Improved Discriminability of Severe Lung Injury and Atelectasis in Thoracic Trauma at Low keV Virtual Monoenergetic Images from Photon-Counting Detector CT
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Declaration
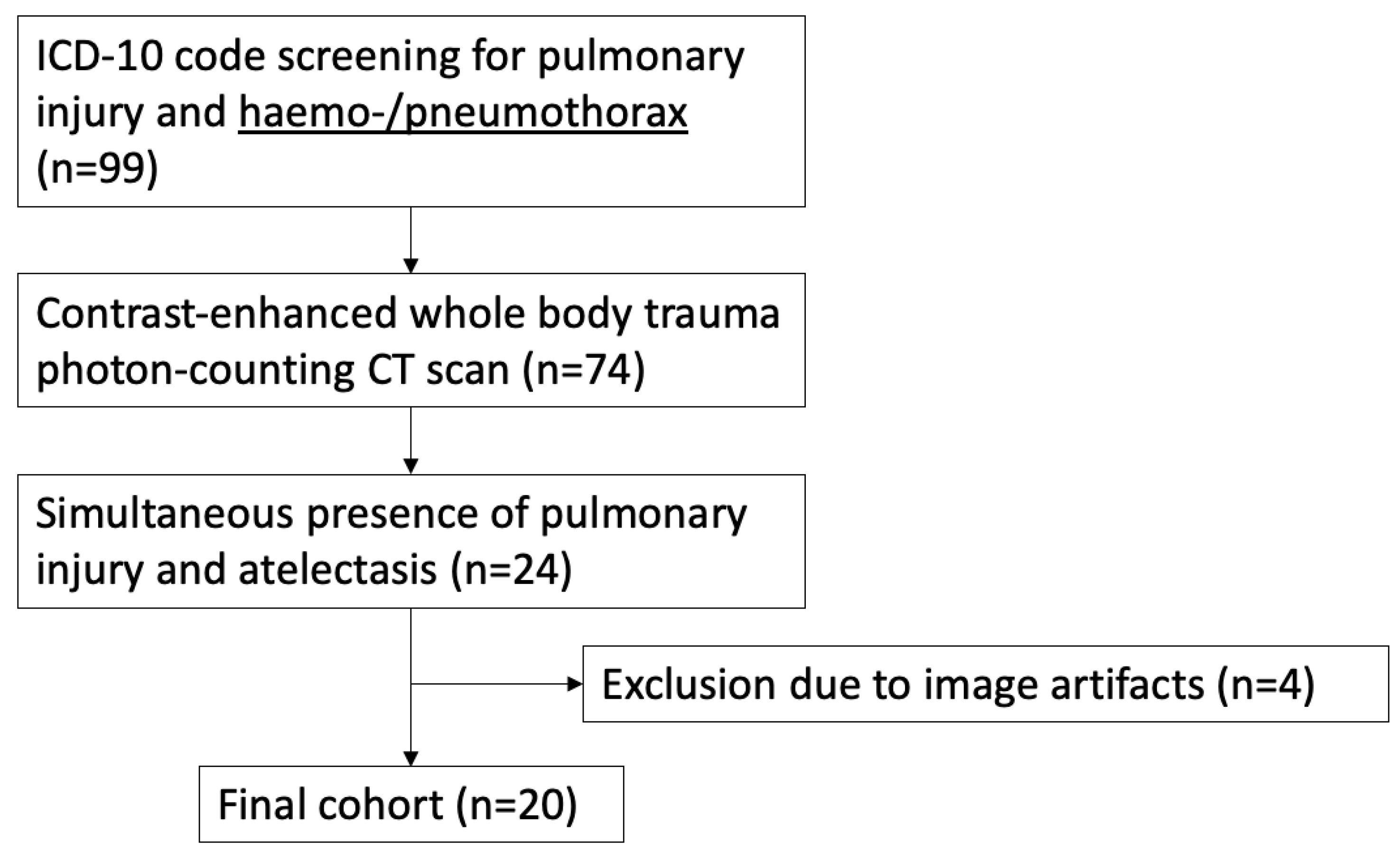
2.2. Study Population
2.3. Data Acquisition
2.4. Objective and Subjective Image Parameters
2.5. Data Analysis
3. Results
3.1. Patient Demographics and Dose Exposure
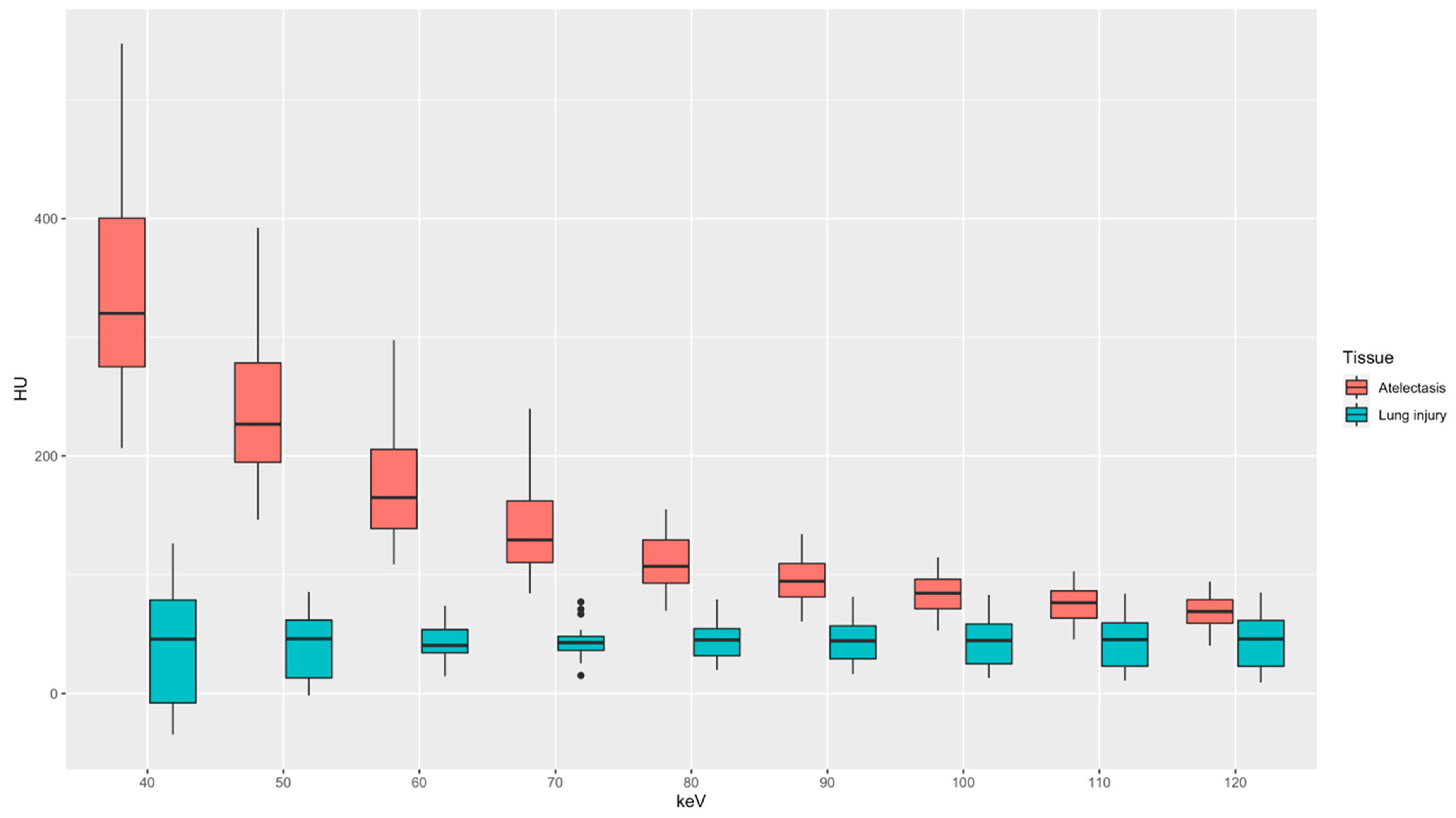
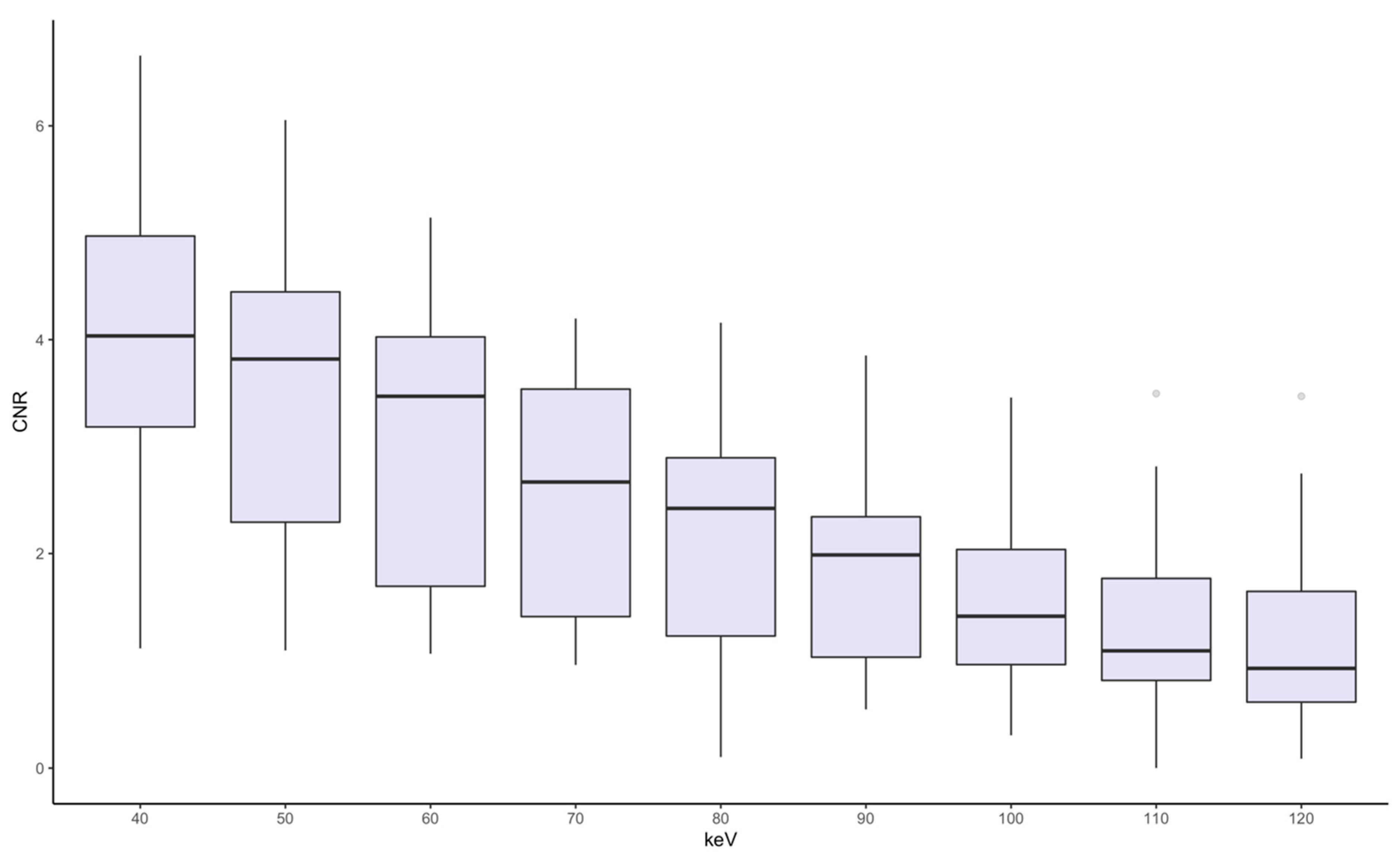
3.2. Quantitative Image Analysis
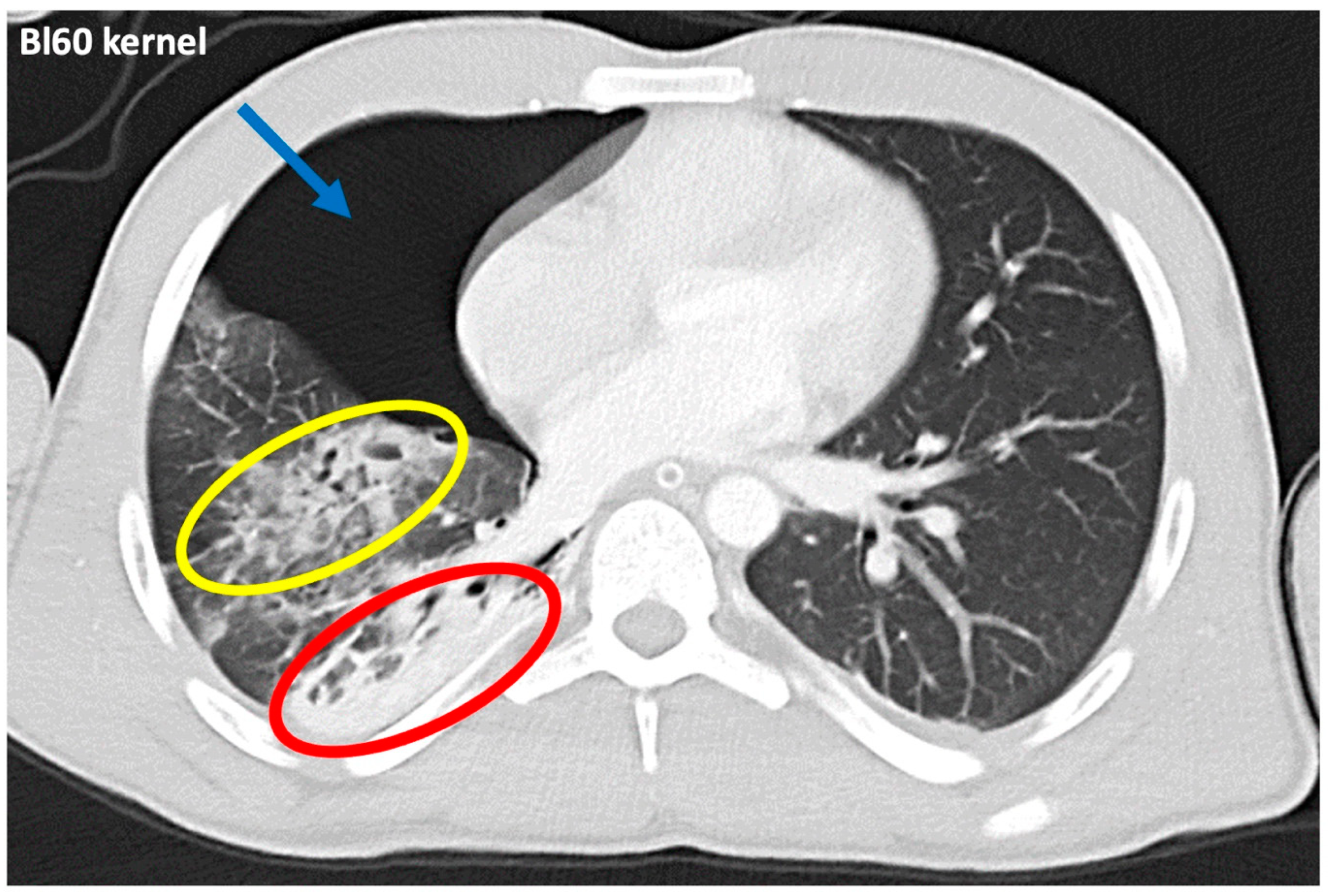
3.3. Qualitative Image Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulz-Drost, S.; Finkbeiner, R.; Lefering, R.; Grosso, M.; Krinner, S.; Langenbach, A. Lung Contusion in Polytrauma: An Analysis of the TraumaRegister DGU. Thorac. Cardiovasc. Surg. 2021, 69, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Remmers, D.; Rice, J.; Ebisch, M.; Krettek, C.; Tscherne, H. Appraisal