Superscan Pattern on Bone Scintigraphy: A Comprehensive Review
Abstract
1. Introduction
2. Definition
3. History and Prevalence
4. Diagnostic Challenges
5. False-Negative Interpretations
6. Elucidating the Mechanisms Underlying a Superscan Pattern on Bone Scintigraphy
7. Etiologies
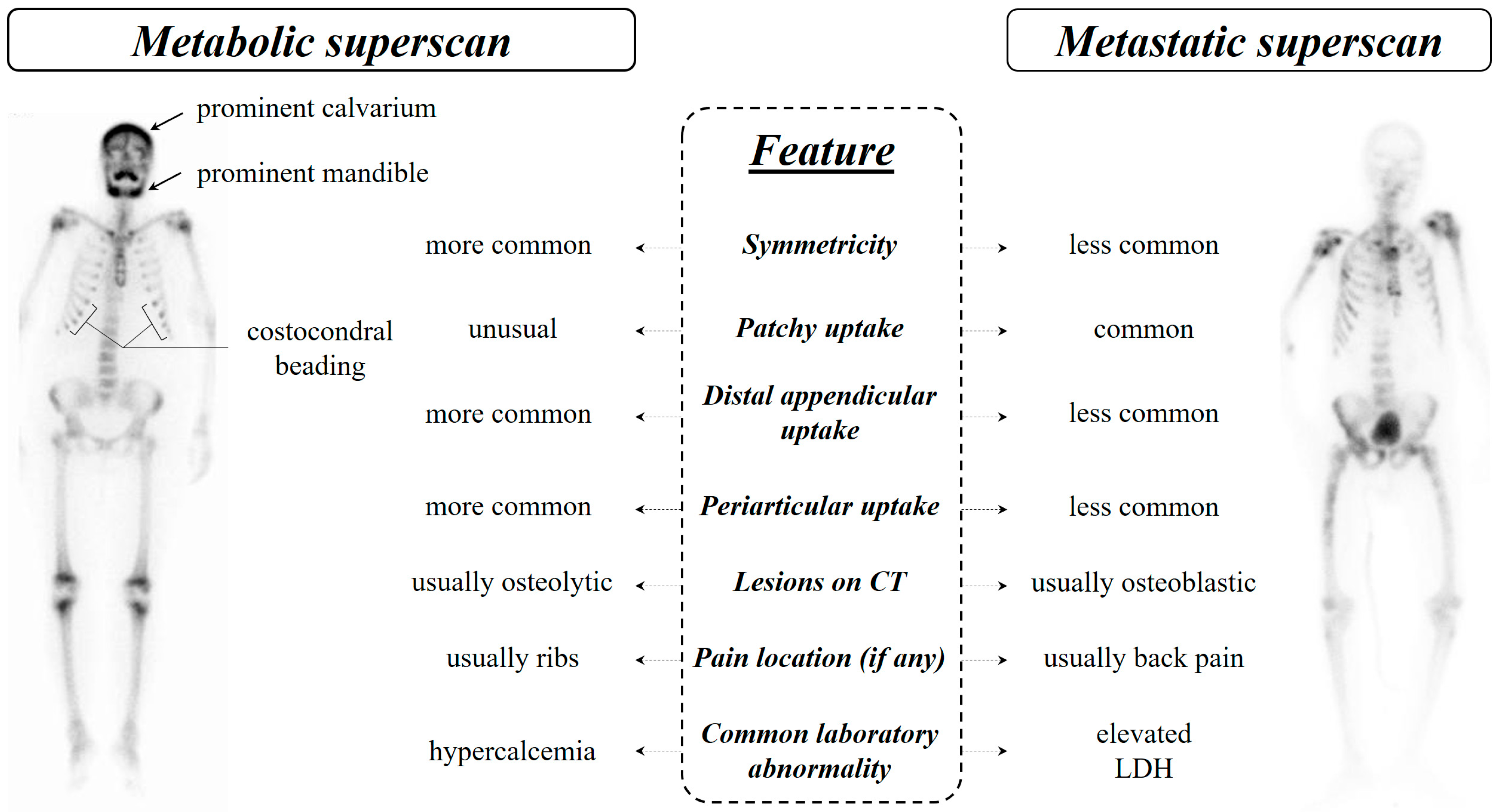
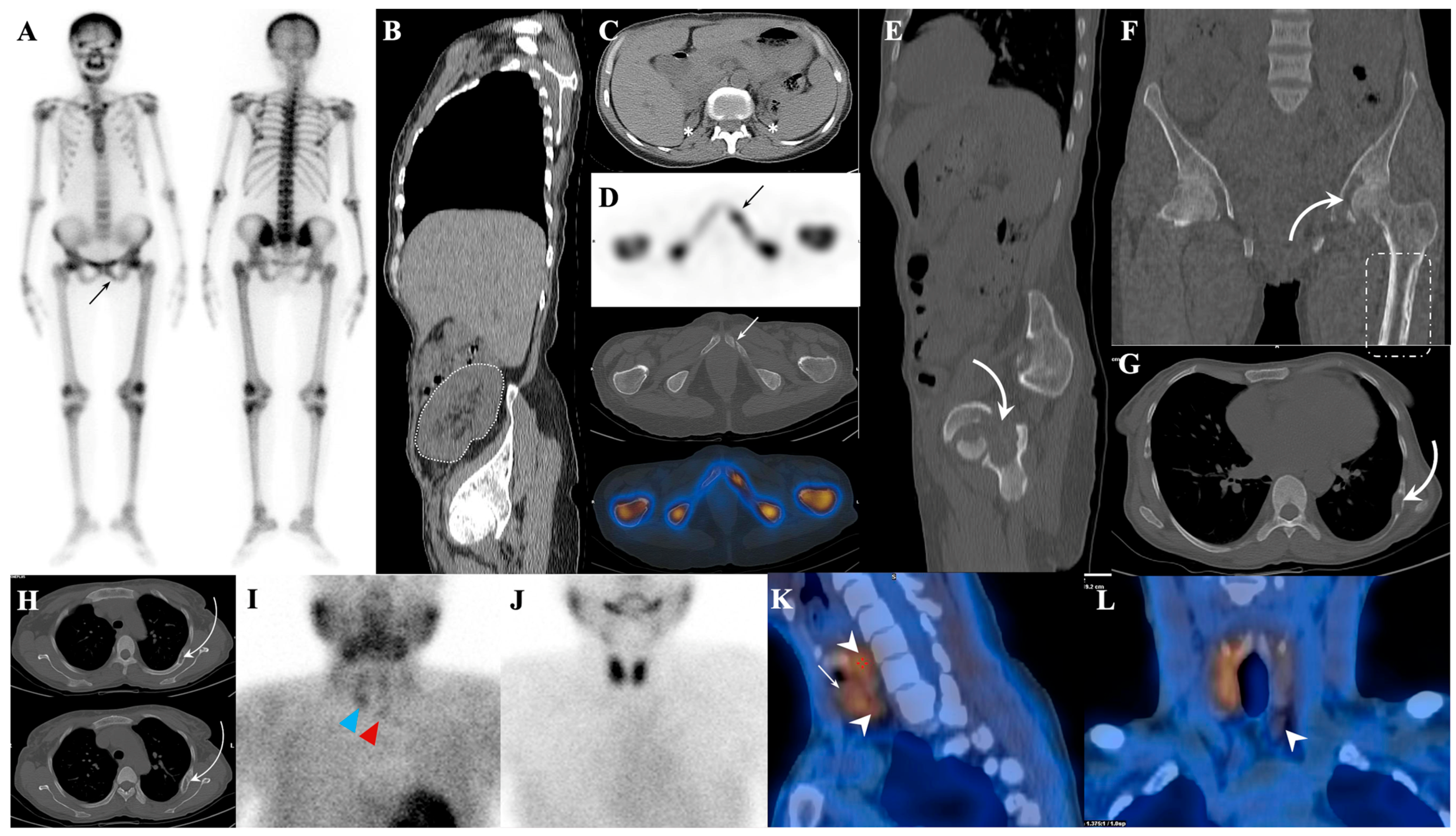
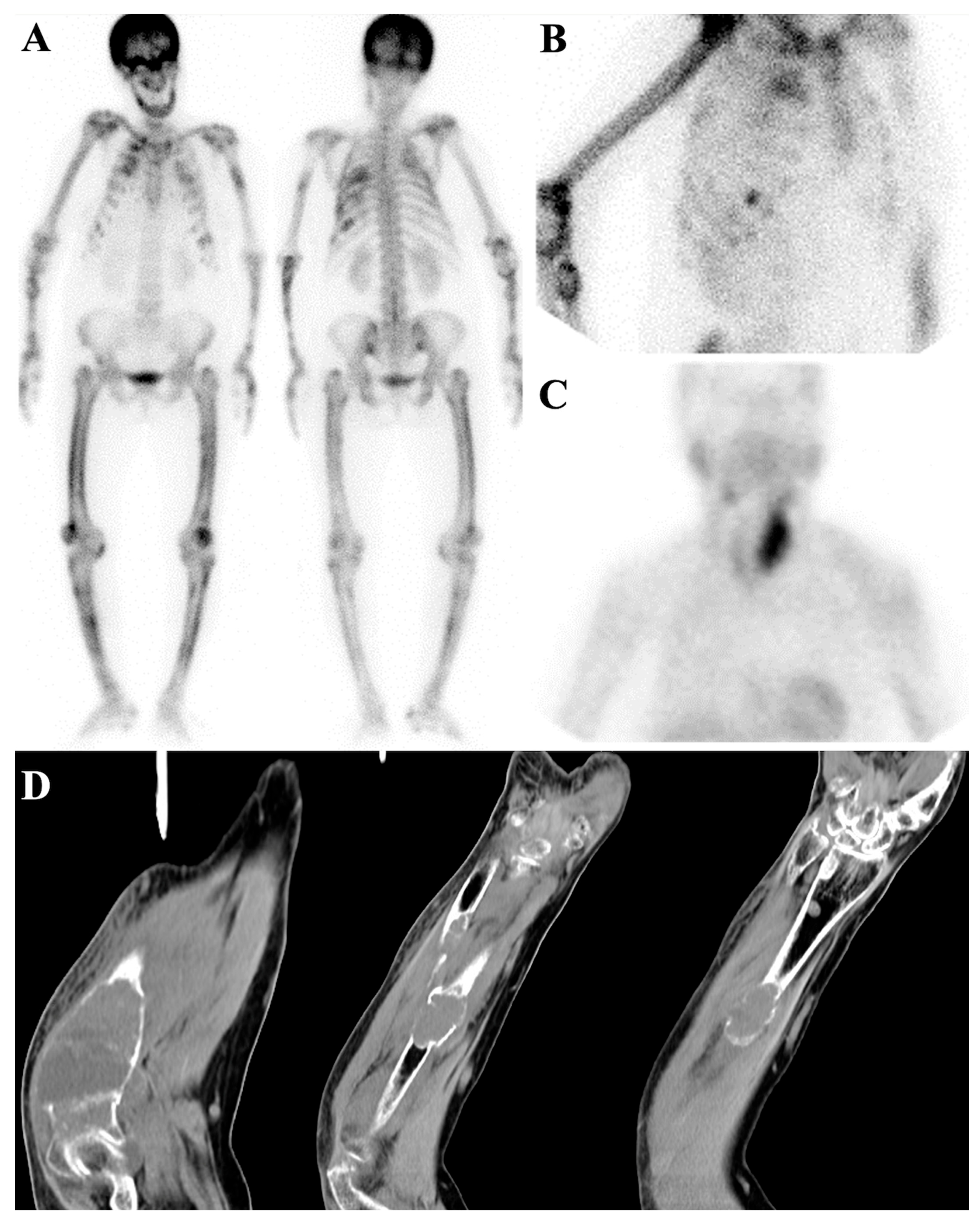
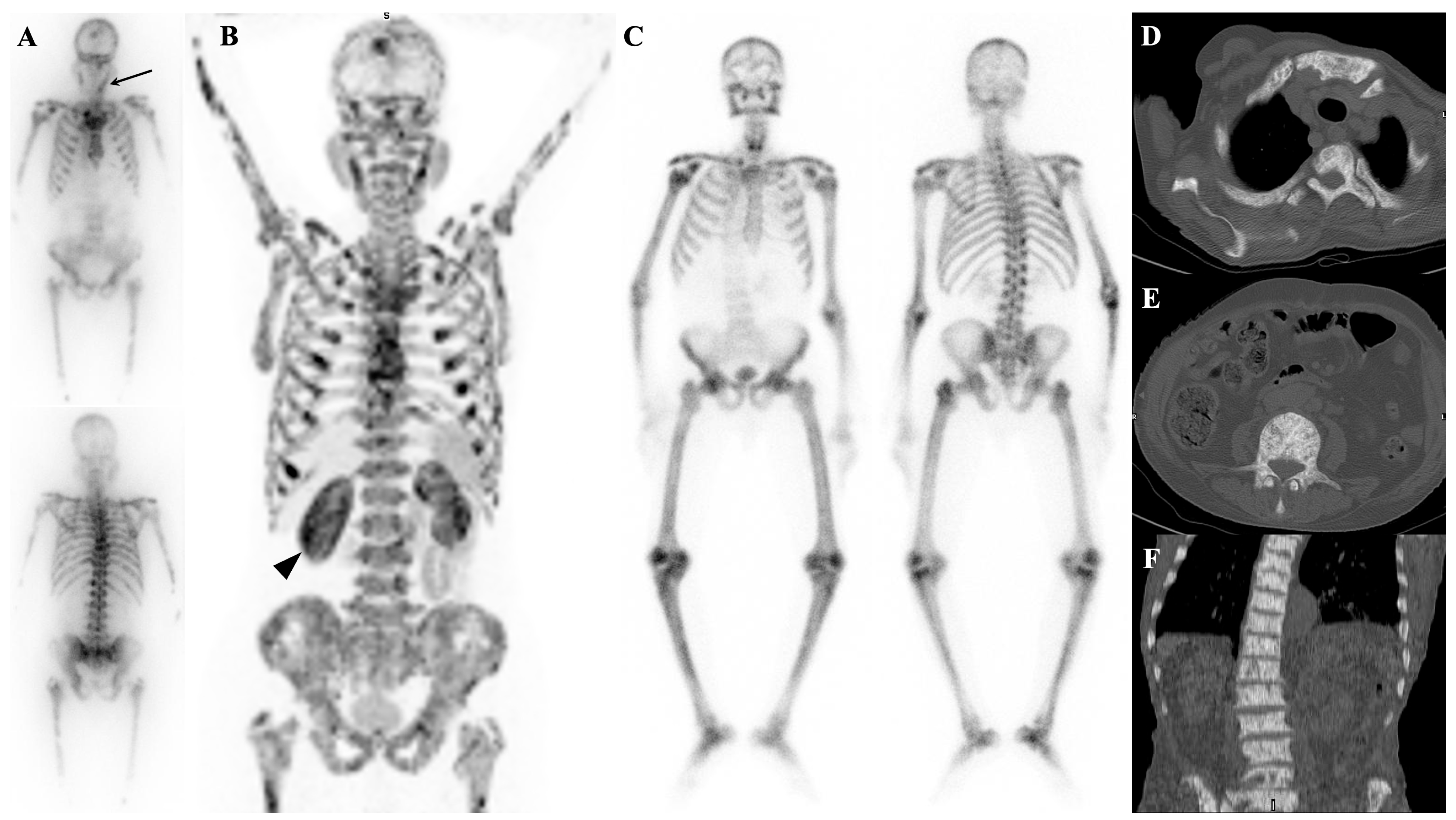
7.1. Metastatic Superscan
7.1.1. Superscan Pattern in Patients with Prostate Cancer
7.1.2. Dynamic Changes in Prostate Cancer Patients with Superscan
7.2. Metabolic Superscan
7.3. Distinguishing Metabolic Superscan from Metastatic Superscan
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manohar, P.R.; Rather, T.; Khan, S.; Malik, D. Skeletal metastases presenting as superscan on technetium 99 m methylene diphosphonate whole body bone scintigraphy in different type of cancers: A 5-year retro-prospective study. World J. Nucl. Med. 2017, 16, 39–44. [Google Scholar] [PubMed]
- Manov, J.J.; Roth, P.J.; Kuker, R. Clinical pearls: Etiologies of superscan appearance on Fluorine-18-Fludeoxyglucose positron emission tomography-computed tomography. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2017, 32, 259. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.A.; Shah, M.N.M. ‘Sub-superscan’in Bone Scan–An Important Feature of Extensive Bone Metastases. Malays. J. Med. Health Sci. 2020, 16, 68–70. [Google Scholar]
- Buckley, O.; O’Keeffe, S.; Geoghegan, T.; Lyburn, I.D.; Munk, P.L.; Worsley, D.; Torreggiani, W.C. 99mTc bone scintigraphy superscans: A review. Nucl. Med. Commun. 2007, 28, 521–527. [Google Scholar] [CrossRef]
- Harshman, L.K.; Latifi, H.R.; Griffeth, L.K. Visualization of discrete sacral foramina as an ancillary sign of superscan. Clin. Nucl. Med. 2011, 36, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Soerdjbalie-Maikoe, V.; Pelger, R.C.; À Nijeholt, G.A.L.; Arndt, J.-W.; Zwinderman, A.H.; Bril, H.; Papapoulos, S.E.; Hamdy, N.A. Bone scintigraphy predicts the risk of spinal cord compression in hormone-refractory prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 958–963. [Google Scholar] [CrossRef]
- Soloway, M.S.; Hardeman, S.W.; Hickey, D.; Todd, B.; Soloway, S.; Raymond, J.; Moinuddin, M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988, 61, 195–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Zheng, Y.; Wang, K.; Xia, W.; Wang, J.; Ge, P. Retrospective validation of bone risk stratification criteria for men with de novo metastatic hormone-naive prostate cancer in China. PeerJ 2023, 11, e14500. [Google Scholar] [CrossRef]
- Osmond, J.D., III; Pendergrass, H.P.; Potsaid, M.S. Accuracy of 99mTc-diphosphonate bone scans and roentgenograms in the detection of prostate, breast and lung carcinoma metastases. Am. J. Roentgenol. 1975, 125, 972–977. [Google Scholar] [CrossRef]
- Sy, W.M.; Patel, D.; Faunce, H. Significance of absent or faint kidney sign on bone scan. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1975, 16, 454–456. [Google Scholar]
- Frankel, R.S.; Johnson, K.W.; Mabry, J.J.; Johnston, G.S. “Normal” bone radionuclide image with diffuse skeletal lymphoma: A case report. Radiology 1974, 111, 365–366. [Google Scholar] [CrossRef]
- Thrupkaew, A.K.; Henkin, R.E.; Quinn, J.L., III. False negative bone scans in disseminated metastatic disease. Radiology 1974, 113, 383–386. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, Y.; Li, Y.; Tian, J.; Ren, J.; Cao, J.; Jin, L. SPECT/CT imaging characteristics and clinical analysis of 81 cases of super scan. J. Nucl. Med. 2021, 62 (Suppl. 1), 1165. [Google Scholar]
- Wilson, M.; Calhoun, F.; Gaines, J.; Goldsmith, S. Patterns of diffusely increased skeletal radiopharmaceutical uptake. Australas. Radiol. 1981, 25, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Constable, A.; Cranage, R. Recognition of the superscan in prostatic bone scintigraphy. Br. J. Radiol. 1981, 54, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Higano, C.; Saad, F.; Sartor, O.; Conti, P.; George, D.; Sternberg, C.; Shore, N.; Sade, J.; Bellmunt, J. Clinical outcomes and patient (pt) profiles in REASSURE: An observational study of radium-223 (Ra-223) in metastatic castration-resistant prostate cancer (mCRPC). Nukl. -Nucl. 2021, 60, L14. [Google Scholar]
- Montilla-Soler, J.L.; Makanji, R. Skeletal scintigraphy. Cancer Control 2017, 24, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Anscombe, A.; Walkden, S. An interesting bone scan in multiple myeloma—? myeloma superscan. Br. J. Radiol. 1983, 56, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.-M.; Ramchand, S.k.; Grossmann, M. Pitfalls in bone density monitoring in prostate cancer during anti-resorptive treatment. Osteoporos. Int. 2018, 29, 1665–1670. [Google Scholar] [CrossRef]
- Ebrahim, T.; Hadebe, B.; Aldous, C.; Tinarwo, P.; Nyakale, N. Segmented linear correlations between bone scan index and prostate cancer biomarkers, alkaline phosphatase, and prostate specific antigen in patients with a Gleason score ≥ 7. Medicine 2022, 101, e29515. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Kim, D.Y.; Lee, D.S.; Chung, J.-K.; Lee, M.C.; Koh, C.-S. Absent or faint renal uptake on bone scan: Etiology and significance in metastatic bone disease. Clin. Nucl. Med. 1991, 16, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, A.Z.; Hellman, R.S.; Timins, M.E.; Collier, B.D.; Anderson, T.; Isitman, A.T. Diagnostic bone scanning in oncology. Semin. Nucl. Med. 1997, 27, 107–141. [Google Scholar] [CrossRef]
- Fogelman, I.; Bessent, R.G.; Turner, J.G.; Citrin, D.L.; Boyle, I.T.; Greig, W.R. The use of whole-body retention of Tc-99m diphosphonate in the diagnosis of metabolic bone disease. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1978, 19, 270–275. [Google Scholar]
