Distribution of Immunomodulation, Protection and Regeneration Factors in Cleft-Affected Bone and Cartilage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Patients and Controls
2.2. Tissue Preparation, Routine Staining and Immunohistochemistry
2.3. Statistical Methods
3. Results
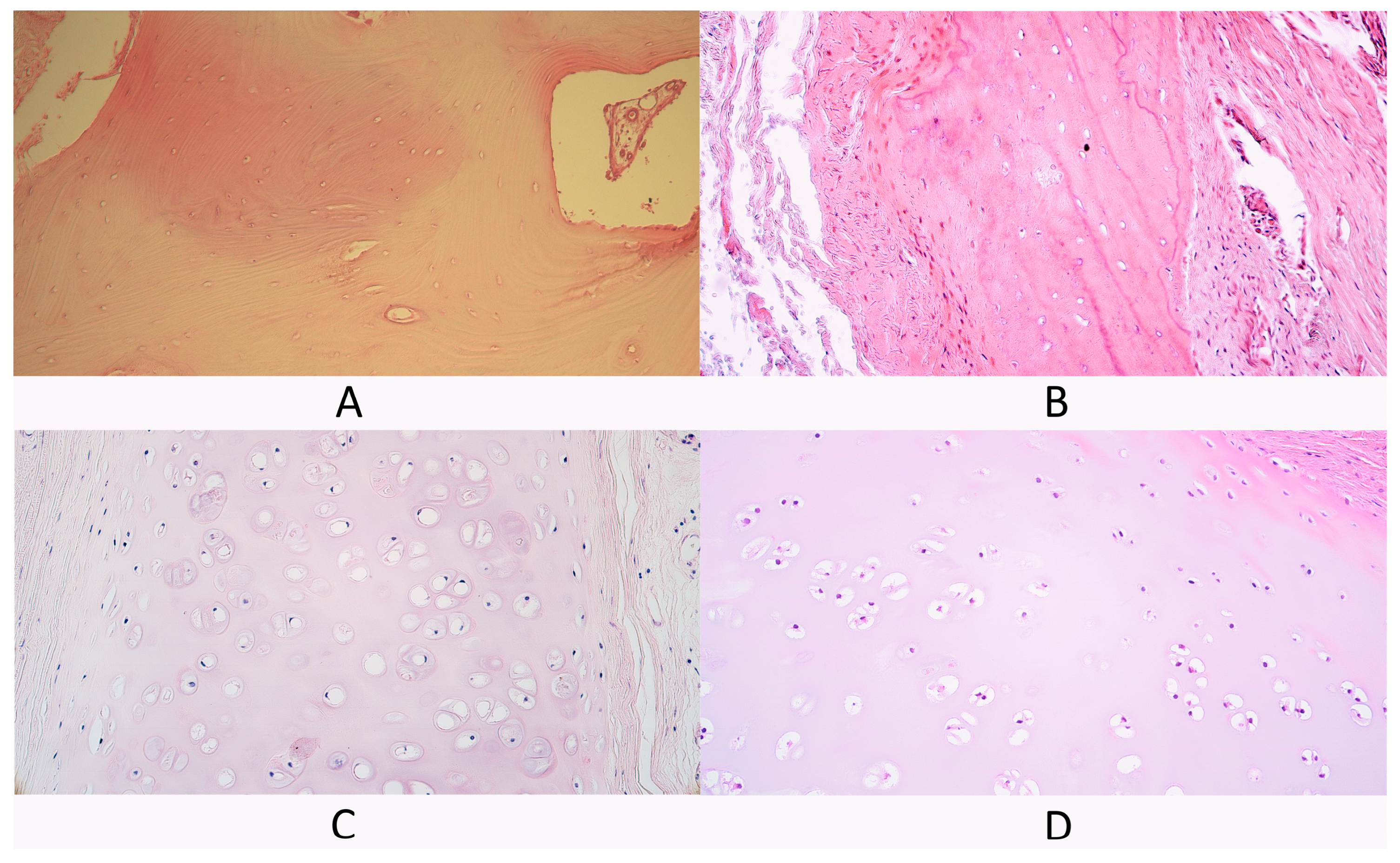
3.1. Routine Hematoxylin and Eosin Staining
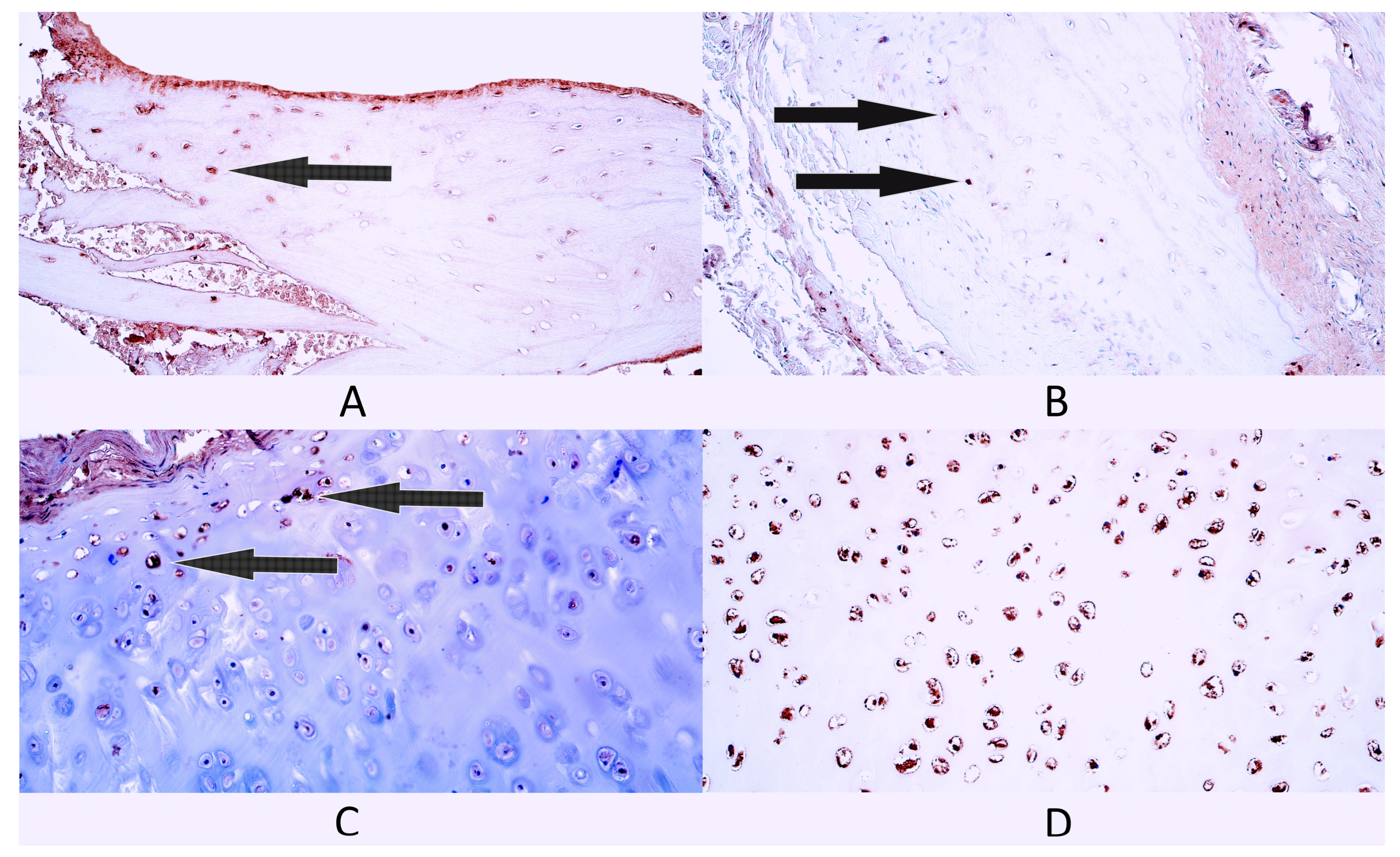
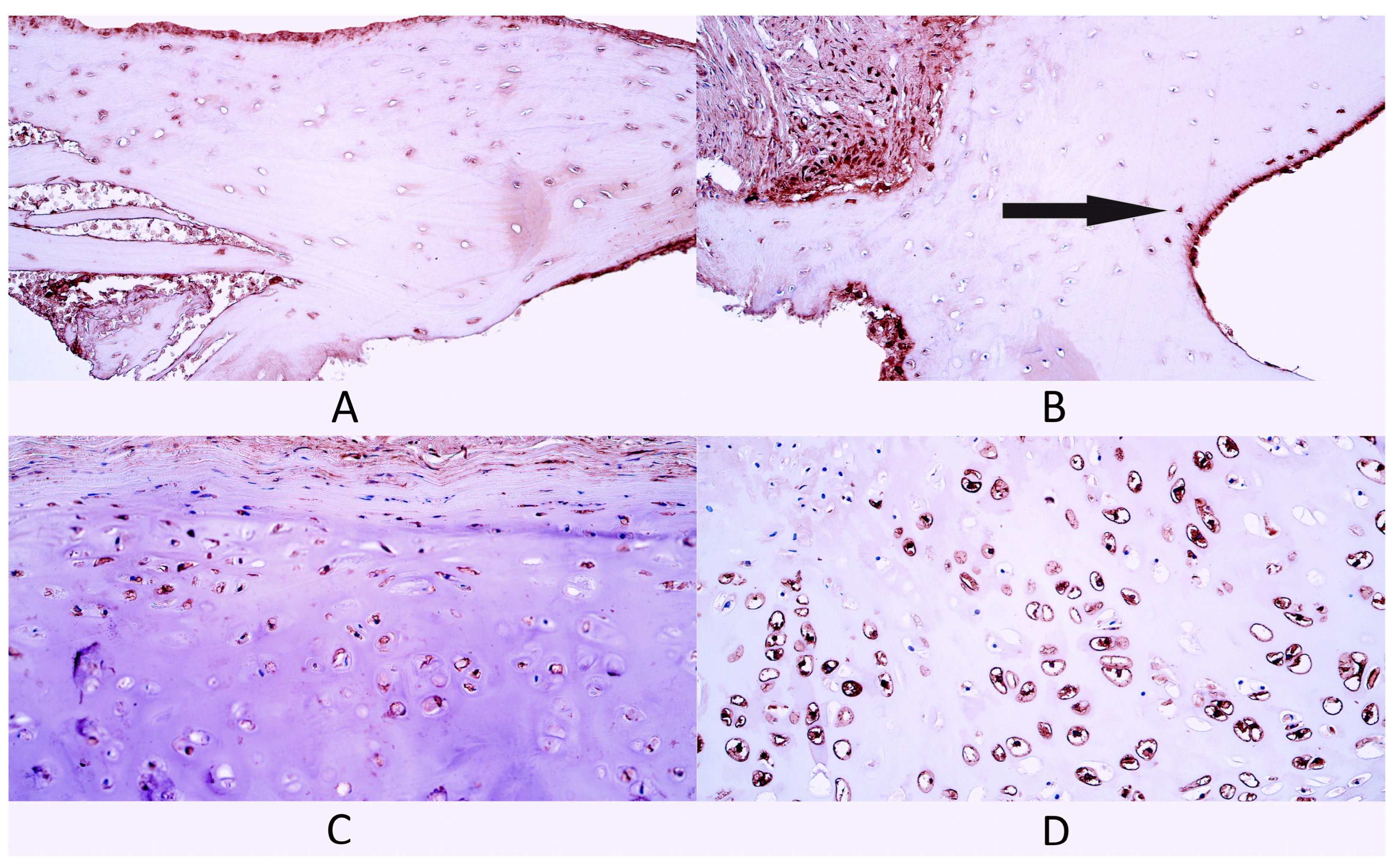
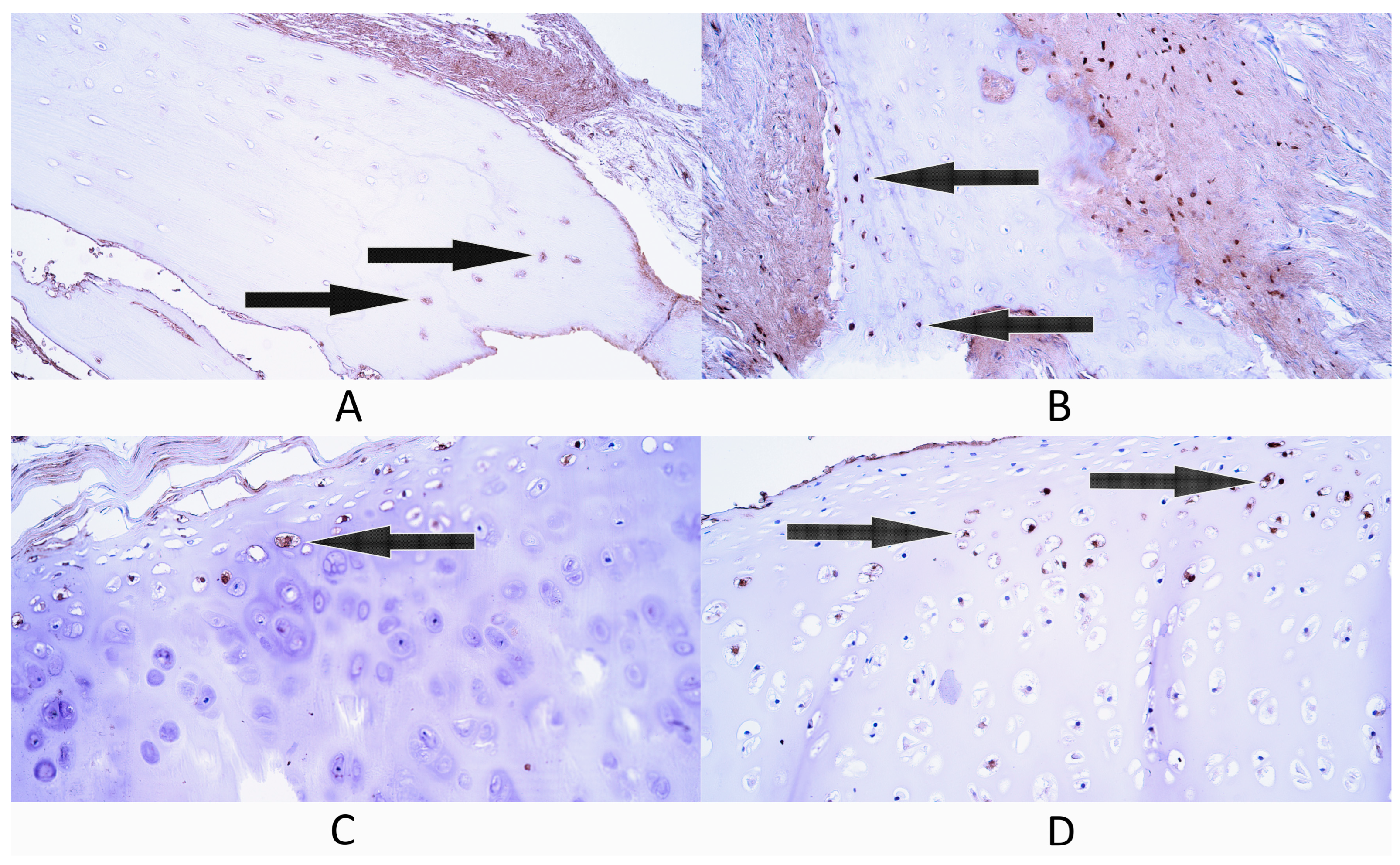
3.2. Immunohistochemistry
3.2.1. Gal-10
3.2.2. NF-κB p65
3.2.3. HSP60
3.2.4. HSP70
3.2.5. LL-37
3.2.6. Col-I
3.2.7. BMP-2/4
3.3. Correlations
4. Discussion
4.1. Immunohistochemistry Evaluation
4.2. Correlation Analysis
4.3. Limitations of the Study
5. Conclusions
- The statistically significant increase in HSP70-positive chondrocytes in cleft-affected cartilage could indicate that HSP70 could provide protective action in cleft-affected cartilage against stressors like inflammation possibly by enhancing the production of anti-inflammatory factors within the cartilage due to the presence of orofacial cleft and disrupted tissue homeostasis.
- The statistically significant increase in Col-I-positive osteocytes in cleft-affected bone tissue could indicate increased osteocyte functional activity, which might be explained by increased bone remodeling capacity due to the presence of a tissue defect caused by the orofacial cleft.
- Within evaluated bone tissue groups, multiple statistically significant positive correlations were detected in the bone tissue control group, mainly involving Gal-10, BMP-2/4 and Col-I, while cleft-affected bone tissue had a single statistically significant negative correlation between the number of Gal-10- and Col-I-positive osteocytes, indicating notable differences in factor activity and interactions between the evaluated bone tissue groups. Similarly, differences in statistically significant correlations between factor-positive cells were found in cleft tissue groups—within hyaline cartilage controls, negative correlations were detected involving heat shock proteins, while cleft-affected cartilage had positive correlations, which could mean that molecular interactions between the evaluated factors in orofacial supportive tissue are changed due to the presence of orofacial cleft.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vyas, T.; Gupta, P.; Kumar, S.; Gupta, R.; Gupta, T.; Singh, H. Cleft of Lip and Palate: A Review. J. Fam. Med. Prim. Care 2020, 9, 2621. [Google Scholar] [CrossRef]
