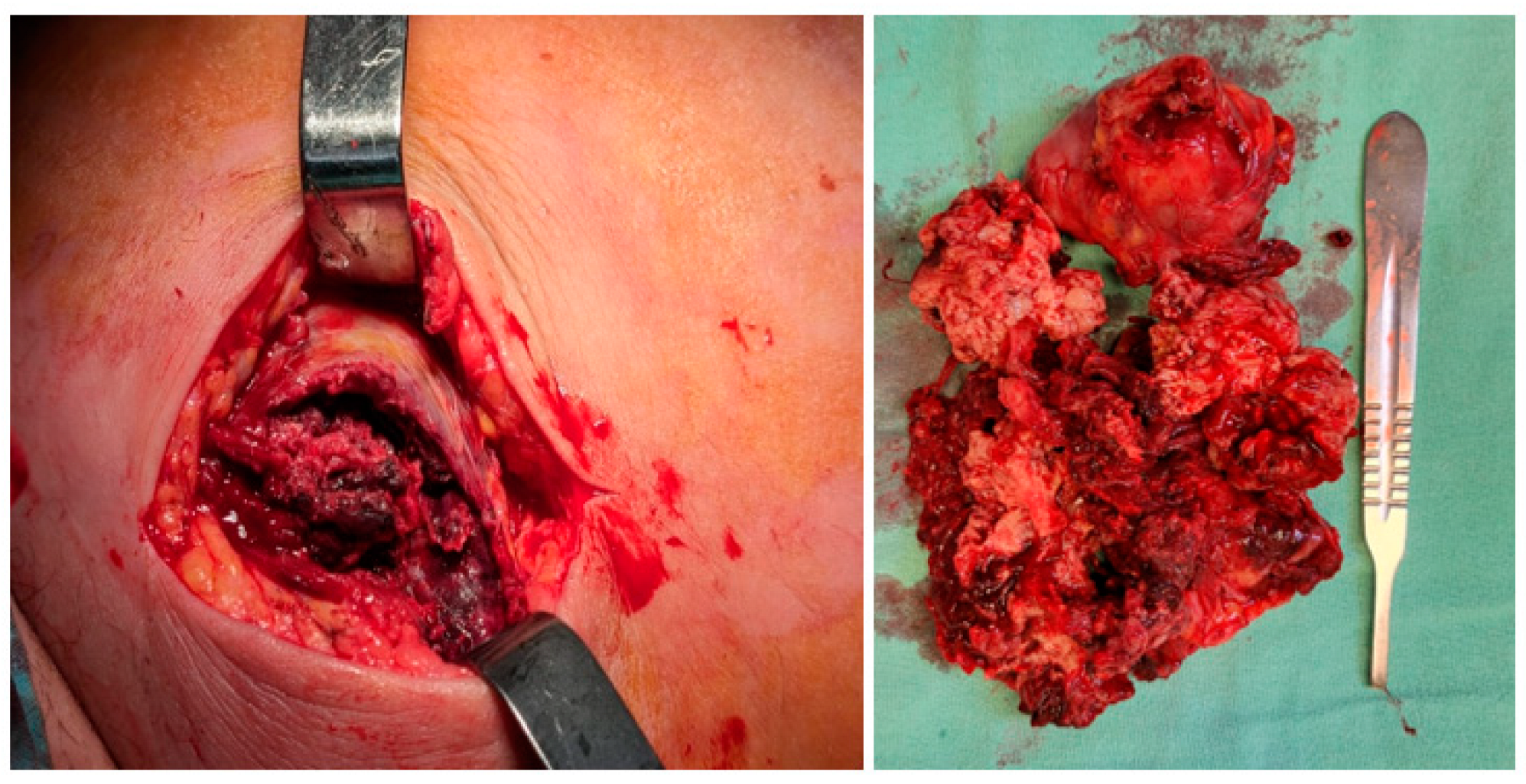
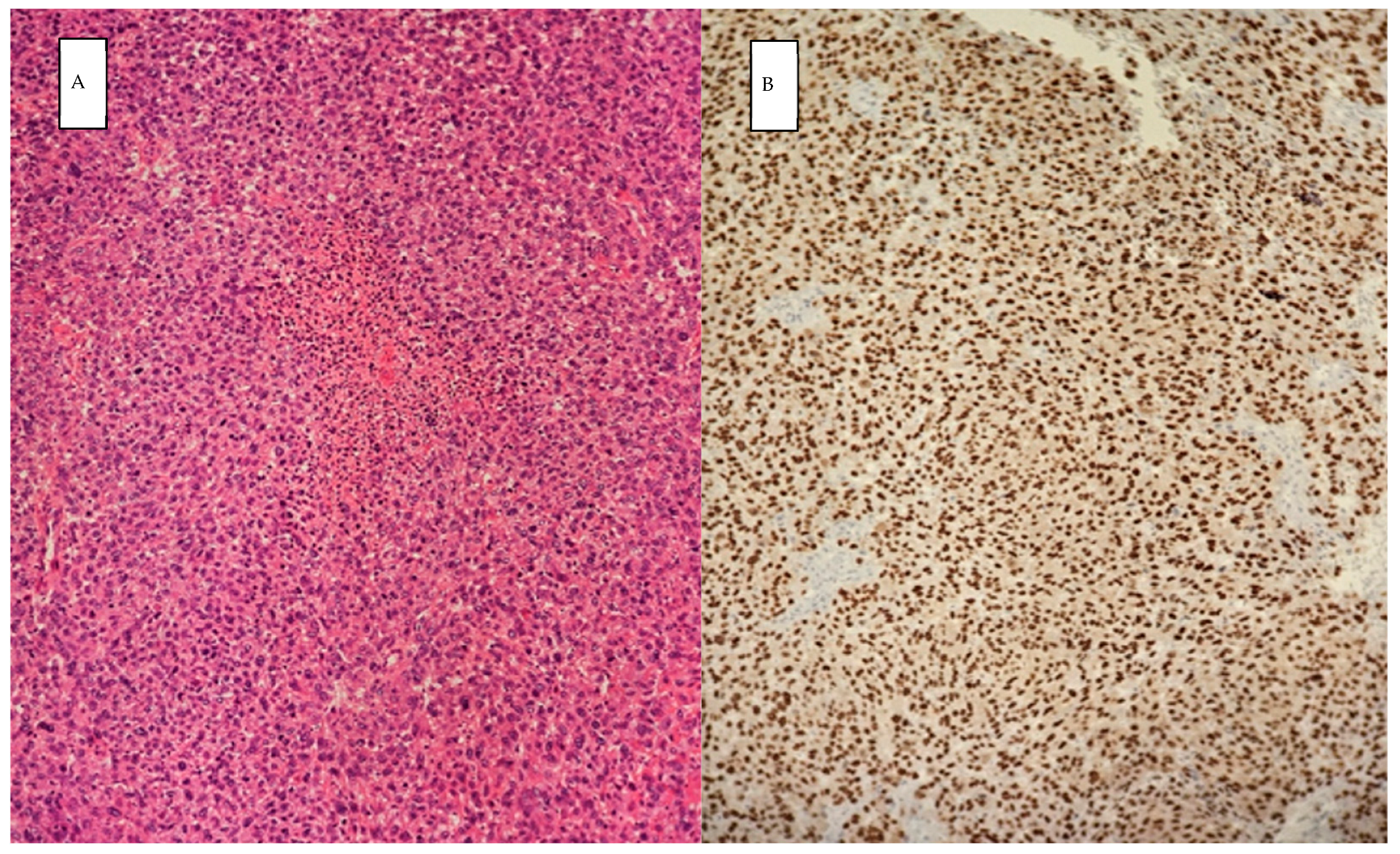
2. Case Presentation
3. Discussion and Literature Review
3.1. Non-Cutaneous Malignant Melanomas
| Gastrointestinal tract | Gastroesophageal junction primary melanoma | Hussein Agha Y et al., 2020 [18] |
| Primary gastric melanoma | Sohail et al., 2024 [10], Schizas D et al., 2021 [9] | |
| Primary small bowel melanoma | Wu F et al., 2022 [19] | |
| Primary rectal melanoma | Ugonabo O et al., 2022 [20] | |
| Urogenital tract | Primary gallbladder melanoma | Peison B et al., 1976 [21], Hatanaka N. et al., 1993 [22], Dong XD, et al., 1999 [23] |
| Primary urethral melanoma | Nguyen Q et al., 2023 [24] | |
| Primary malignant melanoma of the uterine cervix | Duggal R et al., 2010 [25] | |
| Respiratory tract | Primary pulmonary melanoma | Feng Y et al., 2016 [26], Filippini A. et al., 2015 [27], Dos Santos C. L et al., 2013 [28] |
| Uveal melanoma | Choroidal melanoma | Shields CL, 2014 [29], Singh P [30] |
| Melanoma of the ciliary body | Shields CL, 2012 [31] | |
| Iris melanoma | Shields CL, 2012 [31] | |
| Breast | Parenchymal melanoma without skin involvement | Drueppel D et al., 2015 [14], Koh J et al., 2019 [15] |
| Adrenal gland | Primary adrenal melanoma | González-Sáez L et al., 2011 [16] |
| Malignant primary melanocytic tumors of the central nervous system | Primary meningeal malignant melanoma | Lang-Orsini M et al., 2021 [17] |
| Primary leptomeningeal melanomatosis (melanocytosis) | Noronha C et al., 2019 [32] |
3.2. MUP Diagnosis
| MUP Site | Clinical Presentations | References | |
|---|---|---|---|
| Lymph nodes | Asymptomatic palpable lump Limb edema Superior vena cava syndrome (facial edema, collateral venous circulation in the neck) | Nakamura et al., 2023 [35] Doyle et al., 2023 [36] Phan et al., 2021 [37] Bankar et al., 2015 [38] Eltawansy et al., 2015 [39] Andrianandrasana et al., 2023 [82] | |
| Soft tissues | Ulcerated skin nodules Subcutaneous swellings Hypertrophic lesions Numbness and pain in the limbs | Babu et al., 2022 [40] Sirvan et al., 2019 [41] Spoto et al., 2018 [94] Liu et al., 2024 [95] | |
| Gastro-intestinal tract | Gastroesophageal junction | Progressive dysphagia | Averbukh et al., 2019 [44] |
| Stomach | Vomiting Early satiety Anorexia Unintentional weight loss Anemia Nausea Fatigue | Yan et al., 2023 [45] Takahashi et al., 2020 [46] Myrou et al., 2021 [96] Cortellini et al., 2021 [97] | |
| Intestines | Gastrointestinal hemorrhage Bowel obstruction, intussusception Vomiting Weight loss Abdominal pain | Sirvan et al., 2019 [41] Vrable et al., 2017 [48] Wu et al., 2022 [49] Reddy et al., 2014 [50] Mui et al., 2019 [98] De Monti et al., 2018 [99] Stagnitti et al., 2014 [100] | |
| Pancreas | Upper abdominal pain Fever Jaundice | Ben Slama et al., 2017 [56] | |
| Liver | Asymptomatic Hepatomegaly Ascites Lower limb edema Abdominal distention Loss of appetite Nausea Weakness Progressive weight loss Fever Sweating Upper right quadrant pain | Yan et al., 2023 [45] Yuan et al., 2023 [51] Wang et al., 2023 [52] Cheng et al., 2021 [53] Tiong et al., 2023 [54] | |
| Gallbladder | Acute cholecystitis Hemobilia | Dong et al., 1999 [23] Onozawa et al., 2014 [55] | |
| Nervous system | Central nervous system | Dizziness Headaches Diplopia Seizures Visual auras Ataxia Acute confusion Magnetic gait Muscle weakness Urinary incontinence Hydrocephalus (nausea, vomiting) Leptomeningeal disease Weight loss | Doyle et al., 2023 [36] Padilla et al., 2023 [57] Nguyen et al., 2022 [58] Garcia-Ramiu et al., 2022 [59] Sawalha et al., 2023 [61] Kuriakose et al., 2015 [65] Mremi et al., 2021 [101] Takagi et al., 2020 [102] |
| Peripheral nervous system | Paresthesia Numbness Paraplegia Cauda equina syndrome | Naing et al., 2004 [62] Chen et al., 2022 [63] Takagi et al., 2020 [102] | |
| Mediastinum | Dry cough Chest pain Weight loss | Pujani et al., 2017 [64] | |
| Heart | Dyspnea Fatigue Weight loss | Kuriakose et al., 2015 [65] | |
| Lung | Cough Thoracic pain Horner’s syndrome | El Haj et al., 2021 [66] Tsaknis et al., 2021 [67] Gebauer et al., 2020 [68] Yamamoto et al., 2023 [69] | |
| Respiratory tract | Nasopharyngeal | Cerumen impaction Epistaxis Impaired hearing | Azoury et al., 2015 [70] |