- Kondakov, A.; Kharina, D.; Servuli, E.; Znamenskiy, I. “Hot Kidneys” Artifact on Bone Scan: Incidence and Approach to Quantification. In Proceedings of the 28th Annual EANM Congress of the European Association of Nuclear Medicine, Berlin/Heidelberg, Germany, 19–23 October 2024; p. 42. [Google Scholar]
- Mironov, S.; Ansheles, A.; Shulgin, D.; Sergienko, V. Extraosseous abnormalities and artifacts in skeletal scintigraphy. Vestn. Rentgenol. I Radiol. 2016, 97, 85–94. [Google Scholar] [CrossRef]
- Afzal, M.; Imran, M.; Akhtar, M.; Iqbal, M. Increased renal parenchymal retention of 99mTc-MDP (hot kidneys) in two patients of Hepatocellular Cancer: One with and other without osseous metastases. Eur. J. Med. Case Rep. 2017, 1, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Huang, M.Y.; Hsieh, J.S.; Hou, M.F.; Chou, S.H.; Lin, C.L. Discordant findings of skeletal metastasis between Tc99m MDP bone scans and F18 FDG PET/CT imaging for advanced breast and lung cancers—Two case reports and literature review. Kaohsiung J. Med. Sci. 2007, 23, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.-J.; Tan, T.H.; Chew, M.T. Superscan: Superiority of xSPECT/CT over OSEM SPECT/CT in bone scans of prostate cancer patients. Radiat. Phys. Chem. 2021, 178, 108998. [Google Scholar] [CrossRef]
- Hain, S.F.; Fogelman, I. Nuclear Medicine. In Imaging of the Hip & Bony Pelvis: Techniques and Applications; Davies, A.M., Johnson, K.J., Whitehouse, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 59–71. [Google Scholar]
- Silberstein, E.B.; Schroder, L.E. Minimal bone scan findings in the presence of wide-spread osteoblastic disease on skeletal radiography. Clin. Nucl. Med. 1990, 15, 816–817. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Costo, S.; Lheureux, S.; Allouache, N.; Bardet, S. Ivory vertebra appearing photopenic on Tc-99m MDP bone scan: Demonstration by SPECT/CT. Clin. Nucl. Med. 2008, 33, 479–481. [Google Scholar] [CrossRef]
- Barber, R.; Hartman, N.; Peters, A. Anemia and polycythemia. In Clinical Nuclear Medicine; CRC Press: Boca Raton, FL, USA, 2006; pp. 763–772. [Google Scholar]
- Fogelman, I. 3 Bone scanning and photon absorptiometry in metabolic bone disease. Baillière’s Clin. Endocrinol. Metab. 1988, 2, 59–86. [Google Scholar] [CrossRef]
- Nordin, B.E.C. Diagnostic procedures in disorders of calcium metabolism. Clin. Endocrinol. 1978, 8, 55–67. [Google Scholar] [CrossRef]
- Eriksen, E.; Mosekilde, L.; Melsen, F. Trabecular bone remodeling and bone balance in hyperthyroidism. Bone 1985, 6, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P. Heritable forms of rickets and osteomalacia. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 765–787. [Google Scholar]
- Caglar, M.; Tuncel, M.; Yildiz, E.; Karabulut, E. Bone scintigraphy as a gatekeeper for the detection of bone metastases in patients with prostate cancer: Comparison with Ga-68 PSMA PET/CT. Ann. Nucl. Med. 2020, 34, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Enevoldsen, L.H.; Heaf, J.; Højgaard, L.; Zerahn, B.; Hasbak, P. Increased technetium-99 m hydroxy diphosphonate soft tissue uptake on bone scintigraphy in chronic kidney disease patients with secondary hyperparathyroidism: Correlation with hyperphosphataemia. Clin. Physiol. Funct. Imaging 2017, 37, 131–136. [Google Scholar] [CrossRef]
- Lawal, I.; Ankrah, A.; Ololade, K.; Modiselle, M.; Sathekge, M. Renal osteodystrophy presenting as a metabolic superscan on F-18 FDG PET/CT: A case report. Medicine 2017, 96, e8471. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Schembri, G.P. Combined 18F-FDG PET/CT and 68Ga DOTATATE PET/CT “superscan” in metastatic pancreatic neuroendocrine tumor. Clin. Nucl. Med. 2017, 42, 108–109. [Google Scholar] [CrossRef]
- Cheng, G.; Alavi, A.; Lim, E.; Akers, S.R. Superscan-like hypermetabolic lesions on delayed FDG PET/CT imaging in a patient with lung cancer. Clin. Nucl. Med. 2012, 37, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Bailly, M.; Besse, H.; Kerdraon, R.; Metrard, G.; Gauvain, S. 18F-FDG PET/CT superscan in prostate cancer. Clin. Nucl. Med. 2014, 39, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Liu, R.S.; Liao, S.Q.; Wang, S.J. F-18 FDG PET superscan. Clin. Nucl. Med. 2006, 31, 28–29. [Google Scholar] [CrossRef]
- Yousefi-Koma, A.; Shiravand, Y.; Qutbi, M. Diffuse Skeletal Uptake on 18 F-Fluoro-2-deoxy-d-glucose Positron Emission Tomography/Computed Tomography Scan in a Patient with Acute Lymphoblastic Leukemia: A Typical Superscan Pattern Resembling NaF Positron Emission Tomography Scan. Indian J. Nucl. Med. 2019, 34, 326–328. [Google Scholar]
- Qureshi, P.A.A.A.; Aleem, J.; Niazi, I.K.; Hassan, A.; Bashir, H. Superscan on 18F-FDG PET-CT imaging for lymphoma: How frequent is it? JPMA J. Pak. Med. Assoc. 2022, 72, 1845–1847. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Zhai, L.; Geng, H.; Wang, P.; Zhang, W. 18F-FDG PET/CT Findings in a Patient With Neutrophilic Leukemoid Reaction Associated With Multiple Myeloma. Clin. Nucl. Med. 2020, 45, 405–406. [Google Scholar] [CrossRef]
- Law, W.P.; Emmett, S.; Jackson, P. 18F-FDG superscan caused by extensive bone marrow involvement in hemophagocytic lymphohistiocytosis. Clin. Nucl. Med. 2017, 42, 617–619. [Google Scholar] [CrossRef]
- Saeed, H.; Hassan, A.; Saeed, H.; Gill, S.M. 18F FDG PET-CT Super scan: Paediatric Rhabdomyosarcoma. JPMA J. Pak. Med. Assoc. 2022, 72, 1880–1881. [Google Scholar] [PubMed]
- Malhotra, G.; Swami, A.; Shah, P.; Mittal, N.; Gandhi, S.J.; Tiwari, B.; Jatale, P.V.; Asopa, R.V. F-18 fluorodeoxyglucose positron emission tomography “super scan” in a patient of metastatic primitive neuroectodermal tumor of the kidney. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2012, 27, 115. [Google Scholar] [CrossRef]
- Tan, T.H.; Wong, T.H.; Hassan, S.Z.A.; Lee, B.N. Unusual bone superscan, MIBG superscan, and 68Ga DOTATATE PET/CT in metastatic pheochromocytoma. Clin. Nucl. Med. 2015, 40, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.E.; Barron, B.J. MIBG superscan of metastatic paraganglioma occurring with neurofibromatosis type 1. Clin. Nucl. Med. 2013, 38, 459–462. [Google Scholar] [CrossRef]
