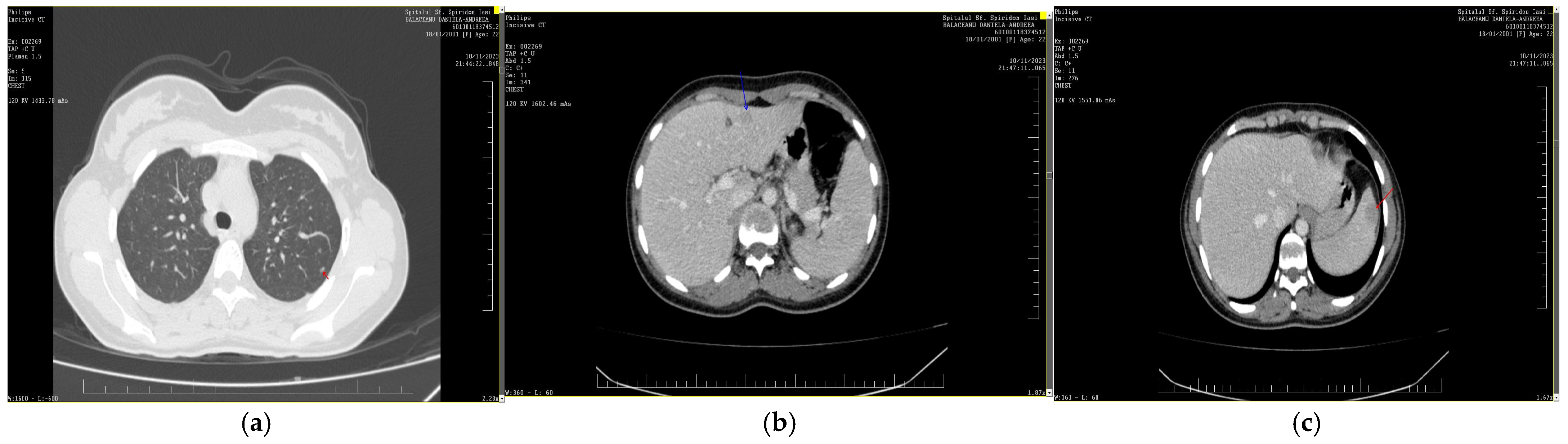
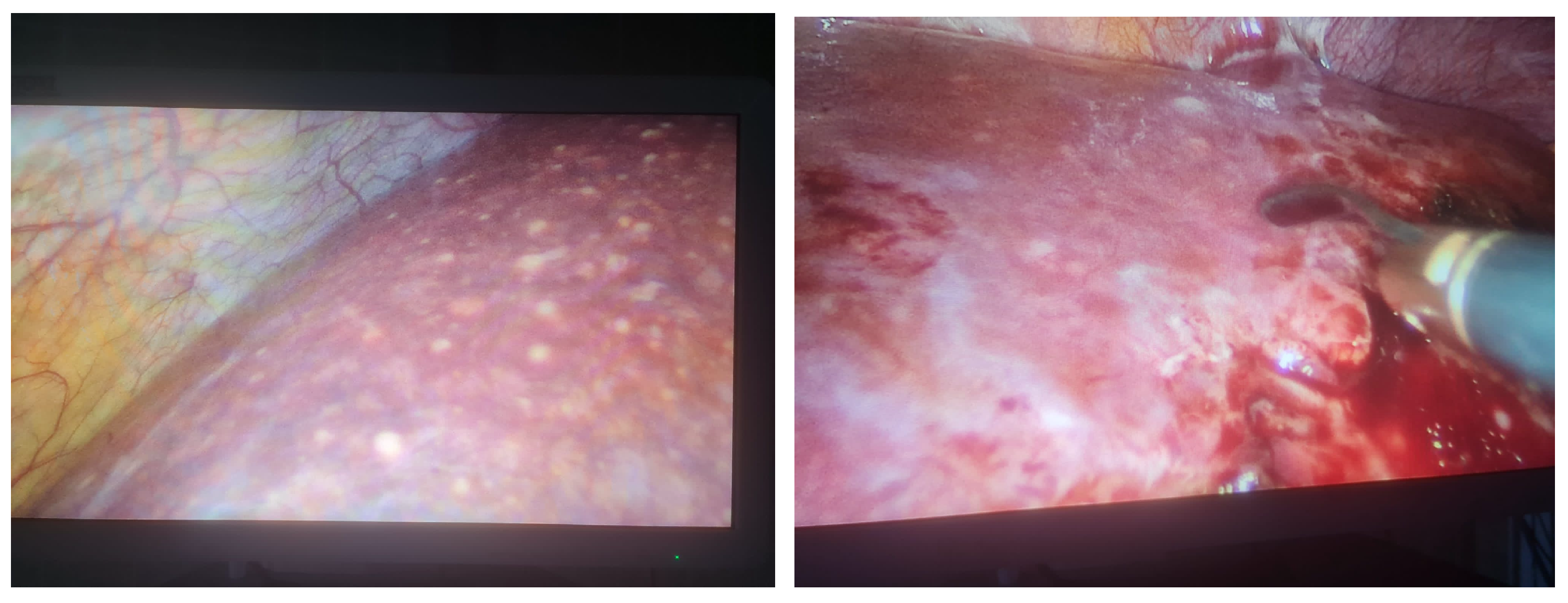
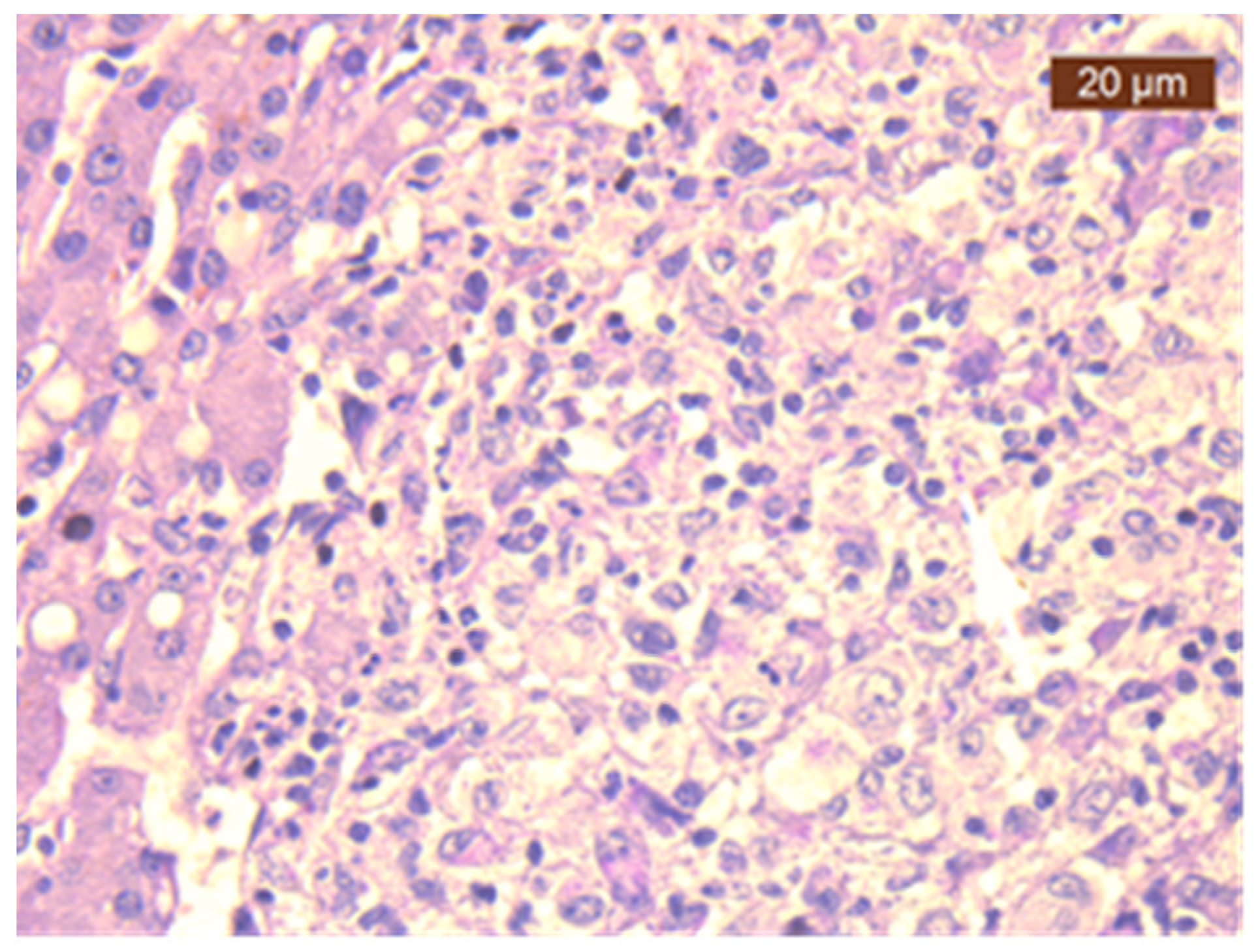
Disseminated Histoplasmosis Diagnosed in an Immunocompetent Patient from a Non-Endemic Area: Neglected or Emerging Disease?