| Endobronchial | Cough Hemoptysis | Kim et al., 2023 [71] | |
| Genitourinary system | Melanuria Hematospermia | Diamantopoulos et al., 2023 [72] Meng et al., 2000 [73] Fabiani et al., 2016 [74] | |
| Placenta | - | Garcia-Ramiu et al., 2022 [59] | |
| Bones | Radiculopathy Lower back pain Dysuria Motor deficit Paresis | Tang et al., 2019 [76] Kakutani et al., 2008 [77] Mathew et al., 2017 [78] Tang et al., 2020 [79] | |
| Bone marrow | General weakness Loss of appetite Vomiting Progressive thrombocytopenia | Matsumoto et al., 2021 [80] Suzuki et al., 2014 [81] | |
| Muscles | Lumps Trismus Antalgic positions of limbs | Tsaknis et al., 2021 [67] Andrianandrasana et al., 2023 [82] Grech et al., 2020 [83] Dalle Carbonare et al., 2017 [84] Rastrelli et al., 2014 [85] | |
| Breast | Asymptomatic Palpable nodule | Kim et al., 2017 [86] Agosto-Arroyo et al., 2017 [103] El-Tani et al., 2016 [104] | |
| Salivary glands | Asymmetric swelling Snoring complaints | Gorris et al., 2021 [87] Scott et al., 2016 [88] | |
| Adrenal glands | Asthenia Weight loss Recent-onset vitiligo Dorsal pain | Drouet et al., 2017 [89] Blanco et al., 2014 [90] | |
| Choroid | Progressive loss of vision | Rieth et al., 2021 [91] | |
| Paraneoplastic presentations | Opsoclonus–myoclonus syndrome Paraneoplastic vitelliform maculopathy | Mondragón et al., 2019 [92] Rahimi et al., 2018 [93] | |
3.3. Staging and Prognosis
3.4. Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, J.F.; Gerstenblith, M.R. Melanoma of Unknown Primary. In Noncutaneous Melanoma; Scott, J.F., Gerstenblith, M.R., Eds.; Codon Publications: Brisbane, Australia, 2018. [Google Scholar]
- Boussios, S.; Rassy, E.; Samartzis, E.; Moschetta, M.; Sheriff, M.; Pérez-Fidalgo, J.A.; Pavlidis, N. Melanoma of unknown primary: New perspectives for an old story. Crit. Rev. Oncol. Hematol. 2021, 158, 103208. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, T.; Bowden, L.; Berg, J.W. Malignant melanoma of unknown primary origin. Surg. Gynecol. Obstet. 1963, 117, 341–345. [Google Scholar]
- Rousset, P.; Dalle, S.; Mortier, L.; Dereure, O.; Dalac, S.; Dutriaux, C.; Leccia, M.-T.; Legoupil, D.; Brunet-Possenti, F.; De Quatrebarbes, J.; et al. Impact of systemic therapies in metastatic melanoma of unknown primary: A study from MELBASE, a French multicentric prospective cohort. J. Am. Acad. Dermatol. 2023, 88, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Tiodorovic, D.; Stojkovic-Filipovic, J.; Marghoob, A.; Argenziano, G.; Puig, S.; Malvehy, J.; Tognetti, L.; Pietro, R.; Akay, B.N.; Zalaudek, I.; et al. Dermatoscopic patterns of cutaneous metastases: A multicentre cross-sectional study of the International Dermoscopy Society. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Lubner, M.G.; Menias, C.O.; Mellnick, V.M.; Kennedy, T.A.; Bhalla, S.; Pickhardt, P.J. Clinical and Imaging Features of Noncutaneous Melanoma. AJR Am. J. Roentgenol. 2017, 208, 942–959. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar]
- Falcone, R.; Verkhovskaia, S.; Di Pietro, F.R.; Poti, G.; Samela, T.; Carbone, M.L.; Morelli, M.F.; Zappalà, A.R.; di Rocco, Z.C.; Morese, R.; et al. Primary Mucosal Melanoma: Clinical Experience from a Single Italian Center. Curr. Oncol. 2024, 31, 588–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schizas, D.; Tomara, N.; Katsaros, I.; Sakellariou, S.; Machairas, N.; Paspala, A.; Tsilimigras, D.I.; Papanikolaou, I.S.; Mantas, D. Primary gastric melanoma in adult population: A systematic review of the literature. ANZ J. Surg. 2021, 91, 269–275. [Google Scholar] [CrossRef]
- Sohail, Z.; Samar, M.R.; Qureshi, N.J.; Arshad, S.; Zaki, A. A Curious Case of Primary Gastric Mucosal Melanoma. Korean J. Gastroenterol. 2024, 83, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Safioleas, M.; Agapitos, E.; Kontzoglou, K.; Stamatakos, M.; Safioleas, P.; Mouzopoulos, G.; Kostakis, A. Primary melanoma of the gallbladder: Does it exist? Report of a case and review of the literature. World J. Gastroenterol. 2006, 12, 4259–4261. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Zarkavelis, G.; Andrianopoulou, A.; Papoudou-Bai, A.; Stefanou, D.; Boussios, S.; Pentheroudakis, G. Primary Pulmonary Malignant Melanoma: Report of an Important Entity and Literature Review. Case Rep. Oncol. Med. 2017, 2017, 8654326. [Google Scholar] [CrossRef] [PubMed]