- Bohdiewicz, P.; Gallegos, E.; Fink-Bennett, D. Raccoon eyes and the MIBG super scan: Scintigraphic signs of neuroblastoma in a case of suspected child abuse. Pediatr. Radiol. 1995, 25, S90–S92. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.; Jois, A.; Singh, H.; Bora, G.S.; Basher, R.K.; Mittal, B.R. Metastatic neuroblastoma in adult patient, presenting as a super scan on 68Ga-DOTANOC PET/CT imaging. Clin. Nucl. Med. 2017, 42, 697–699. [Google Scholar] [CrossRef]
- Basu, S.; Abhyankar, A. The use of 99mTc-HYNIC-TOC and 18F-FDG PET/CT in the evaluation of duodenal neuroendocrine tumor with atypical and extensive metastasis responding dramatically to a single fraction of PRRT with 177Lu-DOTATATE. J. Nucl. Med. Technol. 2014, 42, 296–298. [Google Scholar] [CrossRef][Green Version]
- Karfis, I.; Stavraka, A.; Lyra, M.; Gouliamos, A.; Limouris, G. Superscan Pattern in In-111 Octreotide Scintigraphies due to Primary Breast Carcinoma Cases. In European Journal of Nuclear Medicine and Molecular Imaging; Springer: New York, NY, USA, 2010; p. S440. [Google Scholar]
- Urso, L.; Nieri, A.; Borgia, F.; Malorgio, A.; Bartolomei, M. Superscan-Like Pattern on 18F-Choline PET/CT in a Patient with Essential Thrombocythemia. Clin. Nucl. Med. 2022, 48, e131–e132. [Google Scholar] [CrossRef]
- Taralli, S.; Cocciolillo, F.; Alitto, A.R.; Caldarella, C. Bone marrow activation after chemotherapy presenting as diffuse skeletal uptake on 18F-fluorocholine PET/CT. Clin. Nucl. Med. 2021, 46, e498–e500. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Macapinlac, H.A.; Lu, Y. Superscan 18F-fluciclovine PET/CT of PSA-negative prostate cancer bone metastases. Clin. Nucl. Med. 2019, 44, 337–338. [Google Scholar] [CrossRef]
- Moghadam, S.Z.; Askari, E.; Aryana, K. 99mTc-PSMA Left Behind: A Call for Collaboration. Nucl. Med. Mol. Imaging 2022, 56, 218–219. [Google Scholar] [CrossRef]
- Jochumsen, M.R.; Madsen, M.A.; Arveschoug, A.K. Skeletal “Superscan” with 11C-Methionine PET/CT in Polycythemia Vera. Clin. Nucl. Med. 2022, 48, e198–e199. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Chen, Y.; Cai, L.; Yu, Z.; Wu, W.; Yang, J.; Ou, S. A case of high-turnover renal osteodystrophy and vascular calcification in a patient with uremia diagnosed by 18F-NaF PET/CT. Int. Urol. Nephrol. 2023, 55, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Luo, Y.; Cao, X.; Ma, Y.; Li, F. Multiple myeloma presenting as a superscan on 68Ga-Pentixafor PET/CT. Clin. Nucl. Med. 2018, 43, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Q.; Luo, Q.; Chen, L.; Huang, Z. The Manifestation of a Patient With Myelofibrosis in 68Ga-DOTA-FAPI-04 PET/CT Mimicking “Super Bone Imaging”. Clin. Nucl. Med. 2022, 47, 1056–1058. [Google Scholar] [CrossRef]
- Ndlovu, H.; Lawal, I.O.; Mokoala, K.M.G.; Sathekge, M.M. Imaging Molecular Targets and Metabolic Pathways in Breast Cancer for Improved Clinical Management: Current Practice and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 1575. [Google Scholar] [CrossRef]
- Shimada, F.; Misawa, M.; Suzuki, T. “Superscan” in diffusion-weighted imaging with background body suppression magnetic resonance imaging. CMAJ 2021, 193, E48. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Feng, B.; Jiang, Y.; Zhao, Q. Analysis of imaging features, clinical manifestations, age and laboratory examination indexes of SPECT/CT super bone scan. Int. J. Radiat. Med. Nucl. Med. 2022, 46, 325–333. [Google Scholar]
- Pang, Y.; Huang, H.; Fu, L.; Zhao, L.; Chen, H. 68Ga-FAPI PET/CT detects gastric signet-ring cell carcinoma in a patient previously treated for prostate cancer. Clin. Nucl. Med. 2020, 45, 632–635. [Google Scholar] [CrossRef]
- Kohno, M.; Miyama, K.; Gohbara, A.; Onuki, T.; Sugiura, S.; Ikeda, I. Disseminated carcinomatosis of the bone marrow with urothelial carcinoma. Nihon Hinyokika Gakkai Zasshi. Jpn. J. Urol. 2015, 106, 114–117. [Google Scholar]
- Sy, W.; Mottola, O.; Lao, R.; Smith, A.; Freund, H. Unusual bone images in hyperparathyroidism. Br. J. Radiol. 1977, 50, 740–744. [Google Scholar] [CrossRef]
- Bhan, A.; Rao, A.D.; Rao, D.S. Osteomalacia as a result of vitamin D deficiency. Endocrinol. Metab. Clin. 2010, 39, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-S.; Chung, H.W. Metabolic Bone Disease. In Atlas of Nuclear Medicine in Musculoskeletal System: Case-Oriented Approach; Springer: Berlin/Heidelberg, Germany, 2022; pp. 157–178. [Google Scholar]
- Sellami, M.; Riahi, H.; Maatallah, K.; Ferjani, H.; Bouaziz, M.C.; Ladeb, M.F. Skeletal fluorosis: Don’t miss the diagnosis! Skelet. Radiol. 2020, 49, 345–357. [Google Scholar] [CrossRef]
- Hansen, J.A.; Naghavi-Behzad, M.; Gerke, O.; Baun, C.; Falch, K.; Duvnjak, S.; Alavi, A.; Høilund-Carlsen, P.F.; Hildebrandt, M.G. Diagnosis of bone metastases in breast cancer: Lesion-based sensitivity of dual-time-point FDG-PET/CT compared to low-dose CT and bone scintigraphy. PLoS ONE 2021, 16, e0260066. [Google Scholar] [CrossRef]
- Cheng, T.; Holman, B. Increased skeletal: Renal uptake ratio: Etiology and characteristics. Radiology 1980, 136, 455–459. [Google Scholar] [CrossRef]
- Parihar, A.S.; Sood, A.; Lukose, T.T.; Seam, R.K.; Mittal, B.R. Metabolic bone superscan in carcinoma breast with occult Graves’ disease: Looking beyond skeletal metastases. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2018, 33, 145. [Google Scholar]
- Fogelman, I.; McKillop, J.H.; Boyle, I.T.; Greig, W.R. Absent kidney sign associated with symmetrical and uniformly increased uptake of radiopharmaceutical by the skeleton. Eur. J. Nucl. Med. 1977, 2, 257–259. [Google Scholar] [CrossRef]
- Choi, C.W.; Lee, D.S.; Chung, J.-K.; Lee, M.C.; Kim, N.K.; Choi, K.W.; Koh, C.-S. Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin. Nucl. Med. 1995, 20, 310–314. [Google Scholar] [CrossRef]