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Darling, S.T. A protozoön general infection producing pseudotubercles in the lungs and focal necroses in the liver, spleen and lymphnodes. J. Am. Med. Assoc. XLVI 1906, 17, 1283–1285. [Google Scholar] [CrossRef]
- Vite-Garín, T.; Estrada-Bárcenas, D.A.; Cifuentes, J.; Taylor, M.L. The importance of molecular analyses for understanding the genetic diversity of Histoplasma capsulatum: An overview. Rev. Iberoam. Micol. 2014, 31, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Elalouf, A. Infections after organ transplantation and immune response’. Transplant. Immunol. 2023, 77, 101798. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Ponce, M.G.; Gendlina, I.; Nosanchuk, J.D. Histoplasma Capsulatum: Mechanisms for Pathogenesis. Curr. Top. Microbiol. Immunol. 2019, 422, 157–191. [Google Scholar]
- Myint, T.; Leedy, N.; Villacorta Cari, E.; Wheat, L.J. HIV-Associated Histoplasmosis: Current Perspectives. HIV AIDS 2020, 12, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, R.; Mazzoni, A.; Nanetti, A.; Chiodo, F. Histoplasmosis capsulati and duboisii in Europe: The impact of the HIV pandemic, travel and immigration. Eur. J. Epidemiol. 1994, 10, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannis, D.; Di Lorenzo, A.; Zaçe, D.; Benvenuto, D.; Moccione, M.; Muratore, G.; Giacalone, M.L.; Montagnari, G.; Carnevale, L.; Mulas, T.; et al. Histoplasmosis in patients living with HIV in Europe: Review of literature. Front. Microbiol. 2024, 15, 1418530. [Google Scholar] [CrossRef] [PubMed]
- Adenis, A.A.; Aznar, C.; Couppié, P. Histoplasmosis in HIV-Infected Patients: A Review of New Developments and Remaining Gaps. Curr. Trop. Med. Rep. 2014, 1, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Araúz, A.B.; Papineni, P. Histoplasmosis. Infect. Dis. Clin. N. Am. 2021, 35, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.; Benzamin Md Nahar, L.; Rana, M.; Aishy, A.S. Hepatic histoplasmosis: An update. J. Clin. Transl. Hepatol. 2022, 10, 726–729. [Google Scholar] [CrossRef]
- Akram, S.M.; Koirala, J. Histoplasmosis; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- White, P.L. Developments in Fungal Serology. Curr. Fungal Infect. Rep. 2023, 1, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.; Wheat, J.L.; Hage, C. Pulmonary Histoplasmosis: A Clinical Update. J. Fungi 2023, 9, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Paes, R.; Bernardes-Engemann, A.R.; da Silva Motta, B.; Pizzini, C.V.; de Abreu Almeida, M.; de Medeiros Muniz, M.; Dias, R.A.B.; Zancopé-Oliveira, R.M. Immunologic diagnosis of endemic mycoses. J. Fungi 2022, 8, 993. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A. Histoplasmosis: A clinical and laboratory update. Clin. Microbiol. Rev. 2007, 20, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.M.; Hage, C.A. Laboratory Diagnostics for Histoplasmosis. J. Clin. Microbiol. 2017, 55, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Mazi, P.B.; Arnold, S.R.; Baddley, J.W.; Bahr, N.C.; Beekmann, S.E.; McCarty, T.P.; Polgreen, P.M.; Rauseo, A.M.; Spec, A. Management of Histoplasmosis by Infectious Disease Physicians. Open Forum Infect. Dis. 2022, 9, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciortescu, I.; Nemteanu, R.; Chiriac, I.M.; Zaharia, S.; Coseru, A.I.; Dumitrascu, D.L.; Vasilescu, A.; Danciu, M.; Ochisor, C.; Plesa, A. Disseminated Histoplasmosis Diagnosed in an Immunocompetent Patient from a Non-Endemic Area: Neglected or Emerging Disease? Diagnostics 2024, 14, 2219. https://doi.org/10.3390/diagnostics14192219
Ciortescu I, Nemteanu R, Chiriac IM, Zaharia S, Coseru AI, Dumitrascu DL, Vasilescu A, Danciu M, Ochisor C, Plesa A. Disseminated Histoplasmosis Diagnosed in an Immunocompetent Patient from a Non-Endemic Area: Neglected or Emerging Disease? Diagnostics. 2024; 14(19):2219. https://doi.org/10.3390/diagnostics14192219
Chicago/Turabian StyleCiortescu, Irina, Roxana Nemteanu, Ilinca Maria Chiriac, Silvia Zaharia, Alexandru Ionut Coseru, Diana Lacramioara Dumitrascu, Alin Vasilescu, Mihai Danciu, Catalina Ochisor, and Alina Plesa. 2024. "Disseminated Histoplasmosis Diagnosed in an Immunocompetent Patient from a Non-Endemic Area: Neglected or Emerging Disease?" Diagnostics 14, no. 19: 2219. https://doi.org/10.3390/diagnostics14192219
APA StyleCiortescu, I., Nemteanu, R., Chiriac, I. M., Zaharia, S., Coseru, A. I., Dumitrascu, D. L., Vasilescu, A., Danciu, M., Ochisor, C., & Plesa, A. (2024). Disseminated Histoplasmosis Diagnosed in an Immunocompetent Patient from a Non-Endemic Area: Neglected or Emerging Disease? Diagnostics, 14(19), 2219. https://doi.org/10.3390/diagnostics14192219