- Smarius, B.; Loozen, C.; Manten, W.; Bekker, M.; Pistorius, L.; Breugem, C. Accurate Diagnosis of Prenatal Cleft Lip/Palate by Understanding the Embryology. World J. Methodol. 2017, 7, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Farber, S.J.; Maliha, S.G.; Gonchar, M.N.; Kantar, R.S.; Shetye, P.R.; Flores, R.L. Effect on Facial Growth of the Management of Cleft Lip and Palate. Ann. Plast. Surg. 2019, 83, e72–e76. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Losee, J.E. The Impact of Cleft Lip and Palate Repair on Maxillofacial Growth. Int. J. Oral Sci. 2015, 7, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Su, J. A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research. Molecules 2018, 23, 2931. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; Wang, H.; Silva, T.P.; Imoto, Y.; Fujieda, S.; Fukuchi, M.; Miyabe, Y.; Hirokawa, M.; Ueki, S.; Weller, P.F. Galectin-10, the Protein That Forms Charcot-Leyden Crystals, Is Not Stored in Granules but Resides in the Peripheral Cytoplasm of Human Eosinophils. J. Leukoc. Biol. 2020, 108, 139–149. [Google Scholar] [CrossRef]
- Pichler, K.M.; Weinmann, D.; Schmidt, S.; Kubista, B.; Lass, R.; Martelanz, L.; Alphonsus, J.; Windhager, R.; Gabius, H.-J.; Toegel, S. The Dysregulated Galectin Network Activates NF-ΚB to Induce Disease Markers and Matrix Degeneration in 3D Pellet Cultures of Osteoarthritic Chondrocytes. Calcif. Tissue Int. 2021, 108, 377–390. [Google Scholar] [CrossRef]
- Marsich, E.; Mozetic, P.; Ortolani, F.; Contin, M.; Marchini, M.; Vetere, A.; Pacor, S.; Semeraro, S.; Vittur, F.; Paoletti, S. Galectin-1 in Cartilage: Expression, Influence on Chondrocyte Growth and Interaction with ECM Components. Matrix Biol. 2008, 27, 513–525. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-ΚB P65 and Strategies for Therapeutic Manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Parrondo, R.; de las Pozas, A.; Reiner, T.; Rai, P.; Perez-Stable, C. NF-ΚB Activation Enhances Cell Death by Antimitotic Drugs in Human Prostate Cancer Cells. Mol. Cancer 2010, 9, 182. [Google Scholar] [CrossRef]
- Boyce, B.F.; Yao, Z.; Xing, L. Functions of Nuclear Factor ΚB in Bone. Ann. N. Y. Acad. Sci. 2010, 1192, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, A.; Strait, K.; Zhang, H.; Nanes, M.S.; Weitzmann, M.N. Endogenous TNFα Lowers Maximum Peak Bone Mass and Inhibits Osteoblastic Smad Activation through NF-ΚB. J. Bone Miner. Res. 2007, 22, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Hirata, M.; Saito, T.; Ito, S.; Hosaka, Y.; Chung, U.-I.; Kawaguchi, H. NF-ΚB Family Member RELA/P65 in Chondrocytes Controls Skeletal Growth and Osteoarthritis Development by Inhibiting Chondrocyte Apoptosis. Osteoarthr. Cartil. 2013, 21, S14. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Hsp60 Post-Translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef]