- Fogelman, I.; Citrin, D.L.; Turner, J.G.; Hay, I.D.; Bessent, R.G.; Boyle, I.T. Semi-quantitative interpretation of the bone scan in metabolic bone disease: Definition and validation of the metabolic index. Eur. J. Nucl. Med. 1979, 4, 287–289. [Google Scholar] [CrossRef]
- Fogelman, I. The Bone Scan in Paget’s Disease. In Bone Scanning in Clinical Practice; Springer: London, UK, 1987. [Google Scholar] [CrossRef]
- Durr-e-Sabih, U.R.; Khan, U.; Sabih, Q.; Rahim, K.; Imran, M.B. Missed diagnosis of oxalosis with disastrous consequences. Case reports of a father and daughter. Eur. J. Med. Case Rep. 2022, 6, 52–57. [Google Scholar] [CrossRef]
- Manier, S.M.; Van Nostrand, D. Super bone scan. Semin. Nucl. Med. 1984, 14, 46–47. [Google Scholar] [CrossRef]
- Rabelink, N.M.; Westgeest, H.M.; Bravenboer, N.; Jacobs, M.A.; Lips, P. Bone pain and extremely low bone mineral density due to severe vitamin D deficiency in celiac disease. Arch. Osteoporos. 2011, 6, 209–213. [Google Scholar] [CrossRef][Green Version]
- Fischler, R.; Lecoq, A.-L.; Briot, K.; Trabado, S.; Besson, F.; Bouziotis, J.; Goujard, C.; Salenave, S.; Leboulleux, S.; Carreira, E. Skeletal consequences of long-term replacement therapy with human recombinant PTH (1-34) in chronic hypoparathyroidism. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2022; Volume 81. [Google Scholar]
- Bensaid, C.; Rahali, J.; Guerrouj, H.; Ghfir, I.; Çaoui, M.; Aouad, N.B.R. Fixation homogène et symétrique du HMDP-99mTc dans le cadre d’un bilan d’extension: Penser au superscan métastatique (à propos d’un cas). Médecine Nucléaire 2022, 46, 86. [Google Scholar] [CrossRef]
- Ohashi, K.; Smith, H.S.; Jacobs, M.P. “ Superscan” appearance in distal renal tubular acidosis. Clin. Nucl. Med. 1991, 16, 318–320. [Google Scholar] [CrossRef]
- Abdelrazek, S.; Szumowski, P.; Rogowski, F.; Kociura-Sawicka, A.; Mojsak, M.; Szorc, M. Bone scan in metabolic bone diseases. Review. Nucl. Med. Rev. 2012, 15, 124–131. [Google Scholar]
- Arora, S.; Dhull, V.; Mukherjee, A.; Tulsyan, S.; Behera, A.; Tripathi, M. Metastatic superscan on 99m Tc-methylene diphosphonate bone scintigraphy in pediatric neuroblastoma. Indian J. Nucl. Med. IJNM 2015, 30, 286. [Google Scholar]
- Zuo, Z.; Zhang, Q.; Wu, W.; Li, X.; Zhang, L.; Wang, J.; Guo, Z.; Hu, S.; Liu, H. Sclerosing extramedullary hematopoietic tumor of the colon: A case report and literature review. Exp. Ther. Med. 2023, 25, 132. [Google Scholar] [CrossRef]
- Mairal, E.; Tissot, H.; Verger, A. Un cluster de fractures à l’âge adulte: La médecine nucléaire enquête. Nucl. Med. 2021, 45, 200. [Google Scholar] [CrossRef]
- Pinto-Lopes, P.; Fonseca, F.A.; Silva, R.; Hafe, P.v.; Fonseca, E. Indolent systemic mastocytosis limited to the bone: A case report and review of the literature. Sao Paulo Med. J. 2013, 131, 198–204. [Google Scholar] [CrossRef]
- Wu, Y.-w.; Seto, H.; Shimizu, M.; Kageyama, M.; Watanabe, N.; Kakishita, M. Postgastrectomy osteomalacia with pseudofractures assessed by repeated bone scintigraphy. Ann. Nucl. Med. 1995, 9, 29–32. [Google Scholar] [CrossRef]
- Washington, T.; Vora, A.; Mihailescu, D. A case of hypercalcemia associated with Castleman disease. Endocr. Pract. 2010, 16, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Bhadada, S.K.; Dhiman, V.; Mukherjee, S.; Aggarwal, S.; Bal, A.; Sukumar, S.P.; Sood, A.; Sharma, D.C.; Khandelwal, N.; Bhansali, A. Fibrogenesis imperfecta ossium and response to human growth hormone: A potential therapy. J. Clin. Endocrinol. Metab. 2017, 102, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.J.; Shuler, S.E.; Witherspoon, L.R.; Neely, H.R. A retrospective analysis of renal abnormalities detected on bone scans. Clin. Nucl. Med. 1980, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, R.; Li, J.; Cao, X.; Luo, Y. Characterizing Rosai-Dorfman disease with [18F] FDG PET/CT: A retrospective analysis of a single-center study. Eur. Radiol. 2023, 33, 6492–6501. [Google Scholar] [CrossRef]
- Chatterjee, P.; Mukherjee, A.; Mitra, D.; Nautiyal, A.; Roy, A. Superscan on Methylene Diphosphonate Skeletal Scintigraphy in Prostatic Adenocarcinoma: A Common Finding but Rare Etiology. Indian J. Nucl. Med. 2017, 32, 369–371. [Google Scholar]
- Kulkarni, M.; Soni, A.; Shetkar, S.; Amer, M.; Mulavekar, A.; Joshi, P. Coexistent superscan and Lincoln sign on bone scintigraphy. Clin. Nucl. Med. 2017, 42, 630–632. [Google Scholar] [CrossRef]
- Mou, B.; Cruz-Lim, E.M. Recurrent Extraneural Metastatic Medulloblastoma in an Adult Presenting with a Superscan and Treated With Radium-223. Cureus 2023, 15, e34732. [Google Scholar] [CrossRef] [PubMed]
- Tokushima, K.; Ikeda, T.; Kobayashi, F.; Kurosaki, M. A variant alkaline phosphatase-producing gastric carcinoma with super bone scan. Dig. Dis. Sci. 1997, 42, 66. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Chen, F.; Han, Y.; Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br. J. Cancer 2021, 124, 1912–1920. [Google Scholar] [CrossRef]
- Hensel, J.; Thalmann, G.N. Biology of bone metastases in prostate cancer. Urology 2016, 92, 6–13. [Google Scholar] [CrossRef]
- Abramson, E.; Gajardo, H.; Kukreja, S. Hypocalcemia in cancer. Bone Miner. 1990, 10, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Riancho, J.; Arjona, R.; Valle, R.; Sanz, J.; González-Macías, J. The clinical spectrum of hypocalcaemia associated with bone metastases. J. Intern. Med. 1989, 226, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Ulmert, D.; Solnes, L.; Thorek, D.L. Contemporary approaches for imaging skeletal metastasis. Bone Res. 2015, 3, 15024. [Google Scholar] [CrossRef]
- Massie, J.D.; Sebes, J.I. The headless bone scan: An uncommon manifestation of metastatic superscan in carcinoma of the prostate. Skelet. Radiol. 1988, 17, 111–113. [Google Scholar] [CrossRef]
- Moreno-Ballesteros, A.; León-Asuero-Moreno, I.; Marín-Melero, I.; García-Gómez, F.J. Hyperostosis frontalis interna by bone scintigraphy. Jpn. J. Clin. Oncol. 2021, 51, 664–665. [Google Scholar] [CrossRef] [PubMed]