- Branisteanu, D.C.; Bogdanici, C.M.; Branisteanu, D.E.; Maranduca, M.A.; Zemba, M.; Balta, F.; Branisteanu, C.I.; Moraru, A.D. Uveal melanoma diagnosis and current treatment options (Review). Exp. Ther. Med. 2021, 22, 1428. [Google Scholar] [CrossRef] [PubMed]
- Drueppel, D.; Schultheis, B.; Solass, W.; Ergonenc, H.; Tempfer, C.B. Primary malignant melanoma of the breast: Case report and review of the literature. Anticancer Res. 2015, 35, 1709–1713. [Google Scholar] [PubMed]
- Koh, J.; Lee, J.; Jung, S.Y.; Kang, H.S.; Yun, T.; Kwon, Y. Primary Malignant Melanoma of the Breast: A Report of Two Cases. J. Pathol. Transl. Med. 2019, 53, 119–124. [Google Scholar] [CrossRef]
- González-Sáez, L.; Pita-Fernández, S.; Lorenzo-Patiño, M.J.; Arnal-Monreal, F.; Machuca-Santacruz, J.; Romero-González, J. Primary melanoma of the adrenal gland: A case report and review of the literature. J. Med. Case Rep. 2011, 5, 273. [Google Scholar] [CrossRef]
- Lang-Orsini, M.; Wu, J.; Heilman, C.B.; Kravtsova, A.; Weinstein, G.; Madan, N.; Arkun, K. Primary meningeal melanoma masquerading as neurofibromatosis type 2: Illustrative case. J. Neurosurg. Case Lessons 2021, 2, CASE21444. [Google Scholar] [CrossRef]
- Hussein Agha, Y.; Parker, N.A.; Alderson, J. Case Report: Primary melanoma of the gastroesophageal junction. F1000Research 2020, 9, 490. [Google Scholar] [CrossRef]
- Graças, A.M.; Souza, W.P.; Canut, A.C.A.; Franciss, M.Y.; Zilberstein, B. Primary Small Bowel Melanoma: A Case Report and Review of Literature. Front. Surg. 2022, 9, 792243. [Google Scholar] [CrossRef]
- Ugonabo, O.; Mohamed, M.; Ezeh, E.; Simmons, J.; Cuda, J.; Ghavimi, S. A Rare Metastatic Primary Rectal Melanoma in a Geriatric Male. J. Med. Cases 2022, 13, 369–373. [Google Scholar] [CrossRef]
- Peison, B.; Rabin, L. Malignant melanoma of the gallbladder: Report of three cases and review of the literature. Cancer 1976, 37, 2448–2454. [Google Scholar] [CrossRef]
- Hatanaka, N.; Miyata, M.; Kamiike, W.; Okumura, K.; Hashimoto, T.; Yamaguchi, T.; Kishino, Y.; Sakurai, M.; Matsuda, H. Radical resection of primary malignant melanoma of the gallbladder with multiple metastases: Report of a case. Surg. Today Jpn. J. Surg. 1993, 23, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.D.; DeMatos, P.; Prieto, V.G.; Seigler, H.F. Melanoma of the gallbladder: A review of cases seen at Duke University Medical Center. Cancer 1999, 85, 32–39. [Google Scholar] [CrossRef]
- Nguyen, Q.; Nguyen, H.T.; Bui, X.T.; Bui, V.Q.; Nguyen, T.D. Primary malignant melanoma of the male urethra: Case report and review of literature. Int. J. Surg. Case Rep. 2023, 110, 108697. [Google Scholar] [CrossRef] [PubMed]
- Duggal, R.; Srinivasan, R. Primary amelanotic melanoma of the cervix: Case report with review of literature. J. Gynecol. Oncol. 2010, 21, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, J.; Yang, Q.; Xiong, W.; Zhen, G.; Xu, Y.; Zhang, Z.; Zhang, H. Pulmonary melanoma and “crazy paving” patterns in chest images: A case report and literature review. BMC Cancer 2016, 16, 592. [Google Scholar] [CrossRef]
- Filippini, A.; Zorzi, F.; Bna’, C.; Arnaboldi, A.; Sabatini, T. Dark sputum: An atypical presentation of primary pulmonary malignant melanoma. Respir. Med. Case Rep. 2015, 15, 118–120. [Google Scholar] [CrossRef]
- Dos Santos, C.L.; Fernandes, L.R.; Meruje, M.; Barata, F. Primary pulmonary melanoma: The unexpected tumour. BMJ Case Rep. 2013, 2013, bcr2013200706. [Google Scholar] [CrossRef]
- Shields, C.L.; Manalac, J.; Das, C.; Ferguson, K.; Shields, J.A. Choroidal melanoma: Clinical features, classification, and top 10 pseudomelanomas. Curr. Opin. Ophthalmol. 2014, 25, 177–185. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A. Choroidal melanoma. Oman J. Ophthalmol. 2012, 5, 3–9. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Mashayekhi, A.; Shields, J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina 2012, 32, 1363–1372. [Google Scholar] [CrossRef]
- Noronha, C.; Rocha, L. Meningeal melanocytosis: A challenging diagnosis. Lancet Oncol. 2019, 20, e343. [Google Scholar] [CrossRef] [PubMed]