- Okamoto, T.; Yamamoto, H.; Kudo, I.; Matsumoto, K.; Odaka, M.; Grave, E.; Itoh, H. HSP60 Possesses a GTPase Activity and Mediates Protein Folding with HSP10. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Domínguez-Horta, M.d.C.; Serrano-Díaz, A.; Hernández-Cedeño, M.; Martínez-Donato, G.; Guillén-Nieto, G. A Peptide Derived from HSP60 Reduces Proinflammatory Cytokines and Soluble Mediators: A Therapeutic Approach to Inflammation. Front. Immunol. 2023, 14, 1162739. [Google Scholar] [CrossRef]
- Spierings, J.; van Eden, W. Heat Shock Proteins and Their Immunomodulatory Role in Inflammatory Arthritis. Rheumatology 2017, 56, 198–208. [Google Scholar] [CrossRef]
- Wang, F.-S.; Wu, R.-W.; Ko, J.-Y.; Tai, M.-H.; Ke, H.-C.; Yeh, D.-W.; Wu, S.-L.; Chen, M.-W. Heat Shock Protein 60 Protects Skeletal Tissue against Glucocorticoid-Induced Bone Mass Loss by Regulating Osteoblast Survival. Bone 2011, 49, 1080–1089. [Google Scholar] [CrossRef]
- Lian, W.-S.; Ko, J.-Y.; Chen, Y.-S.; Ke, H.-C.; Wu, S.-L.; Kuo, C.-W.; Wang, F.-S. Chaperonin 60 Sustains Osteoblast Autophagy and Counteracts Glucocorticoid Aggravation of Osteoporosis by Chaperoning RPTOR. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Sun, Y.-C.; Li, W.-C.; Wang, F.-S. Chaperonin 60 Regulation of SOX9 Ubiquitination Mitigates the Development of Knee Osteoarthritis. J. Mol. Med. 2016, 94, 755–769. [Google Scholar] [CrossRef]
- Kim, J.Y.; Barua, S.; Huang, M.Y.; Park, J.; Yenari, M.A.; Lee, J.E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells 2020, 9, 2020. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 Chaperone Network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, B.M.; Albinhassan, T.H.; Alzahrani, R.A.; Bouchama, A.; Mohammad, S.; Alomari, A.A.; Bin-Jumah, M.N.; AlSuhaibani, E.S.; Malik, S.S. Profiling the Hsp70 Chaperone Network in Heat-Induced Proteotoxic Stress Models of Human Neurons. Biology 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Cappucci, U.; Noro, F.; Casale, A.M.; Fanti, L.; Berloco, M.; Alagia, A.A.; Grassi, L.; Le Pera, L.; Piacentini, L.; Pimpinelli, S. The Hsp70 Chaperone Is a Major Player in Stress-Induced Transposable Element Activation. Proc. Natl. Acad. Sci. USA 2019, 116, 17943–17950. [Google Scholar] [CrossRef]
- Notsu, K.; Nakagawa, M.; Nakamura, M. Ubiquitin-like Protein MNSFβ Noncovalently Binds to Molecular Chaperone HSPA8 and Regulates Osteoclastogenesis. Mol. Cell. Biochem. 2016, 421, 149–156. [Google Scholar] [CrossRef]
- Hang, K.; Ye, C.; Chen, E.; Zhang, W.; Xue, D.; Pan, Z. Role of the Heat Shock Protein Family in Bone Metabolism. Cell Stress Chaperones 2018, 23, 1153–1164. [Google Scholar] [CrossRef]
- Guo, Y.; Stampoultzis, T.; Karami, P.; Nasrollahzadeh, N.; Rana, V.K.; Pioletti, D.P. HSP70—A Key Regulator in Chondrocyte Homeostasis under Naturally Coupled Hydrostatic Pressure-Thermal Stimuli. Osteoarthr. Cartil. 2024, 32, 896–908. [Google Scholar] [CrossRef]