- Ermiş, F.; Erkan, M.E.; Besir, F.H.; Oktay, M.; Kutlucan, A.; Aydın, Y. Osteoblastic metastasis from signet ring cell gastric cancer in a young male. Turk J Gastroenterol 2014, 25, 284–286. [Google Scholar] [CrossRef]
- Tago, M.; Tokushima, Y.; Oie, S.; Yamashita, S.i. Slight brightness and osteosclerotic changes of bones on plain X-ray could be clues to the diagnosis of disseminated carcinomatosis of bone marrow. Clin. Case Rep. 2019, 7, 1458–1459. [Google Scholar] [CrossRef]
- Liu, R.-S.; Chu, Y.-K.; Chu, L.-S.; Yeh, S.-H.; Yen, S.-H.; Chen, K.Y.; Chen, Y.-K.; Shen, Y.-Y. Superscan in patients with nasopharyngeal carcinoma. Clin. Nucl. Med. 1996, 21, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, K.; Parida, G.K.; Thavnani, R.; Patro, P.S.S.; Agrawal, K. An unusual case of glioblastoma multiforme, presenting as skeletal superscan. Indian J. Nucl. Med. 2022, 37, 268. [Google Scholar] [CrossRef] [PubMed]
- Kamaleshwaran, K.K.; Mittal, B.R.; Harisankar, C.N.B.; Bhattacharya, A.; Singh, S.K.; Mandal, A.K. Predictive value of serum prostate specific antigen in detecting bone metastasis in prostate cancer patients using bone scintigraphy. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2012, 27, 81. [Google Scholar]
- Nakashima, J.; Ozu, C.; Nishiyama, T.; Oya, M.; Ohigashi, T.; Asakura, H.; Tachibana, M.; Murai, M. Prognostic value of alkaline phosphatase flare in patients with metastatic prostate cancer treated with endocrine therapy. Urology 2000, 56, 843–847. [Google Scholar] [CrossRef]
- Sureka, S.K.; Maheshwari, R.; Agnihotri, S.; Mitash, N.; Ahmad, S.; Mandhani, A. Predictors for progression of metastatic prostate cancer to castration-resistant prostate cancer in Indians. Indian J. Med. Res. 2016, 143, S68. [Google Scholar] [PubMed]
- Rizzo, C.; Vella, S.; Cachia, M.J. Refractory hypocalcaemia complicating metastatic prostatic carcinoma. Case Rep. 2015, 2015, bcr2015210003. [Google Scholar] [CrossRef]
- Venz, S.; Cordes, M.; Friedrichs, R.; Hosten, N.; Neumann, K.; Langer, R.; Nagel, R.; Felix, R. Assessment of functional displacement of bone marrow by osteoplastic metastases from prostatic carcinoma with bone marrow scintigraphy. Radiol. Diagn. 1993, 34, 358–363. [Google Scholar]
- Tucci, M.; Mosca, A.; Lamanna, G.; Porpiglia, F.; Terzolo, M.; Vana, F.; Cracco, C.; Russo, L.; Gorzegno, G.; Tampellini, M. Prognostic significance of disordered calcium metabolism in hormone-refractory prostate cancer patients with metastatic bone disease. Prostate Cancer Prostatic Dis. 2009, 12, 94–99. [Google Scholar] [CrossRef]
- Moghadam, S.Z.; Askari, E.; Divband, G.; Shakeri, S.; Aryana, K. Efficacy, safety and prognostic factors affecting overall survival among metastatic prostate cancer patients undergoing treatment with 177Lu-PSMA-617: A single center study. Rev. Española De Med. Nucl. E Imagen Mol. (Engl. Ed. ) 2022, 41, 239–246. [Google Scholar]
- Paone, G.; Nitzsche, E.U. Radiometabolic Therapy of Bone Metastases. In Nuclear Medicine Therapy; Giovanella, L., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Windsor, P. Predictors of Response to Strontium-89 (Metastron®) in Skeletal Metastases from Prostate Cancer: Report of a Single Centre’s 10-Year Experience. Clin. Oncol. 2001, 13, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ubieto, M.; Abos, M.; Tardin, A.; Razola, P.; Prats, E.; Garcia, F.; Polo, E.; Yubero, A.; Banzo, J. Treatment of bone metastatic pain with Sm153-EDTMP. Evaluation of the analgesic response and the existence of differences according to the primary tumor and the metastatic pattern. Rev. Esp. Med. Nucl. 2005, 24, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bączyk, M. Radioisotope therapy of bone metastases. Nucl. Med. Rev. 2011, 14, 96–104. [Google Scholar] [CrossRef]
- Handkiewicz-Junak, D.; Poeppel, T.D.; Bodei, L.; Aktolun, C.; Ezziddin, S.; Giammarile, F.; Delgado-Bolton, R.C.; Gabriel, M. EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 846–859. [Google Scholar] [CrossRef]
- Oguma, Y.; Hosono, M.; Okajima, K.; Inoue, E.; Nakamatsu, K.; Doi, H.; Matsuura, T.; Inada, M.; Uehara, T.; Wada, Y. Investigation into the Optimal Strategy of Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer. Radiation 2022, 2, 273–284. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Gafita, A.; Fendler, W.P.; Hui, W.; Sandhu, S.; Weber, M.; Esfandiari, R.; Calais, J.; Rauscher, I.; Rathke, H.; Tauber, R. Efficacy and safety of 177Lu-labeled prostate-specific membrane antigen radionuclide treatment in patients with diffuse bone marrow involvement: A multicenter retrospective study. Eur. Urol. 2020, 78, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Hope, T.A.; Antonarakis, E.S.; Bodei, L.; Calais, J.; Iravani, A.; Jacene, H.; Koo, P.J.; Morgans, A.K.; Osborne, J.R.; Tagawa, S.T. SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-PSMA-617 Radionuclide Therapy. J. Nucl. Med. 2023, 64, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J. EANM procedure guidelines for radionuclide therapy with 177 Lu-labelled PSMA-ligands (177 Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Lawal, I.O.; Morgenstern, A.; Vorster, M.; Knoesen, O.; Mahapane, J.; Hlongwa, K.N.; Maserumule, L.C.; Ndlovu, H.; Reed, J.D.; Popoola, G.O. Hematologic toxicity profile and efficacy of [225Ac] Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3581–3592. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for therapy of prostate cancer. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2020; pp. 133–140. [Google Scholar]
- Giammarile, F. The MIBG super scan. Pediatr. Radiol. 1997, 27, 193. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Wang, H.; Robertson, A.; Armstrong, W.R.; Zaum, R.; Weber, M.; Yagubbayli, F.; Kratochwil, C.; Grogan, T.R.; Nguyen, K. Tumor sink effect in 68Ga-PSMA-11 PET: Myth or reality? J. Nucl. Med. 2022, 63, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, D.; Nishi, M.; Satoh, T.; Shitara, T.; Matsumoto, K.; Fujita, T.; Yoshida, K.; Iwamura, M. Bone dissemination of prostate cancer after holmium laser enucleation of the prostate: A case report and a review of the literature. Int. J. Urol. 2014, 21, 215–217. [Google Scholar] [CrossRef]