of early evaluation of blunt chest trauma: Development of a standardized scoring system for initial clinical decision making. J. Trauma 2000, 49, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Drost, S.; Oppel, P.; Grupp, S.; Krinner, S.; Langenbach, A.; Lefering, R.; Mauerer, A. Bony injuries of the thoracic cage in multiple trauma: Incidence, concomitant injuries, course and outcome. Unfallchirurg 2016, 119, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.M.; Dubose, J.J. Pulmonary contusion: An update on recent advances in clinical management. World J. Surg. 2010, 34, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Ketai, L.; Primack, S. Thoracic Trauma. In Diseases of the Chest, Breast, Heart and Vessels 2019–2022: Diagnostic and Interventional Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Požgain, Z.; Kristek, D.; Lovrić, I.; Kondža, G.; Jelavić, M.; Kocur, J.; Danilović, M. Pulmonary contusions after blunt chest trauma: Clinical significance and evaluation of patient management. Eur. J. Trauma Emerg. Surg. 2018, 44, 773–777. [Google Scholar] [CrossRef]
- Neudecker, J.; Schulz-Drost, S.; Walles, T. Treatment of Persistent Parenchymal Lung Injuries in Thoracic Trauma: Lung Laceration, Pleural Fistula and Pneumothorax. Zentralbl. Chir. 2023, 148, 93–104. [Google Scholar] [CrossRef]
- Störmann, P.; Krämer, S.; Raab, S.; Kalverkamp, S.; Graeff, P. Pathophysiology, Diagnostics and Therapy of Pulmonary Contusion—Recommendations of the Interdisciplinary Group on Thoracic Trauma of the Section NIS of the German Society for Trauma Surgery (DGU) and the German Society for Thoracic Surgery (DGT). Zentralbl. Chir. 2023, 148, 50–56. [Google Scholar] [CrossRef]