- Tos, T.; Klyver, H.; Drzewiecki, K.T. Extensive screening for primary tumor is redundant in melanoma of unknown primary. J. Surg. Oncol. 2011, 104, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Kamposioras, K.; Pentheroudakis, G.; Pectasides, D.; Pavlidis, N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit. Rev. Oncol. Hematol. 2011, 78, 112–126. [Google Scholar]
- Nakamura, M.; Ohnishi, K.; Uchida, F.; Saito, T.; Kitagawa, Y.; Matsuoka, R.; Yanagawa, T.; Sakurai, H. Proton beam therapy for cervical lymph node metastasis in an octogenarian with melanoma of unknown primary: A case report. Int. Cancer Conf. J. 2023, 12, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; O’Sullivan, B.; Watchorn, R.E.; Eustace, K. Melanoma of unknown primary: A case series. Ir. J. Med. Sci. 2023, 192, 65–66. [Google Scholar] [CrossRef]
- Phan, M.B.; Phan, J.; Nguyen, C.; He, J.; Nguyen, Q.D. Melanoma with an Unknown Primary in an Asymptomatic Elderly Male with Unilateral Lymphadenopathy. Cureus 2021, 13, e15140. [Google Scholar] [CrossRef]
- Bankar, S.; Patkar, S.; Desai, S.; Shrikhande, S.V. Unusual presentation of melanoma of unknown primary origin: A case report and review of literature. J. Cancer Res. Ther. 2015, 11, 1025. [Google Scholar] [CrossRef]
- Eltawansy, S.A.; Panasiti, R.; Hasanien, S.; Lourdusamy, D.; Sharon, D. Metastatic malignant melanoma of the inguinal lymph node with unknown primary lesion. Case Rep. Med. 2015, 2015, 879460. [Google Scholar] [CrossRef]
- Babu, A.K.; Mizaj, Z.; Gowda, V.; Jaleel, A.; John, N.M.; Nair Santhamma, S.G.; John, S. Metastatic Melanoma with an Unknown Primary. Indian J. Dermatol. 2022, 67, 627. [Google Scholar] [CrossRef] [PubMed]
- Sirvan, S.S.; Eren, H.; Yazar, S.K.; Gunenc, A.C.; Ye, K.; Irmak, F.; Tuncel, D. Approach to Patients with Malignant Melanoma of Unknown Primary Origin. Şişli Etfal Hastan. Tıp Bülteni 2019, 53, 125–131. [Google Scholar] [CrossRef]
- Hariga, C.S.; Achim, S.C.; Savu, A.C.; Enache, V.; Jecan, C.R. A case of extrapleural solitary fibrous tumor of the thigh with eight years follow-up. Rom. J. Morphol. Embryol. 2016, 57, 303–306. [Google Scholar] [PubMed]
- Kohoutova, D.; Worku, D.; Aziz, H.; Teare, J.; Weir, J.; Larkin, J. Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options. Cells 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Averbukh, L.D.; Mavilia, M.G.; Aujla, A.K. Secondary Gastrointestinal Melanoma of Unknown Origin: A Case Report of a Rare Entity. Cureus 2019, 11, e4720. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.H.; Huang, J.; Chiang, J.; Kwan, K.W.C. Metastatic Gastric Mucosal Melanoma: A Rare Case Presenting with Diffuse Gastric Polyposis. Cureus 2023, 15, e43740. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Sugita, S.; Kagaya, H.; Morita, T. Surgically treated gastric melanoma of unknown primary: A case report from a 10-year survivor. Pathol. Int. 2020, 70, 786–792. [Google Scholar] [CrossRef]
- Blecker, D.; Abraham, S.; Furth, E.E.; Kochman, M.L. Melanoma in the gastrointestinal tract. Am. J. Gastroenterol. 1999, 94, 3427–3433. [Google Scholar] [CrossRef]
- Vrable, A.; Chang, R. Malignant melanoma of the small bowel presenting with intussusception in a woman: A case report. Melanoma Manag. 2017, 4, 99–104. [Google Scholar] [CrossRef]
- Wu, F.; Lee, M.S.; Kim, D.E. Small bowel obstruction caused by hemorrhagic metastatic melanoma: Case report and literature review. J. Surg. Case Rep. 2022, 2022, rjac395. [Google Scholar] [CrossRef]
- Reddy, P.; Walker, C.; Afonso, B. A rare case of metastatic malignant melanoma to the colon from an unknown primary. Case Rep. Gastrointest. Med. 2014, 2014, 312902. [Google Scholar] [CrossRef]
- Yuan, Z.; Guo, H.-Y.; Lu, W.-T.; Wang, Y.-H.; He, J.; Zhang, F.; Che, J.-Y.; Qiao, F. Report on a case of liver-originating malignant melanoma of unknown primary. Open Life Sci. 2023, 18, 20220750. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Kang, Y. Diffuse hepatic infiltration of malignant melanoma of unknown primary origin. Asian J. Surg. 2023, 46, 2168–2169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Lin, Y.J.; Chiu, S.H.; Shih, Y.L. Combined immune checkpoint inhibitors of CTLA4 and PD-1 for hepatic melanoma of unknown primary origin: A case report. World J. Clin. Cases 2021, 9, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Tiong, J.; Rajagopalan, A.; Jaya, J.; Sritharan, M. Spontaneous rupture of a solitary oligometastatic hepatic melanoma. BMJ Case Rep. 2023, 16, e252367. [Google Scholar] [CrossRef]