- Manier, S.M.; van Nostrand, D. From “hot spots” to “superscan”. Clin. Nucl. Med. 1983, 8, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Chen, Y.-W.; Chang, C.-C.; Yang, W.-C.; Huang, C.-J.; Hou, M.-F. Bone metastasis versus bone marrow metastasis? Integration of diagnosis by 18F-fluorodeoxyglucose positron emission/computed tomography in advanced malignancy with super bone scan: Two case reports and literature review. Kaohsiung J. Med. Sci. 2013, 29, 229–233. [Google Scholar] [CrossRef]
- Saylor, P.J.; Mahmood, U.; Kunawudhi, A.; Smith, M.R.; Palmer, E.L.; Michaelson, M.D. Multitargeted tyrosine kinase inhibition produces discordant changes between 99mTc-MDP bone scans and other disease biomarkers: Analysis of a phase II study of sunitinib for metastatic castration-resistant prostate cancer. J. Nucl. Med. 2012, 53, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; De Bono, J.; Sternberg, C.; Le Moulec, S.; Oudard, S.; De Giorgi, U.; Krainer, M.; Bergman, A.; Hoelzer, W.; De Wit, R. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J. Clin. Oncol. 2016, 34, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C.; Chuang, T.-L.; Lee, M.-S.; Chiou, W.-Y.; Lin, H.-Y.; Hung, S.-K. The Super-scan and flare phenomena in a nasopharyngeal cancer patient: A case report. J. Clin. Med. Res. 2012, 4, 221. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Wibmer, A.G.; Becker, A.S.; Teo, M.Y.; Gönen, M.; Hricak, H.; Scher, H.I.; Morris, M.J.; Vargas, H.A. Correlation between Imaging-Based Intermediate Endpoints and Overall Survival in Men With Metastatic Castration-Resistant Prostate Cancer: Analysis of 28 Randomized Trials Using the Prostate Cancer Clinical Trials Working Group (PCWG2) Criteria in 16,511 Patients. Clin. Genitourin. Cancer 2022, 20, 69–79. [Google Scholar] [PubMed]
- Kovacsne, A.; Kozon, I.; Bentestuen, M.; Zacho, H.D. Frequency of superscan on bone scintigraphy: A systematic review. Clin. Physiol. Funct. Imaging 2023, 43, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yakupoglu, U.; Karayalcin, B.; Passadakis, P.; Tam, P.; Memmos, D.; Katopodis, K.; Ozener, C.; Akcicek, F.; Camsari, T.; Ates, K. Correlation of superscan findings with clinical parameters in renal osteodystrophy of peritoneal dialysis patients. In Nephrology Dialysis Transplantation; Oxford Univ Press: Oxford, UK, 2005; p. V349. [Google Scholar]
- Cook, G.J.; Gnanasegaran, G.; Chua, S. Miscellaneous indications in bone scintigraphy: Metabolic bone diseases and malignant bone tumors. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2010; pp. 52–61. [Google Scholar]
- Shakeri, S.; Khosravi, S.; Divband, G.; Sadeghi, R.; Shafiei, S. Whole-body blood pool metastatic superscan (bone hunger pattern). Clin. Nucl. Med. 2019, 44, 475–476. [Google Scholar] [CrossRef]
- Sarı, F.; Karayalçın, B.; Süleymanlar, G.; Akçiçek, F.; Ataman, R.; Akpolat, T.; Bozfakioğlu, S.; Gültekin, M.; Ersoy, F. 99mTc MDP Bone Scan Findings in CKD-MBD: Could the “Superscan” Image be Useful in Excluding Adynamic Bone Disease? Turk. J. Nephrol. 2021, 30, 308–315. [Google Scholar] [CrossRef]
- Lenz, O.; Sancassani, R.; Bottino, C.; Fornoni, A. Reversible bone pain and symmetric bone scan uptake in a dialysis patient treated with cinacalcet: A case report. J. Med. Case Rep. 2010, 4, 191. [Google Scholar] [CrossRef]
- Khor, L.K.; Tan, K.B.; Loi, H.Y.; Lu, S.-J. Nephrogenic systemic fibrosis in a patient with renal failure demonstrating a “reverse superscan” on bone scintigraphy. Clin. Nucl. Med. 2013, 38, 203–204. [Google Scholar] [CrossRef]
- Ozcan-Kara, P.; Mahmoudian, B.; Erbas, B.; Erbas, T. McCune–Albright syndrome associated with acromegaly and bipolar affective disorder. Eur. J. Intern. Med. 2007, 18, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U. Causes of superscan picture on bone scintigraphy. JCPSP-J. Coll. Physicians Surg. Pak. 2001, 11, 765–769. [Google Scholar]
- Boro, H.; Goyal, A.; Naik, S.S.; Tandon, N. Primary Sjögren’s syndrome manifesting as sclerotic metabolic bone disease. BMJ Case Rep. CP 2021, 14, e237987. [Google Scholar] [CrossRef]
- Kim, S.E.; Cho, J.T.; Lee, D.S.; Chung, J.-K.; Kim, S.; Lee, M.C.; Lee, J.S.; Koh, C.-S. Poor renal uptake of Tc-99m DMSA and Tc-99m MDP in a patient with Fanconi syndrome and near normal glomerular filtration rate. Clin. Nucl. Med. 1995, 20, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Cook, F.J.; Seagrove-Guffey, M.; Mumm, S.; Veis, D.J.; McAlister, W.H.; Bijanki, V.N.; Wenkert, D.; Whyte, M.P. Non-endemic skeletal fluorosis: Causes and associated secondary hyperparathyroidism (case report and literature review). Bone 2021, 145, 115839. [Google Scholar] [CrossRef]
- Khoury, J.; Jerushalmi, J.; Cohen, H.I.; Loberant, N.; Zaina, A. Super scan leading to definitive diagnosis in a patient with recurrent syncope. Clin. Nucl. Med. 2003, 28, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Zanotti, R.; Bonadonna, P.; Artuso, A.; Caruso, B.; Schena, D.; Vecchiato, D.; Bonifacio, M.; Viapiana, O.; Gatti, D. Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone 2011, 49, 880–885. [Google Scholar] [CrossRef]
- Sohn, M.-H.; Kim, M.-W.; Lim, S.T.; Kwak, J.-Y.; Yim, C.-Y. Unusual bone scintigraphic findings of secondary myelofibrosis associated with disseminated tuberculosis before and after therapy. Clin. Nucl. Med. 2004, 29, 706–708. [Google Scholar] [CrossRef]
- Reske, S. Recent advances in bone marrow scanning. Eur. J. Nucl. Med. 1991, 18, 203–221. [Google Scholar] [CrossRef]
- Padma, S.; Sundaram, P.S. Rare Association of Schizophrenia and Unilateral Graves’disease with Contralateral Thyroid Hemiagenesis in Two Cases of Mccune-Albright Syndrome (Case Report). 2015. Available online: https://www.sid.ir/paper/689816/en (accessed on 10 May 2023).