- Child, D.F.; Hudson, P.R.; Hunter-Lavin, C.; Mukhergee, S.; China, S.; Williams, C.P.; Williams, J.H.H. Birth Defects and Anti–Heat Shock Protein 70 Antibodies in Early Pregnancy. Cell Stress Chaperones 2006, 11, 101. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. Little Peptide, Big Effects: The Role of LL-37 in Inflammation and Autoimmune Disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef]
- Yang, B.; Good, D.; Mosaiab, T.; Liu, W.; Ni, G.; Kaur, J.; Liu, X.; Jessop, C.; Yang, L.; Fadhil, R.; et al. Significance of LL-37 on Immunomodulation and Disease Outcome. Biomed. Res. Int. 2020, 2020, 8349712. [Google Scholar] [CrossRef]
- Chinipardaz, Z.; Zhong, J.M.; Yang, S. Regulation of LL-37 in Bone and Periodontium Regeneration. Life 2022, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, Y.; Yuan, Q.; Sun, J.; Zhuang, A.; Bi, X. Cathelicidin LL37 Promotes Osteogenic Differentiation in Vitro and Bone Regeneration in Vivo. Front. Bioeng. Biotechnol. 2021, 9, 638494. [Google Scholar] [CrossRef]
- Kittaka, M.; Shiba, H.; Kajiya, M.; Fujita, T.; Iwata, T.; Rathvisal, K.; Ouhara, K.; Takeda, K.; Fujita, T.; Komatsuzawa, H.; et al. The Antimicrobial Peptide LL37 Promotes Bone Regeneration in a Rat Calvarial Bone Defect. Peptides 2013, 46, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Kumbhakarn, S.; Sable, R.; Gondane, P.; Takle, S.; Maity, P. Peptide-Based Biomaterials for Bone and Cartilage Regeneration. Biomedicines 2024, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Najmi, Z.; Kumar, A.; Scalia, A.C.; Cochis, A.; Obradovic, B.; Grassi, F.A.; Leigheb, M.; Lamghari, M.; Loinaz, I.; Gracia, R.; et al. Evaluation of Nisin and LL-37 Antimicrobial Peptides as Tool to Preserve Articular Cartilage Healing in a Septic Environment. Front. Bioeng. Biotechnol. 2020, 8, 561. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 13329. [Google Scholar] [CrossRef]
- Brown, W.E.; Lavernia, L.; Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Human Nasal Cartilage: Functional Properties and Structure-Function Relationships for the Development of Tissue Engineering Design Criteria. Acta Biomater. 2023, 168, 113–124. [Google Scholar] [CrossRef]
- Sasano, Y.; Takahashi, I.; Zhu, J.-X.; Ohtani, H.; Mizoguchi, I.; Kagayama, M. Gene and Protein Expressions of Type I Collagen Are Regulated Tissue-Specifically in Rat Hyaline Cartilages in Vivo. Eur. J. Morphol. 2001, 39, 149–154. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-Based Biomaterials for Bone Tissue Engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Boraschi-Diaz, I.; Wang, J.; Mort, J.S.; Komarova, S.V. Collagen Type I as a Ligand for Receptor-Mediated Signaling. Front. Phys. 2017, 5, 12. [Google Scholar] [CrossRef]