- Onozawa, H.; Saito, M.; Yoshida, S.; Sakuma, T.; Matsuzaki, M.; Katagata, N.; Watanabe, F.; Yamaguchi, Y.; Takenoshita, S.; Nomizu, T. Multiple metastatic malignant melanoma presenting intraluminal gallbladder bleeding. Int. Surg. 2014, 99, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Ben Slama, S.; Bacha, D.; Bayar, R.; Gharbi, L.; Lahmar, A. Pancreatic resection for isolated metastasis from melanoma of unknown primary. Acta Gastroenterol. Belg. 2017, 80, 323–324. [Google Scholar] [PubMed]
- Padilla, C.S.; Ho, V.K.Y.; Mooijenkind, T.W.A.N.; Louwman, M.W.J.; de Vos, F.Y.F.L.; Bekkenk, M.W.; Minnaard, W.A.; Loef, C.; van Zanten, S.E.M.V. Brain metastases in adult patients with melanoma of unknown primary in the Netherlands (2011–2020). J. Neurooncol. 2023, 163, 239–248. [Google Scholar] [CrossRef]
- Nguyen, V.; Aboulenain, S.; Mohammed, S.; Perez Parra, S. A Case of Metastatic CNS Melanoma of Unknown Primary Presenting with Seizures. Case Rep. Med. 2022, 2022, 3099750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Ramiu, K.; Mohsin, I.; Ikeguchi, A.; Newberry, B. An Unusual Case of Malignant Melanoma with Metastasis to the Placenta During Pregnancy. Eur. J. Case Rep. Intern. Med. 2022, 9, 003490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avino, A.; Ion, D.-E.; Gheoca-Mutu, D.-E.; Abu-Baker, A.; Țigăran, A.-E.; Peligrad, T.; Hariga, C.-S.; Balcangiu-Stroescu, A.-E.; Jecan, C.-R.; Tudor, A.; et al. Diagnostic and Therapeutic Particularities of Symptomatic Melanoma Brain Metastases from Case Report to Literature Review. Diagnostics 2024, 14, 688. [Google Scholar] [CrossRef]
- Sawalha, A.; Alkilani, H. Leptomeningeal Disease and Hydrocephalus as the First Presentation of Melanoma. Cureus 2023, 15, e44648. [Google Scholar] [CrossRef]
- Naing, A.; Messina, J.L.; Vrionis, F.R.; Daud, A.I. Uncommon manifestations of common malignancies: Case 3. Malignant melanoma arising from a spinal nerve root. J. Clin. Oncol. 2004, 22, 3194–3195. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Newby, C.; Fakhri, N.; Lammle, M. Metastatic melanoma of unknown origin mimicking neurofibromatosis. Radiol. Case Rep. 2020, 16, 119–122, Correction in Radiol. Case Rep. 2022, 17, 4055–4056. [Google Scholar] [CrossRef] [PubMed]
- Pujani, M.; Hassan, M.J.; Jetley, S.; Raina, P.K.; Kumar, M. Malignant Melanoma Presenting as a Mediastinal Malignant Melanoma Presenting as a Mediastinal Unknown Primary Origin? Mediastinal Kitle Şeklinde Görülen Malign Melanom—Primer ya da Primeri Bilinmeyen Metastatik Melanom? Turk. Patoloji Derg. 2017, 33, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, R.; Melvani, R.; Gangadharan, V.; Cowley, M. Right atrial metastatic melanoma with unknown primaries. Case Rep. Cardiol. 2015, 2015, 483520. [Google Scholar] [CrossRef] [PubMed]
- El Haj, N.I.; Hafidi, S.; Karam, R.; Boubia, S.; Karkouri, M.; Ridai, M. Thoracic metastasis of malignant melanoma of unknown primary: A case report and literature review. Int. J. Surg. Case Rep. 2021, 87, 106383. [Google Scholar] [CrossRef]
- Tsaknis, G.; Naeem, M.; Singh, A.; Vijayakumar, S. Malignant melanoma without primary, presenting as solitary pulmonary nodule: A case report. J. Med. Case Rep. 2021, 15, 347. [Google Scholar] [CrossRef]
- Gebauer, K.; Schirren, J.; Jaeschke, B.; Kaufmann, R.; Meissner, M. Neoadjuvant therapy with vemurafenib in Horner’s syndrome as a very rare first diagnosis of a malignant melanoma of unknown primary. J. Dtsch. Dermatol. Ges. 2020, 18, 47–49. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kuriyama, H.; Kashiwada-Nakamura, K.; Kajihara, I.; Makino, K.; Aoi, J.; Miyashita, A.; Yoshida, C.; Kubo, Y.; Fukushima, S. A case of unknown primary melanoma detected with a BRAF gene mutation from a pleural fluid cell block. J. Dermatol. 2023, 50, e305–e306. [Google Scholar] [CrossRef]