- Wong, Y.C.; Wang, L.J.; Kaewlai, R.; Wu, C.H. Watch Out for the Early Killers: Imaging Diagnosis of Thoracic Trauma. Korean J. Radiol. 2023, 24, 752–760. [Google Scholar] [CrossRef]
- Becher, R.D.; Colonna, A.L.; Enniss, T.M.; Weaver, A.A.; Crane, D.K.; Martin, R.S.; Mowery, N.T.; Miller, P.R.; Stitzel, J.D.; Hoth, J.J. An innovative approach to predict the development of adult respiratory distress syndrome in patients with blunt trauma. J. Trauma Acute Care Surg. 2012, 73, 1229–1235. [Google Scholar] [CrossRef]
- Mahmood, I.; El-Menyar, A.; Younis, B.; Ahmed, K.; Nabir, S.; Ahmed, M.N.; Al-Yahri, O.; Mahmood, S.; Consunji, R.; Al-Thani, H. Clinical Significance and Prognostic Implications of Quantifying Pulmonary Contusion Volume in Patients with Blunt Chest Trauma. Med. Sci. Monit. 2017, 23, 3641–3648. [Google Scholar] [CrossRef]
- Miller, P.R.; Croce, M.A.; Bee, T.K.; Qaisi, W.G.; Smith, C.P.; Collins, G.L.; Fabian, T.C. ARDS after pulmonary contusion: Accurate measurement of contusion volume identifies high-risk patients. J. Trauma 2001, 51, 223–228; discussion 229–230. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.T.; Herr, K.D.; Hamlin, S.A.; Henry, T.; Little, B.P.; Naeger, D.M.; Hanna, T.N. Imaging Manifestations of Chest Trauma. RadioGraphics 2021, 41, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Konietzke, P.; Steentoft, H.H.; Wagner, W.L.; Albers, J.; Dullin, C.; Skornitzke, S.; Stiller, W.; Weber, T.F.; Kauczor, H.U.; Wielpütz, M.O. Consolidated lung on contrast-enhanced chest CT: The use of spectral-detector computed tomography parameters in differentiating atelectasis and pneumonia. Heliyon 2021, 7, e07066. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Bryer, J.; Speerschneider, K. Likert: Analysis and Visualization Likert Items. R Package Version 1.3.5. 2016. Available online: http://github.com/jbryer/likert (accessed on 27 September 2024).