- Shiflett, L.A.; Tiede-Lewis, L.M.; Xie, Y.; Lu, Y.; Ray, E.C.; Dallas, S.L. Collagen Dynamics during the Process of Osteocyte Embedding and Mineralization. Front. Cell Dev. Biol. 2019, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2022, 9, 770143. [Google Scholar] [CrossRef]
- Sasano, Y.; Furusawa, M.; Ohtani, H.; Mizoguchi, I.; Takahashi, I.; Kagayama, M. Chondrocytes Synthesize Type I Collagen and Accumulate the Protein in the Matrix during Development of Rat Tibial Articular Cartilage. Anat. Embryol. 1996, 194, 247–252. [Google Scholar] [CrossRef]
- Gagliano, N.; Carinci, F.; Moscheni, C.; Torri, C.; Pezzetti, F.; Scapoli, L.; Martinelli, M.; Gioia, M.; Stabellini, G. New Insights in Collagen Turnover in Orofacial Cleft Patients. Cleft Palate Craniofac. J. 2010, 47, 393–399. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Alarmo, E.-L.; Huhtala, H.; Korhonen, T.; Pylkkänen, L.; Holli, K.; Kuukasjärvi, T.; Parkkila, S.; Kallioniemi, A. Bone Morphogenetic Protein 4 Expression in Multiple Normal and Tumor Tissues Reveals Its Importance beyond Development. Mod. Pathol. 2013, 26, 10–21. [Google Scholar] [CrossRef]
- Cheng, H.; Gao, X.; Huard, M.; Lu, A.; Ruzbarsky, J.J.; Amra, S.; Wang, B.; Huard, J. Bone Morphogenetic Protein 4 Rescues the Bone Regenerative Potential of Old Muscle-Derived Stem Cells via Regulation of Cell Cycle Inhibitors. Stem Cell Res. Ther. 2022, 13, 1–21. [Google Scholar] [CrossRef]
- Khan, M.P.; Khan, K.; Yadav, P.S.; Singh, A.K.; Nag, A.; Prasahar, P.; Mittal, M.; China, S.P.; Tewari, M.C.; Nagar, G.K.; et al. BMP Signaling Is Required for Adult Skeletal Homeostasis and Mediates Bone Anabolic Action of Parathyroid Hormone. Bone 2016, 92, 132–144. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Swaminathan, S.; Mishina, Y.; Komatsu, Y. Enhanced BMP Signaling Leads to Enlarged Nasal Cartilage Formation in Mice. Biochem. Biophys. Res. Commun. 2023, 678, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Ringe, J.; Häupl, T.; Notter, M.; Manz, R.; Burmester, G.-R.; Sittinger, M.; Kaps, C. BMP2 Initiates Chondrogenic Lineage Development of Adult Human Mesenchymal Stem Cells in High-Density Culture. Differentiation 2003, 71, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.D.; Cooper, G.M.; Marra, K.G. Chondrogenesis, Bone Morphogenetic Protein-4 and Mesenchymal Stem Cells. Osteoarthr. Cartil. 2008, 16, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Buile, D.; Pilmane, M.; Akota, I. Appearance and Distribution of Tissue Remodellation Factors in the Hard Tissue of Patients Affected by Cleft Lip Palate. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2020, 74, 171–180. [Google Scholar] [CrossRef]
- Kauffmann, P.; Kolle, J.; Quast, A.; Wolfer, S.; Schminke, B.; Meyer-Marcotty, P.; Schliephake, H. Two-Stage Palatal Repair in Non-Syndromic CLP Patients Using Anterior to Posterior Closure Is Associated with Minimal Need for Secondary Palatal Surgery. Head Face Med. 2024, 20, 1–8. [Google Scholar] [CrossRef]