- Azoury, S.C.; Crompton, J.G.; Straughan, D.M.; Klemen, N.D.; Reardon, E.S.; Beresnev, T.H.; Hughes, M.S. Unknown primary nasopharyngeal melanoma presenting as severe recurrent epistaxis and hearing loss following treatment and remission of metastatic disease: A case report and literature review. Int. J. Surg. Case Rep. 2015, 10, 232–235. [Google Scholar] [CrossRef]
- Kim, B.C.; Kang, H.K.; Kim, Y.S.; Haw, S.; Kim, H.S.; Kang, J. A rare case of endobronchial melanoma of unknown primary. Respir. Med. Case Rep. 2023, 42, 101811. [Google Scholar] [CrossRef]
- Diamantopoulos, P.; Patavoukas, G.; Garantzioti, A.; Charakopoulos, E.; Kyriakakis, G.; Mikou, P.; Benopoulou, O.; Gogas, H. Melanuria in a patient with BRAF-mutant metastatic melanoma of unknown primary: Insights on the pathophysiology, differential diagnosis, prognosis, and treatment. Am. J. Med. Sci. 2023, 365, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.V.; Werboff, L.H. Hematospermia as the presenting symptom of metastatic malignant melanoma of unknown primary origin. Urology 2000, 56, 330. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, A.; Principi, E.; Filosa, A.; Fioretti, F.; Maurelli, V.; Servi, L.; Mammana, G. Ultrasound features of a metastatic seminal vesicle melanoma: A case report. Arch. Ital. Urol. Androl. 2016, 88, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Samlowski, W.E.; Grossman, D.; Bruggers, C.S.; Harris, R.M.; Zone, J.J.; Noyes, R.D.; Bowen, G.M.; Leachman, S.A. Metastatic melanoma in pregnancy: Risk of transplacental metastases in the infant. J. Clin. Oncol. 2003, 21, 2179–2186, Erratum in J. Clin. Oncol. 2010, 28, 3670. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Su, Y.C. Multiple bone metastases from an amelanotic melanoma of unknown primary origin. Kaohsiung J. Med. Sci. 2019, 35, 125–126. [Google Scholar] [CrossRef]
- Kakutani, K.; Doita, M.; Nishida, K.; Miyamoto, H.; Kurosaka, M. Radiculopathy due to malignant melanoma in the sacrum with unknown primary site. Eur. Spine J. 2008, 17 (Suppl. S2), S271–S274. [Google Scholar] [CrossRef]
- Mathew, C.J.; Karim, F.; Ali, M.; Katsigiorgis, G.; Lerman, V.; Avanesov, K. Primary Malignant Melanoma of the Lumbar Spine. Austin J. Orthop. Rheumatol. 2017, 4, 1047. [Google Scholar]
- Tang, S.; Zuo, J.; Zhang, H.; Wu, Z.; Liang, B. Spinal Metastatic Melanoma with Unknown Primary Lesions Presenting as Radiculopathy: Case Report and Literature Review. World Neurosurg. 2020, 140, 320–324. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kikuchi, K.; Kikuyama, T.; Saito, G.; Adachi, T.; Watanabe, A.; Tsunashima, H.; Tsujikawa, T.; Sato, K.; Doi, S. Disseminated Bone Marrow Carcinomatosis Due to Malignant Melanoma of Unknown Primary Origin. Intern. Med. 2021, 60, 3469–3472. [Google Scholar] [CrossRef]
- Suzuki, T.; Kusumoto, S.; Iida, S.; Tada, T.; Mori, F. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: A case report and review of the literature. Intern. Med. 2014, 53, 325–328. [Google Scholar] [CrossRef]
- Andrianandrasana, N.O.T.F.; Randrianarisoa, R.M.F.; Navoly, P.; Ranaivoson, M.A.C.; Vololontiana, H.M.D.; Rafaramino, F. Melanoma of unknown primary origin with skeletal muscle metastasis: A case report. J. Med. Case Rep. 2023, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.; Mercieca, N.; Calleja-Agius, J.; Abela, R. Metastatic malignant melanoma of unknown primary in temporalis muscle. J. Surg. Case Rep. 2020, 2020, rjaa202. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, M.; Goh, M.X.; AlshiekhAli, Z.; Howlett, D. Metastatic melanoma of unknown primary in the temporalis muscle. BMJ Case Rep. 2017, 2017, bcr2017221577. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Pigozzo, J.; di Maggio, A.; Tosi, A.L.; Sileni, V.C.; Rossi, C.R. Neoadjuvant treatment with dabrafenib of unresectable localizations from occult melanoma. Melanoma Res. 2014, 24, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, K.R.; Seo, B.K.; Woo, O.H.; Lee, J.H.; Cho, S.B. Radiologic findings of metastatic malignant melanoma of the breast: Mammographic, sonographic, dynamic contrast-enhanced breast MRI, and 18F-FDG PET-CT features. Iran. J. Radiol. 2017, 14, e38392. [Google Scholar] [CrossRef]
- Gorris, S.; Perdaens, C.; Delvaux, V.; Poorten, V.V.; Neyns, B.; Baron, I. A Solitary Melanoma Metastasis Confined to the Submandibular Gland. Case Rep. Oncol. 2021, 14, 957–962. [Google Scholar] [CrossRef]