- Adam, S.Z.; Rabinowich, A.; Kessner, R.; Blachar, A. Spectral CT of the abdomen: Where are we now? Insights Imaging 2021, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; van Assen, M.; Tesche, C.; et al. Review of Clinical Applications for Virtual Monoenergetic Dual-Energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef]
- Cester, D.; Eberhard, M.; Alkadhi, H.; Euler, A. Virtual monoenergetic images from dual-energy CT: Systematic assessment of task-based image quality performance. Quant. Imaging Med. Surg. 2021, 12, 726–741. [Google Scholar] [CrossRef]
- Godoy, M.C.; Heller, S.L.; Naidich, D.P.; Assadourian, B.; Leidecker, C.; Schmidt, B.; Vlahos, I. Dual-energy MDCT: Comparison of pulmonary artery enhancement on dedicated CT pulmonary angiography, routine and low contrast volume studies. Eur. J. Radiol. 2011, 79, e11–e17. [Google Scholar] [CrossRef]
- Yu, L.; Leng, S.; McCollough, C.H. Dual-Energy CT–Based Monochromatic Imaging. Am. J. Roentgenol. 2012, 199, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Christner, J.A.; Leng, S.; Wang, J.; Fletcher, J.G.; McCollough, C.H. Virtual monochromatic imaging in dual-source dual-energy CT: Radiation dose and image quality. Med. Phys. 2011, 38, 6371–6379. [Google Scholar] [CrossRef]
- Grant, K.L.; Flohr, T.G.; Krauss, B.; Sedlmair, M.; Thomas, C.; Schmidt, B. Assessment of an advanced image-based technique to calculate virtual monoenergetic computed tomographic images from a dual-energy examination to improve contrast-to-noise ratio in examinations using iodinated contrast media. Investig. Radiol. 2014, 49, 586–592. [Google Scholar] [CrossRef]
- Schabel, C.; Bongers, M.; Sedlmair, M.; Korn, A.; Grosse, U.; Mangold, S.; Claussen, C.D.; Thomas, C. Assessment of the hepatic veins in poor contrast conditions using dual energy CT: Evaluation of a novel monoenergetic extrapolation software algorithm. Rofo 2014, 186, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Trommer, J.; Wichmann, J.L.; Scholtz, J.-E.; Martin, S.S.; Lehnert, T.; Vogl, T.J.; Bodelle, B. Comprehensive Comparison of Virtual Monoenergetic and Linearly Blended Reconstruction Techniques in Third-Generation Dual-Source Dual-Energy Computed Tomography Angiography of the Thorax and Abdomen. Investig. Radiol. 2016, 51, 582–590. [Google Scholar] [CrossRef]
- Sun, E.X.; Wortman, J.R.; Uyeda, J.W.; Lacson, R.; Sodickson, A.D. Virtual monoenergetic dual-energy CT for evaluation of hepatic and splenic lacerations. Emerg. Radiol. 2019, 26, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, G.; Walsh, J.P.; Zhang, Y.; Niu, B.; Macri, F.; Khasanova, E.; Metwally, O.; Murray, N.; Nicolaou, S. Virtual monochromatic reconstructions of dual energy CT in abdominal trauma: Optimization of energy level improves pancreas laceration conspicuity and diagnostic confidence. Emerg. Radiol. 2021, 28, 1–7. [Google Scholar] [CrossRef]