- Kommineni, N.; Reddy, C.V.; Chandra, N.; Reddy, D.S.; Kumar, A.; Reddy, M.V. Mixed Dentition Analysis—Applicability of Two Non-Radiographic Methods for Chennai School Children. J. Int. Soc. Prev. Community Dent. 2014, 4, 133. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Raine, L.; Fanger, H. The Use of Antiavidin Antibody and Avidin-Biotin-Peroxidase Complex in Immunoperoxidase Technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Pilmane, M.; Shine, J.; Iismaa, T.P. Distribution of Galanin Immunoreactivity in the Bronchi of Humans with Tuberculosis. Ann. N. Y. Acad. Sci. 1998, 863, 445–449. [Google Scholar] [CrossRef]
- Seidel, C.L.; Percivalle, E.; Tschaftari, M.; Weider, M.; Strobel, K.; Willershausen, I.; Unertl, C.; Schmetzer, H.M.; Weber, M.; Schneider, M.; et al. Orofacial Clefts Lead to Increased Pro-Inflammatory Cytokine Levels on Neonatal Oral Mucosa. Front. Immunol. 2022, 13, 1044249. [Google Scholar] [CrossRef]
- Pilmane, M.; Sidhoma, E.; Akota, I.; Kazoka, D. Characterization of Cytokines and Proliferation Marker Ki67 in Cleft Affected Lip Tissue. Medicina 2019, 55, 518. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A.K.; Iqbal, H.M.N.; Dhama, K. Heat Shock Proteins: Therapeutic Perspectives in Inflammatory Disorders. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 10, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Jacquier-Sarlin, M.R.; Fuller, K.; Dinh-Xuan, A.T.; Richard, M.-J.; Polla, B.S. Protective Effects of Hsp70 in Inflammation. Experientia 1994, 50, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Yoon, J.-H.; Kim, C.-H. HSP70 Is Associated with the Severity of Inflammation in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, e101–e106. [Google Scholar] [CrossRef]
- Sobol, D.L.; Allori, A.C.; Carlson, A.R.; Pien, I.J.; Watkins, S.E.; Aylsworth, A.S.; Meyer, R.E.; Pimenta, L.A.; Strauss, R.P.; Ramsey, B.L.; et al. Nasal Airway Dysfunction in Children with Cleft Lip and Cleft Palate: Results of a Cross-Sectional Population-Based Study, with Anatomical and Surgical Considerations. Plast. Reconstr. Surg. 2016, 138, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yamaguchi, T.; Furukawa, M. Maxillary Sinus Development and Sinusitis in Patients with Cleft Lip and Palate. Auris Nasus Larynx 2000, 27, 253–256. [Google Scholar] [CrossRef]
- Schlesinger, P.H.; Blair, H.C.; Beer Stolz, D.; Riazanski, V.; Ray, E.C.; Tourkova, I.L.; Nelson, D.J. Cellular and Extracellular Matrix of Bone, with Principles of Synthesis and Dependency of Mineral Deposition on Cell Membrane Transport. Am. J. Physiol. Cell Physiol. 2020, 318, C111–C124. [Google Scholar] [CrossRef]
- Creecy, A.; Damrath, J.G.; Wallace, J.M. Control of Bone Matrix Properties by Osteocytes. Front. Endocrinol. 2021, 11, 578477. [Google Scholar] [CrossRef]