- Scott, J.F.; Thompson, C.L.; Vyas, R.; Honda, K.; Zender, C.; Rezaee, R.; Lavertu, P.; Koon, H.; Cooper, K.D.; Gerstenblith, M.R. Parotid melanoma of unknown primary. J. Cancer Res. Clin. Oncol. 2016, 142, 1529–1537. [Google Scholar] [CrossRef]
- Drouet, C.; Morel, O.; Boulahdour, H. Bilateral Huge Incidentalomas of Isolated Adrenal Metastases From Unknown Primary Melanoma Revealed by 18F-FDG PET/CT. Clin. Nucl. Med. 2017, 42, e51–e53. [Google Scholar] [CrossRef]
- Blanco, R.; Villar, D.R.; Fernández-Pello, S.; Baldissera, J.V.; Diaz, B.; Venta, V.; Menéndez, C.L. Massive bilateral adrenal metastatic melanoma of occult origin: A case report. Anal. Quant. Cytopathol. Histpathol. 2014, 36, 51–54. [Google Scholar]
- Rieth, J.M.; Bowen, R.C.; Milhem, M.M.; Boldt, H.C.; Binkley, E.M. Presumed Melanoma of Unknown Primary Origin Metastatic to the Choroid Mimics Primary Uveal Melanoma. Case Rep. Ophthalmol. 2021, 12, 987–993. [Google Scholar] [CrossRef]
- Mondragón, J.D.; Jiménez-Zarazúa, O.; Vélez-Ramírez, L.N.; Martínez-Rivera, M.A.; Enríquez-Maciel, S.; González-Guzmán, J.; Alvarez-Delgado, M.M.; González-Carrillo, P.L. Paraneoplastic opsoclonus-myoclonus syndrome secondary to melanoma metastasis form occult primary cancer. Case Rep. Neurol. 2019, 11, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Navajas, E.V.; Sarraf, D. Paraneoplastic Vitelliform Maculopathy Associated with Metastatic Melanoma. Retin. Cases Brief Rep. 2018, 12 (Suppl. S1), S102–S104. [Google Scholar] [CrossRef] [PubMed]
- Spoto, S.; Crescenzi, A.; Taffon, C.; Ciccozzi, M.; Costantino, S.; Angeletti, S. Where Did It Start? Subcutaneous Metastatic Melanoma. Am. J. Med. 2018, 131, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.T.; Hsu, C.H.; Chang, D.H. Management of advanced stage IV melanoma of unknown primary origin with multiple visceral metastases. Asian J. Surg. 2024, 47, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Myrou, A.; Aslanidis, T.; Protopapas, A.; Psoma, E.; Kontosis, A.; Koletsa, T. Primary gastric melanoma of unknown origin: A case report and short literature review. Folia Med. (Plovdiv) 2021, 63, 282–286. [Google Scholar] [CrossRef]
- Cortellini, F.; Marasco, G.; Renzulli, M.; Vasuri, F.; Ricciardiello, L. Gastric Melanoma of Unknown Primary. J. Gastrointest. Liver Dis. 2021, 30, 14. [Google Scholar] [CrossRef]
- Mui, M.; Pham, T. Ileocaecal intussusception due to melanoma from an unknown primary. ANZ J. Surg. 2019, 89, E584–E585. [Google Scholar] [CrossRef]
- De Monti, M.; Pacchiarini, L.; Cestaro, G.; Peloni, G.; Fasolini, F. Small bowel intussusception due to malignant melanoma: Primary lesion or metastases? A case report. G. Chir. 2018, 39, 184–187. [Google Scholar]
- Stagnitti, F.; Orsini, S.; Martellucci, A.; Tudisco, A.; Avallone, M.; Aiuti, F.; Di Girolamo, V.; Stefanelli, F.; De Angelis, F.; Di Grazia, C.; et al. Small bowel intussussception due to metastatic melanoma of unknown primary site. Case report. G. Chir. 2014, 35, 246–249. [Google Scholar] [CrossRef]
- Mremi, A.; Goodluck, G.; Sadiq, A.; Lodhia, J. Metastatic malignant melanoma of unknown primary site to the brain: A case report. Int. J. Surg. Case Rep. 2021, 86, 106311. [Google Scholar] [CrossRef]
- Takagi, Y.; Yamada, H.; Ebara, H.; Hayashi, H.; Kidani, S.; Okamoto, S.; Toyooka, K.; Nanpo, K.; Kitano, Y.; Terahata, S.; et al. Thoracic extradural malignant melanoma with unknown primary. BJR Case Rep. 2020, 6, 20200042. [Google Scholar] [CrossRef] [PubMed]
- Agosto-Arroyo, E.; Rosa, M.; Chau, A.; Khazai, L. Concurrent BRAF and PTEN mutations in melanoma of unknown origin presenting as a breast mass. SAGE Open Med. Case Rep. 2017, 5, 2050313X17711064. [Google Scholar] [CrossRef] [PubMed]