- Waller, M.L.; Chowdhury, F.U. The basic science of nuclear medicine. Orthop. Trauma 2016, 30, 201–222. [Google Scholar] [CrossRef]
- Diamond, T.H.; Bryant, C.; Quinn, R.; Mohanty, S.T.; Bonar, F.; Baldock, P.A.; McDonald, M.M. Increased bone formation and accelerated bone mass accrual in a man presenting with diffuse osteosclerosis/high bone mass phenotype and adenocarcinoma of unknown primary. JBMR Plus 2023, 7, e10734. [Google Scholar] [CrossRef] [PubMed]
- Zuckier, L.S.; Martineau, P. Altered biodistribution of radiopharmaceuticals used in bone scintigraphy. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2015; pp. 81–96. [Google Scholar]
- Fogelman, I. The bone scan in metabolic bone disease. In Bone Scanning in Clinical Practice; Springer: London, UK, 1987; pp. 73–87. [Google Scholar]
- Mangaonkar, A.A.; Gupta, H.R.; Bera, B.M.; Barmare, S. Bone marrow fibrosis and metastatic prostate adenocarcinoma. Case Rep. 2012, 2012, bcr2012007348. [Google Scholar] [CrossRef] [PubMed]
- Taywade, S.K.; Damle, N.A.; Tripathi, M.; Agarwal, S.; Aggarwal, S. Synchronous parathyroid adenoma and papillary thyroid cancer detected on 99mTc-sestamibi scintigraphy. Indian J. Endocrinol. Metab. 2016, 20, 878. [Google Scholar]
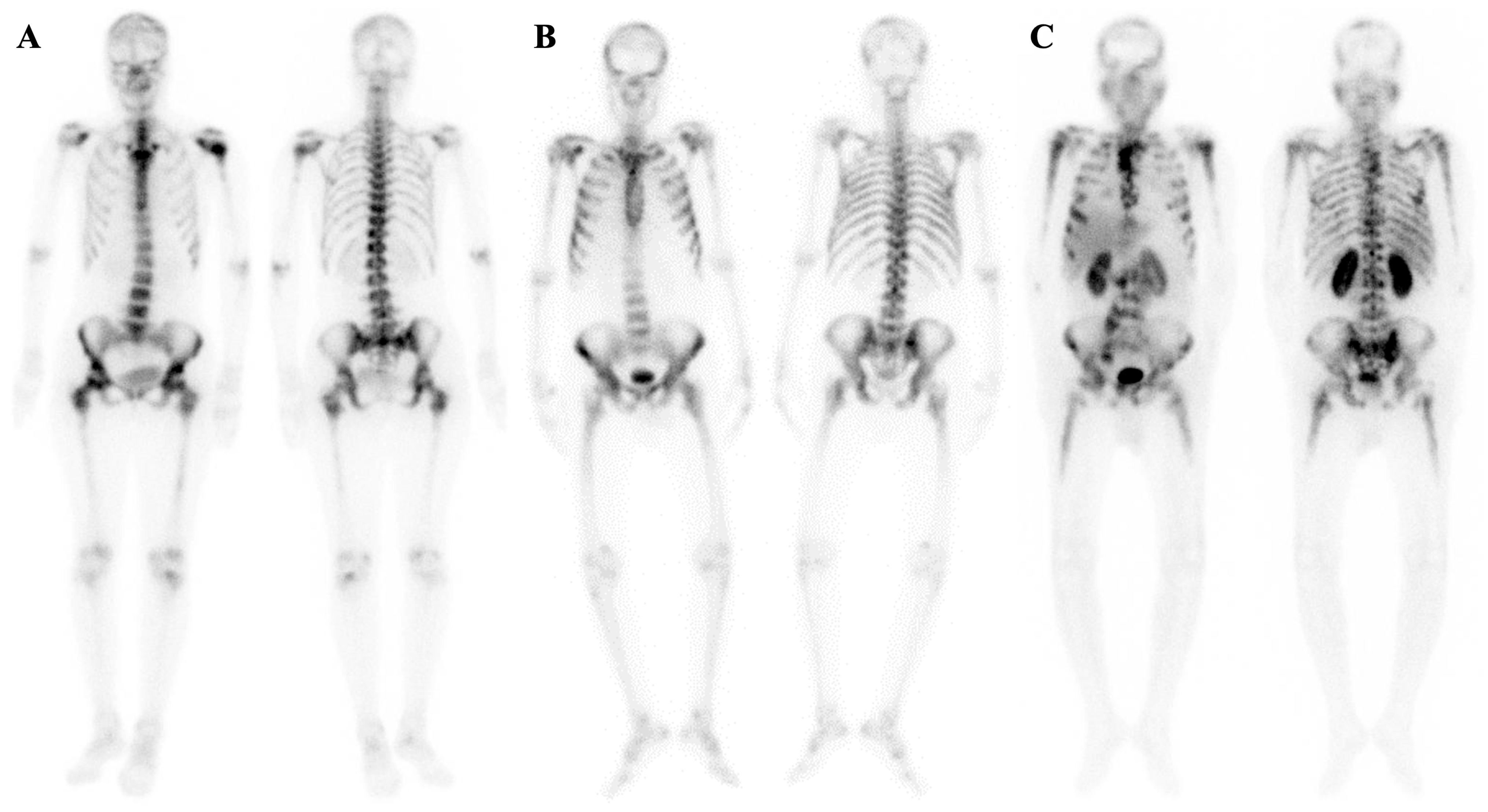
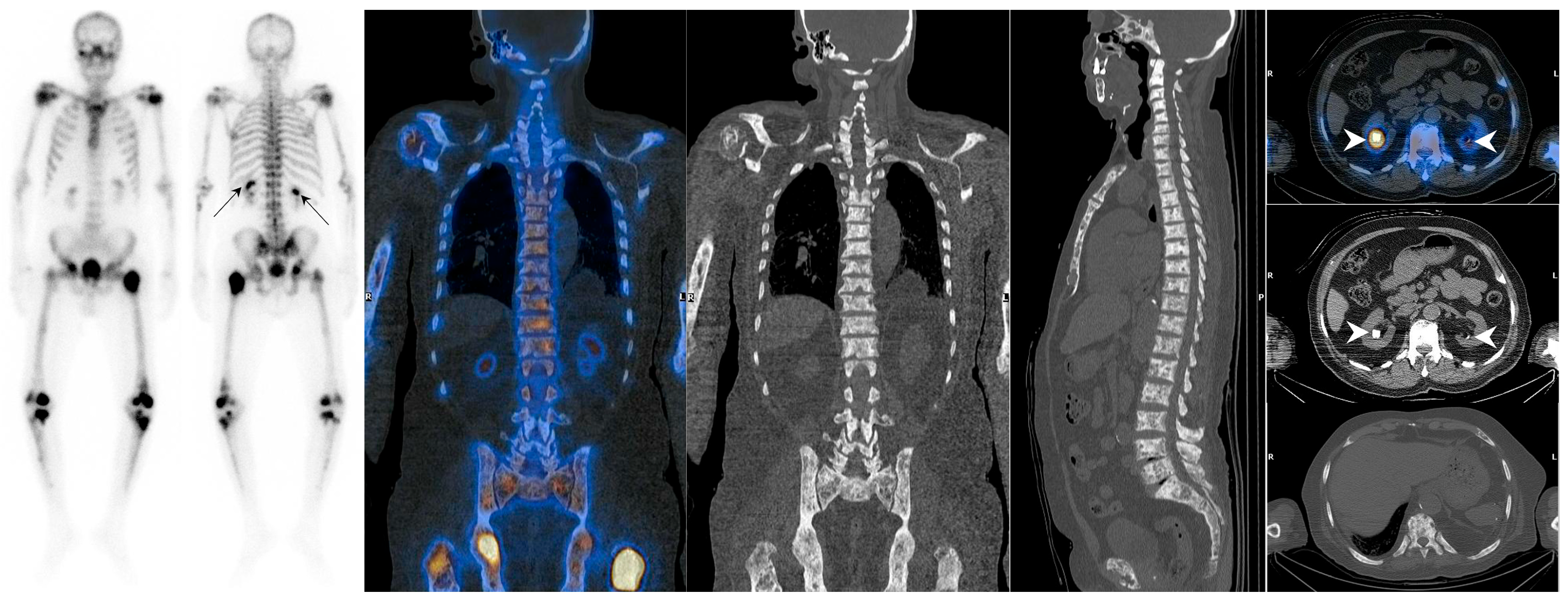
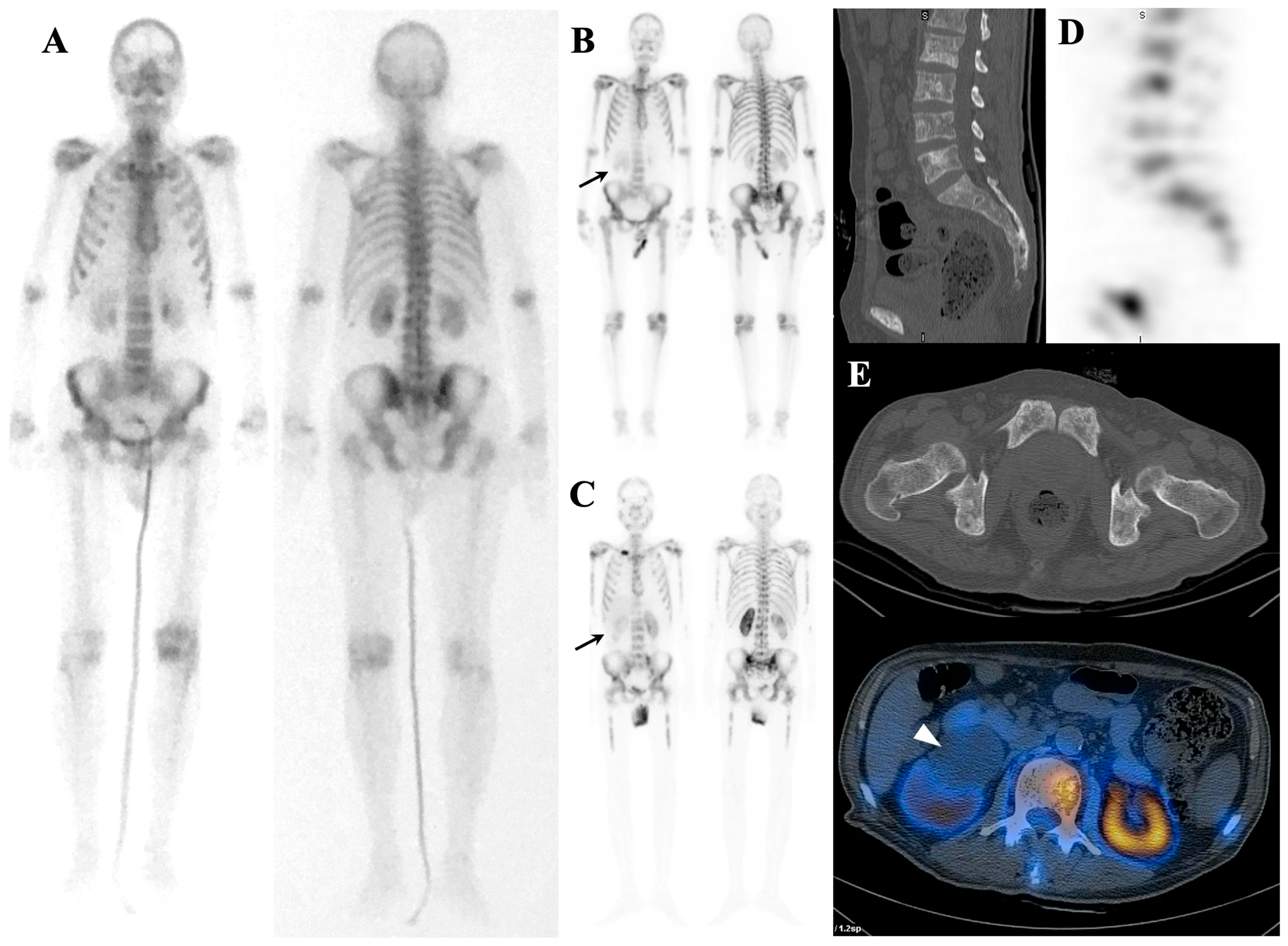
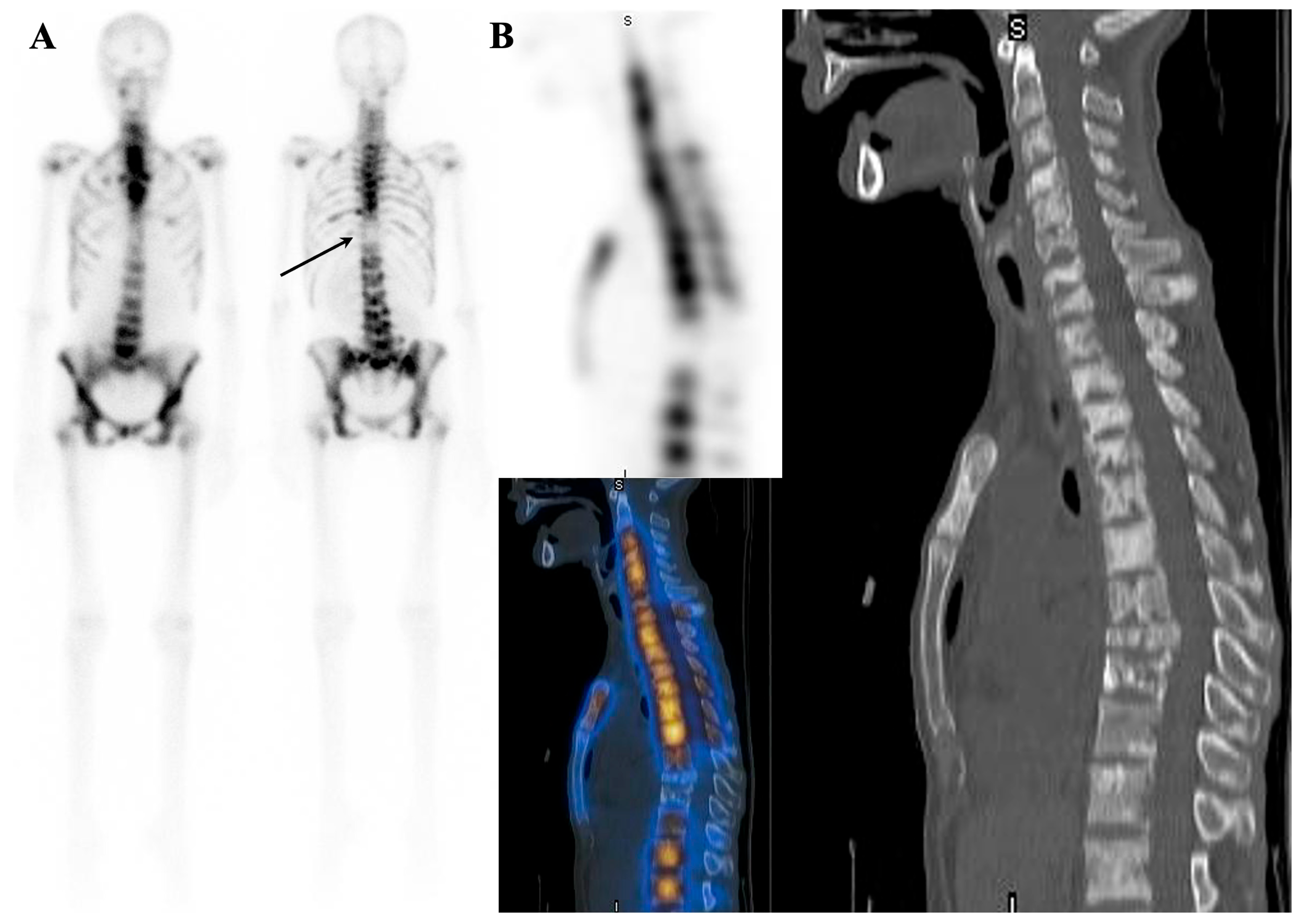

| Mechanism | Examples |
|---|---|
| Chemisorption | |
Contributive factors include the following:
| Myelofibrosis Osteomalacia Hyperthyroidism Hyperparathyroidism, Paget’s disease |
| Bone marrow involvement | |
| Increased tracer uptake due to disseminated bone marrow replacement by tumoral cells | Metastatic superscans |
| Diminished urinary phosphate excretion | |
| Secondary to increased bone resorption or some degrees of renal insufficiencies Increased phosphate levels probably resulting in elevated levels of hydroxyapatite crystals | Metabolic superscans (particularly hyperparathyroidism) |
| Osteomalacia | |
| Immature collagen fibers coupled to bone-seeking tracers | Hereditary metabolic superscans * |
| Metastatic Superscan | Hematologic Disorders | Metabolic Superscan | |||||
|---|---|---|---|---|---|---|---|
| More Common | Less Common | Endocrinopathies | Osteomalacia | Orthopedic Disorders | Rheumatologic Diseases | Miscellaneous | |