- Martín-del-Campo, M.; Rosales-Ibañez, R.; Rojo, L. Biomaterials for Cleft Lip and Palate Regeneration. Int. J. Mol. Sci. 2019, 20, 2176. [Google Scholar] [CrossRef]
- Metzger, C.E.; Narayanan, S.A. The Role of Osteocytes in Inflammatory Bone Loss. Front. Endocrinol. 2019, 10, 285. [Google Scholar] [CrossRef]
- Fukunaga, T.; Yamashiro, T.; Oya, S.; Takeshita, N.; Takigawa, M.; Takano-Yamamoto, T. Connective Tissue Growth Factor MRNA Expression Pattern in Cartilages Is Associated with Their Type I Collagen Expression. Bone 2003, 33, 911–918. [Google Scholar] [CrossRef]
- Osório, J. Galectin-1 Damages Cartilage via Inflammation. Nat. Rev. Rheumatol. 2016, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Fantauzzi, C.B.; Pugliese, G.; Menini, S. Role of Galectin-3 in Bone Cell Differentiation, Bone Pathophysiology and Vascular Osteogenesis. Int. J. Mol. Sci. 2017, 18, 2481. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Flint, J.K.; Rezvani, G.; De Luca, F. Nuclear Factor-ΚB P65 Facilitates Longitudinal Bone Growth by Inducing Growth Plate Chondrocyte Proliferation and Differentiation and by Preventing Apoptosis. J. Biol. Chem. 2007, 282, 33698–33706. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-ΚB: A Key Role in Inflammatory Diseases. J. Clin. Invest. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.N.Y.; Choi, K.-Y. (grace); Piyadasa, H.; Bossert, M.; Uzonna, J.; Klonisch, T.; Mookherjee, N. Human Cathelicidin LL-37-Derived Peptide IG-19 Confers Protection in a Murine Model of Collagen-Induced Arthritis. Mol. Immunol. 2014, 57, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Soares, L.C.; Al-Dalahmah, O.; Hillis, J.; Young, C.C.; Asbed, I.; Sakaguchi, M.; O’Neill, E.; Szele, F.G. Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases. Cells 2021, 10, 3047. [Google Scholar] [CrossRef]
- Vinik, Y.; Shatz-Azoulay, H.; Hiram-Bab, S.; Kandel, L.; Gabet, Y.; Rivkin, G.; Zick, Y. Ablation of the Mammalian Lectin Galectin-8 Induces Bone Defects in Mice. FASEB J. 2018, 32, 2366–2380. [Google Scholar] [CrossRef]
- Tanikawa, R.; Tanikawa, T.; Hirashima, M.; Yamauchi, A.; Tanaka, Y. Galectin-9 Induces Osteoblast Differentiation through the CD44/Smad Signaling Pathway. Biochem. Biophys. Res. Commun. 2010, 394, 317–322. [Google Scholar] [CrossRef]
- Chun, J.N.; Choi, B.; Lee, K.W.; Lee, D.J.; Kang, D.H.; Lee, J.Y.; Song, I.S.; Kim, H.I.; Lee, S.-H.; Kim, H.S.; et al. Cytosolic Hsp60 Is Involved in the NF-ΚB-Dependent Survival of Cancer Cells via IKK Regulation. PLoS ONE 2010, 5, e9422. [Google Scholar] [CrossRef]