- El-Tani, Z.; Duc, C.; Gluecker, T.; Cottier, O. Intramammary metastatic melanoma of unknown primary origin in a 58-year old patient: A case report. J. Med. Case Rep. 2016, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Tchernev, G.; Chokoeva, A.; Popova, L.V. Primary Solitary Melanoma of the Lymphatic Nodes Or a Single Metastasis of Unknown Melanoma: Do We Need a New Staging System? Open Access Maced. J. Med. Sci. 2017, 5, 970–973. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Verleye, L.; Devos, C.; Thiry, N.; Silversmit, G.; Van Damme, N.; De Gendt, C.; Hulstaert, F.; Neyt, M. Survival of patients with unfavorable prognosis cutaneous melanoma with increased use of immunotherapy agents: A population-based study in Belgium. Int. J. Dermatol. 2024, 63, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Verver, D.; Grünhagen, D.J.; van Akkooi, A.C.J.; Aarts, M.J.B.; Berkmortel, F.W.P.J.v.D.; Eertwegh, A.J.M.v.D.; de Groot, J.W.B.; Boers-Sonderen, M.J.; Haanen, J.B.A.G.; Hospers, G.A.P.; et al. Clinical outcome of patients with metastatic melanoma of unknown primary in the era of novel therapy. Cancer Immunol. Immunother. 2021, 70, 3123–3135. [Google Scholar] [CrossRef]
- Rassy, E.; Boussios, S.; Chebly, A.; Farra, C.; Kattan, J.; Pavlidis, N. Comparative genomic characterization of melanoma of known and unknown primary. Clin. Transl. Oncol. 2021, 23, 2302–2308. [Google Scholar] [CrossRef]
- Bae, J.M.; Choi, Y.Y.; Kim, D.S.; Lee, J.H.; Jang, H.S.; Lee, J.H.; Kim, H.; Oh, B.H.; Roh, M.R.; Nam, K.A.; et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: A systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 2015, 72, 59–70. [Google Scholar] [CrossRef]
- De Andrade, J.P.; Wong, P.; O’leary, M.P.; Parekh, V.; Amini, A.; Schoellhammer, H.F.; Margolin, K.A.; Afkhami, M.; Melstrom, L.G. Multidisciplinary Care for Melanoma of Unknown Primary: Experience in the Era of Molecular Profiling. Ann. Surg. Oncol. 2020, 27, 5240–5247. [Google Scholar] [CrossRef]
- Van Beek, E.J.A.H.; Balm, A.J.M.; Nieweg, O.E.; Hamming-Vrieze, O.; Lohuis, P.J.F.M.; Klop, W.M.C. Treatment of Regional Metastatic Melanoma of Unknown Primary Origin. Cancers 2015, 7, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Verver, D.; van der Veldt, A.; van Akkooi, A.; Verhoef, C.; Grünhagen, D.J.; Louwman, W.J. Treatment of melanoma of unknown primary in the era of immunotherapy and targeted therapy: A Dutch population-based study. Int. J. Cancer 2020, 146, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Utter, K.; Goldman, C.; Weiss, S.A.; Shapiro, R.L.; Berman, R.S.; Wilson, M.A.; Pavlick, A.C.; Osman, I. Treatment Outcomes for Metastatic Melanoma of Unknown Primary in the New Era: A Single-Institution Study and Review of the Literature. Oncology 2017, 93, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Chatzipantazi, M.; Schröter, U.; Stockfleth, E.; Gedik, C. Patients with melanoma of unknown primary show better outcome under immune checkpoint inhibitor therapy than patients with known primary: Preliminary results. Oncoimmunology 2019, 8, e1677139. [Google Scholar] [CrossRef]
- Patel, S.P.; Patel, S.P.; Othus, M.; Othus, M.; Chen, Y.; Chen, Y.; Wright, G.P.; Wright, G.P.; Yost, K.J.; Yost, K.J.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordeanu-Diaconescu, E.-M.; Cretu, A.; Grosu-Bularda, A.; Andrei, M.-C.; Hodea, F.-V.; Dumitru, C.-S.; Enache, V.; Creanga, C.-A.; Lascar, I.; Hariga, C.-S. Comprehensive Literature Review on Melanoma of Unknown Primary Site Triggered by an Intriguing Case Report. Diagnostics 2024, 14, 2210. https://doi.org/10.3390/diagnostics14192210
Bordeanu-Diaconescu E-M, Cretu A, Grosu-Bularda A, Andrei M-C, Hodea F-V, Dumitru C-S, Enache V, Creanga C-A, Lascar I, Hariga C-S. Comprehensive Literature Review on Melanoma of Unknown Primary Site Triggered by an Intriguing Case Report. Diagnostics. 2024; 14(19):2210. https://doi.org/10.3390/diagnostics14192210
Chicago/Turabian StyleBordeanu-Diaconescu, Eliza-Maria, Andrei Cretu, Andreea Grosu-Bularda, Mihaela-Cristina Andrei, Florin-Vlad Hodea, Catalina-Stefania Dumitru, Valentin Enache, Cosmin-Antoniu Creanga, Ioan Lascar, and Cristian-Sorin Hariga. 2024. "Comprehensive Literature Review on Melanoma of Unknown Primary Site Triggered by an Intriguing Case Report" Diagnostics 14, no. 19: 2210. https://doi.org/10.3390/diagnostics14192210
APA StyleBordeanu-Diaconescu, E.-M., Cretu, A., Grosu-Bularda, A., Andrei, M.-C., Hodea, F.-V., Dumitru, C.-S., Enache, V., Creanga, C.-A., Lascar, I., & Hariga, C.-S. (2024). Comprehensive Literature Review on Melanoma of Unknown Primary Site Triggered by an Intriguing Case Report. Diagnostics, 14(19), 2210. https://doi.org/10.3390/diagnostics14192210