| Prostate cancer [15] | Urothelial carcinoma [10,68] | Multiple myeloma [18] | Hyperparathyroidism ** [69] | Vitamin D deficiency [70] | Osteopetrosis [71] | Rheumatoid arthritis [4] | Skeletal fluorosis [72] |
| Breast cancer [73] | Esophageal cancer [1,66] | Waldenstrom macroglobulinemia * [74] | Hyperthyroidism [75] | Hypophosphatemic rickets * [70] | Polyostotic fibrous dysplasia [74] | Ankylosing spondylitis [4] | Hypervitaminosis D [76] |
| Lung cancer [1] | Gastric cancer [77] | Leukemia [32] | Acromegaly [78] | Crohn’s disease | Paget’s disease [79] | Oxalosis [80] | |
| Colorectal cancer [66,81] | Lymphoma [11] | Celiac disease [82] | Juvenile Paget’s disease * [4] | Teriparatide injection [83] | |||
| Nasopharyngeal cancer [84] | Myelofibrosis [25] | Renal tubular acidosis [85] | Aluminum toxicity [4,86] | ||||
| Neuroblastoma [87] | Myelosclerosis [88] | Tumor-induced osteomalacia [89] | Vitamin C deficiency [4] | ||||
| Ewing sarcoma [1] | Systemic mastocytosis [90] | Post-gastrectomy osteomalacia [91] | |||||
| Medullary thyroid carcinoma [1] | Castleman disease [92] | Hereditary *** [93] | |||||
| Melanoma [94] | Aplastic anemia [74] | ||||||
| Salivary gland tumor [1] | Rosai–Dorfman disease [95] | ||||||
| Pheochromocytoma [50] | |||||||
| Glioma [96,97] | |||||||
| Medulloblastoma [98] | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari, E.; Shakeri, S.; Roustaei, H.; Fotouhi, M.; Sadeghi, R.; Harsini, S.; Vali, R. Superscan Pattern on Bone Scintigraphy: A Comprehensive Review. Diagnostics 2024, 14, 2229. https://doi.org/10.3390/diagnostics14192229
Askari E, Shakeri S, Roustaei H, Fotouhi M, Sadeghi R, Harsini S, Vali R. Superscan Pattern on Bone Scintigraphy: A Comprehensive Review. Diagnostics. 2024; 14(19):2229. https://doi.org/10.3390/diagnostics14192229
Chicago/Turabian StyleAskari, Emran, Sara Shakeri, Hessamoddin Roustaei, Maryam Fotouhi, Ramin Sadeghi, Sara Harsini, and Reza Vali. 2024. "Superscan Pattern on Bone Scintigraphy: A Comprehensive Review" Diagnostics 14, no. 19: 2229. https://doi.org/10.3390/diagnostics14192229
APA StyleAskari, E., Shakeri, S., Roustaei, H., Fotouhi, M., Sadeghi, R., Harsini, S., & Vali, R. (2024). Superscan Pattern on Bone Scintigraphy: A Comprehensive Review. Diagnostics, 14(19), 2229. https://doi.org/10.3390/diagnostics14192229