- Sato, T.; Chiba, T.; Nakahara, T.; Watanabe, K.; Sakai, S.; Noguchi, N.; Noto, M.; Ueki, S.; Kono, M. Eosinophil-Derived Galectin-10 Upregulates Matrix Metalloproteinase Expression in Bullous Pemphigoid Blisters. J. Dermatol. Sci. 2023, 112, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sunderic, K.; Nicoll, S.B.; Wang, S. Downregulation of Heat Shock Protein 70 Impairs Osteogenic and Chondrogenic Differentiation in Human Mesenchymal Stem Cells. Sci. Rep. 2018, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, C.; Hui, L.; Song, Y.; Fu, Y.; Li, M.; Yang, H.; Wu, J.; Sun, J.; Xu, W.; et al. Cathelicidins Target HSP60 to Restrict CVB3 Transmission via Disrupting the Exosome and Reducing Cardiomyocyte Apoptosis. J. Virol. 2023, 97, e0143322. [Google Scholar] [CrossRef] [PubMed]


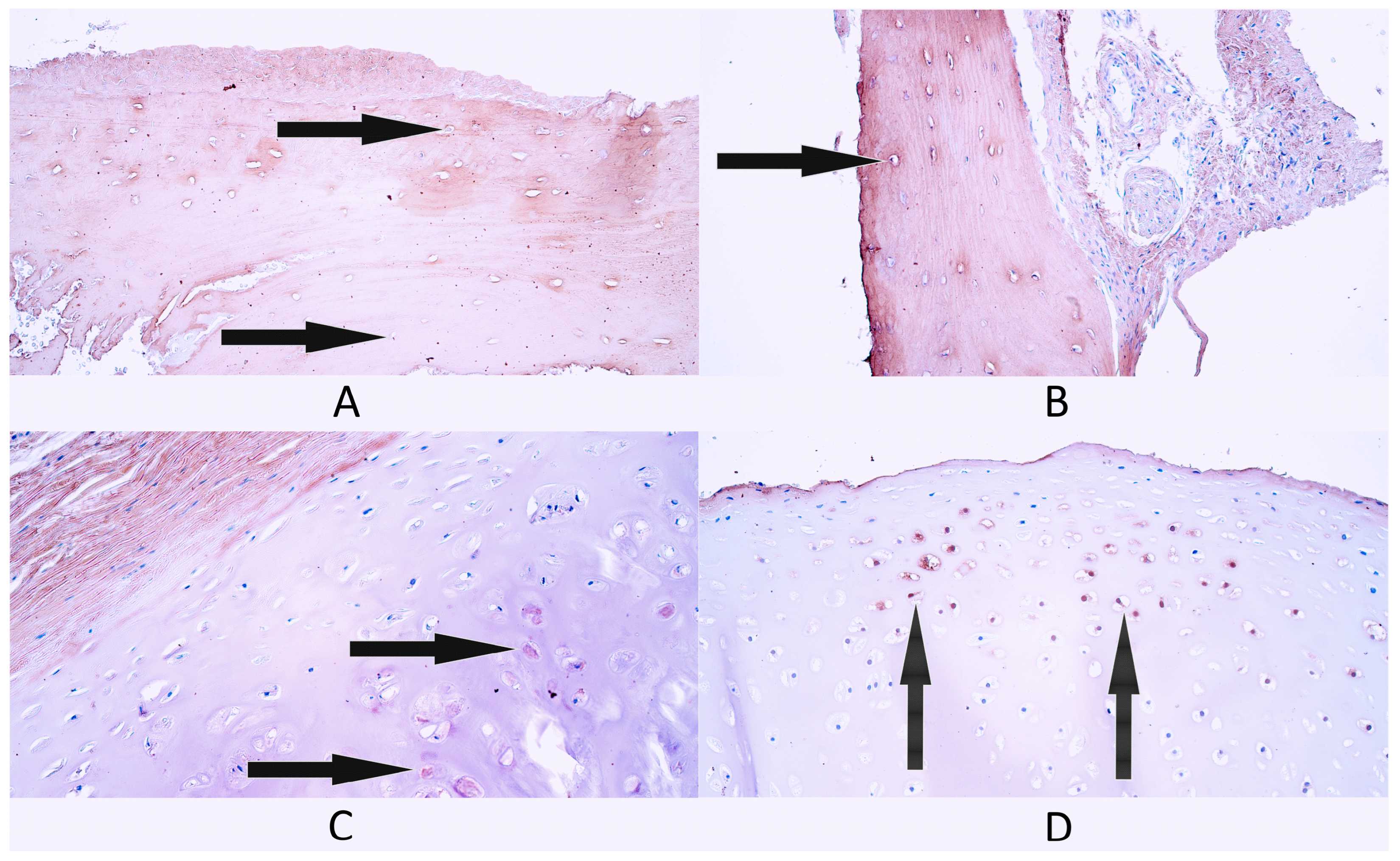
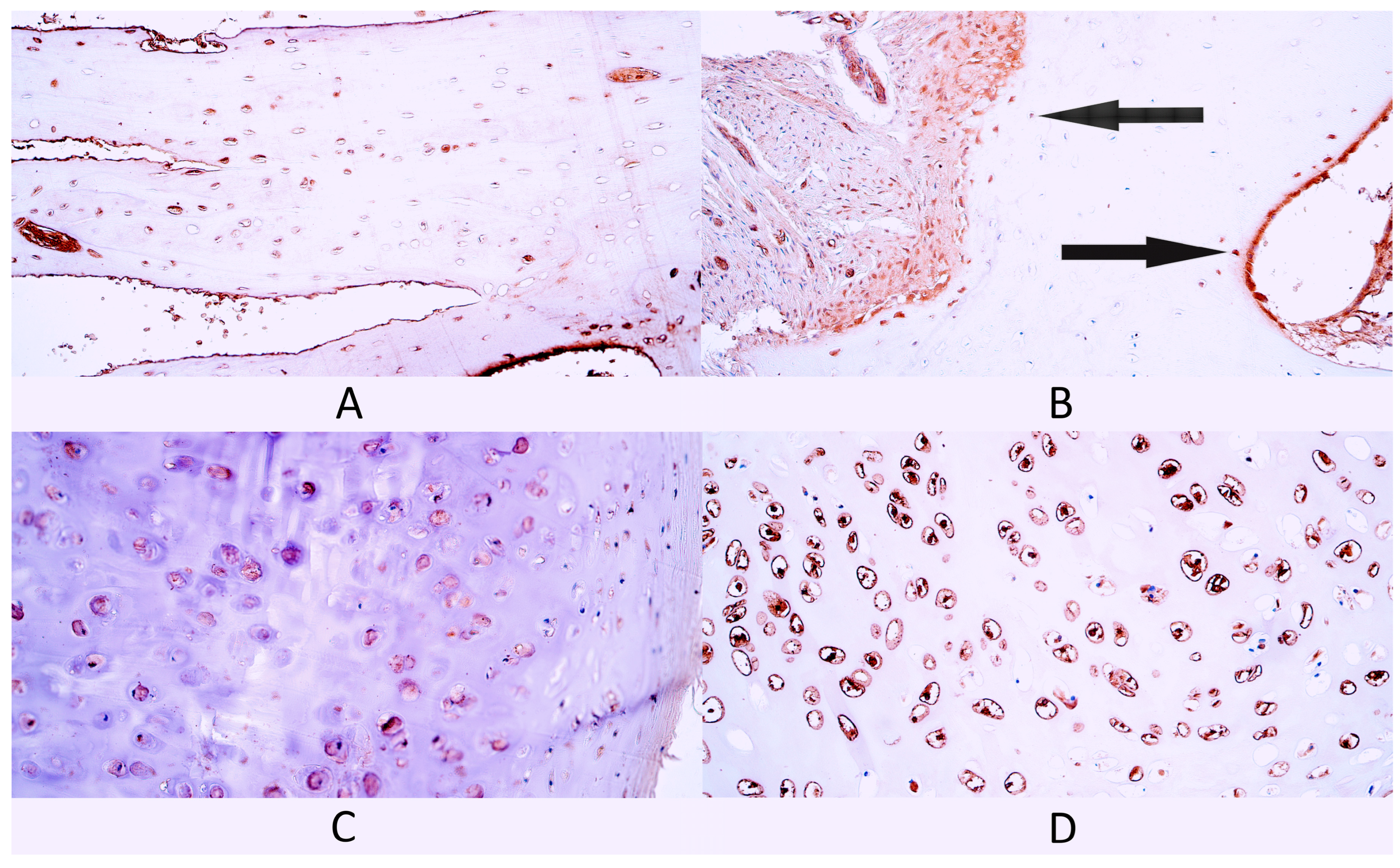
| Patient | Gal-10 | NF-κB p65 | HSP60 | HSP70 | LL-37 | Col-I | BMP-2/4 | |
|---|---|---|---|---|---|---|---|---|
| Bone controls | 1 | + | + | +/++ | ++ | 0/+ | + | ++ |
| 2 | + | 0/+ | + | + | 0/+ | +/++ | ++ | |
| 3 | 0/+ | 0/+ | + | 0/+ | 0/+ | 0/+ | + | |
| 4 | + | + | +/++ | + | 0 | + | ++ | |
| 5 | 0/+ | 0/+ | + | + | 0/+ | 0/+ | + | |
| Median | + | 0/+ | + | + | 0/+ | + | ++ | |
| Cleft- affected bone tissue | 1 | + | + | + | + | 0/+ | ++ | ++/+++ |
| 2 | +/++ | + | ++ | +/++ | + | +/++ | ++ | |
| 3 | + | + | +/++ | +/++ | 0/+ | ++ | +/++ | |
| 4 | + | +/++ | ++ | + | 0/+ | ++ | +/++ | |
| 5 | ++ | +/++ | ++ | ++ | 0/+ | + | ++/+++ | |
| Median | + | + | ++ | +/++ | 0/+ | ++ | ++ | |
| Cartilage controls | 1 | ++/+++ | ++/+++ | ++/+++ | ++ | ++ | 0/+ | +/++ |
| 2 | +/++ | ++ | +/++ | +/++ | +/++ | + | ++/+++ | |
| 3 | ++ | +/++ | +/++ | +/++ | +/++ | +/++ | ++/+++ | |
| 4 | ++ | ++ | +/++ | ++ | +/++ | + | +/++ | |
| 5 | ++ | +/++ | ++ | +/++ | +/++ | 0/+ | ++ | |
| Median | ++ | ++ | +/++ | +/++ | +/++ | + | ++ | |
| Cleft- affected hyaline cartilage | 1 | +/++ | + | +/++ | +++ | 0/+ | + | +/++ |
| 2 | +/++ | +++ | + | +++ | 0 | 0/+ | ++/+++ | |
| 3 | +++ | +++ | ++/+++ | +++ | ++ | +/++ | +++/++++ | |
| 4 | ++ | +++ | ++ | ++/+++ | + | 0/+ | +++ | |
| 5 | +/++ | +++ | ++ | ++/+++ | + | 0/+ | ++ | |
| Median | +/++ | +++ | ++ | +++ | + | 0/+ | ++/+++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaivads, M.; Pilmane, M. Distribution of Immunomodulation, Protection and Regeneration Factors in Cleft-Affected Bone and Cartilage. Diagnostics 2024, 14, 2217. https://doi.org/10.3390/diagnostics14192217
Vaivads M, Pilmane M. Distribution of Immunomodulation, Protection and Regeneration Factors in Cleft-Affected Bone and Cartilage. Diagnostics. 2024; 14(19):2217. https://doi.org/10.3390/diagnostics14192217
Chicago/Turabian StyleVaivads, Mārtiņš, and Māra Pilmane. 2024. "Distribution of Immunomodulation, Protection and Regeneration Factors in Cleft-Affected Bone and Cartilage" Diagnostics 14, no. 19: 2217. https://doi.org/10.3390/diagnostics14192217