- Edwards, R.M.; Godwin, J.D.; Hippe, D.S.; Kicska, G. A Quantitative Approach to Distinguish Pneumonia From Atelectasis Using Computed Tomography Attenuation. J. Comput. Assist. Tomogr. 2016, 40, 746–751. [Google Scholar] [CrossRef]
- Slade, M. Management of pneumothorax and prolonged air leak. Semin. Respir. Crit. Care Med. 2014, 35, 706–714. [Google Scholar] [CrossRef]
- Tomlanovich, M.C. Pulmonary parenchymal injuries. Emerg. Med. Clin. N. Am. 1983, 1, 379–392. [Google Scholar] [CrossRef]
- Bakowitz, M.; Bruns, B.; McCunn, M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 54. [Google Scholar] [CrossRef]
- Anhaus, J.A.; Heider, M.; Killermann, P.; Hofmann, C.; Mahnken, A.H. A New Iterative Metal Artifact Reduction Algorithm for Both Energy-Integrating and Photon-Counting CT Systems. Investig. Radiol. 2024, 59, 526–537. [Google Scholar] [CrossRef]
- Zhou, W.; Bartlett, D.J.; Diehn, F.E.; Glazebrook, K.N.; Kotsenas, A.L.; Carter, R.E.; Fletcher, J.G.; McCollough, C.H.; Leng, S. Reduction of Metal Artifacts and Improvement in Dose Efficiency Using Photon-Counting Detector Computed Tomography and Tin Filtration. Investig. Radiol. 2019, 54, 204–211. [Google Scholar] [CrossRef]
- Matsumoto, K.; Jinzaki, M.; Tanami, Y.; Ueno, A.; Yamada, M.; Kuribayashi, S. Virtual monochromatic spectral imaging with fast kilovoltage switching: Improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 2011, 259, 257–262. [Google Scholar] [CrossRef]
- Große Hokamp, N.; Gilkeson, R.; Jordan, M.K.; Laukamp, K.R.; Neuhaus, V.F.; Haneder, S.; Halliburton, S.S.; Gupta, A. Virtual monoenergetic images from spectral detector CT as a surrogate for conventional CT images: Unaltered attenuation characteristics with reduced image noise. Eur. J. Radiol. 2019, 117, 49–55. [Google Scholar] [CrossRef]
- Atwi, N.E.; Smith, D.L.; Flores, C.D.; Dharaiya, E.; Danrad, R.; Kambadakone, A.; Toshav, A.M. Dual-energy CT in the obese: A preliminary retrospective review to evaluate quality and feasibility of the single-source dual-detector implementation. Abdom. Radiol. (NY) 2019, 44, 783–789. [Google Scholar] [CrossRef]

| ISS | AIS Chest | ||
|---|---|---|---|
| ISS-category | n= | AIS-category | n= |
| 74–50 (critical) | 3 | 5—critical | 5 |
| 49–25 (severe) | 9 | 4—severe | 4 |
| 24–16 (serious) | 4 | 3—serious | 10 |
| 15–05 (moderate) | 4 | 2—moderate | 1 |
| Type of Chest Injury | Frequency | ||
| Pulmonary contusion | 19 | ||
| Pulmonary laceration | 16 | ||
| Haematothorax | 14 | ||
| Pneumothorax | 20 | ||
| Mediastinal hematoma | 2 | ||
| Bronchial rupture | 1 | ||
| Diaphragmatic rupture | 1 | ||
| Pneumomediastinum | 4 | ||
| Soft tissue emphysema | 12 | ||
| Singular rib fracture | 1 | ||
| Serial rib fracture | 18 | ||
| Thoracic spine injury | 7 | ||
| Bony shoulder girdle injury | 12 | ||
| Mean ± SD | ||||
|---|---|---|---|---|
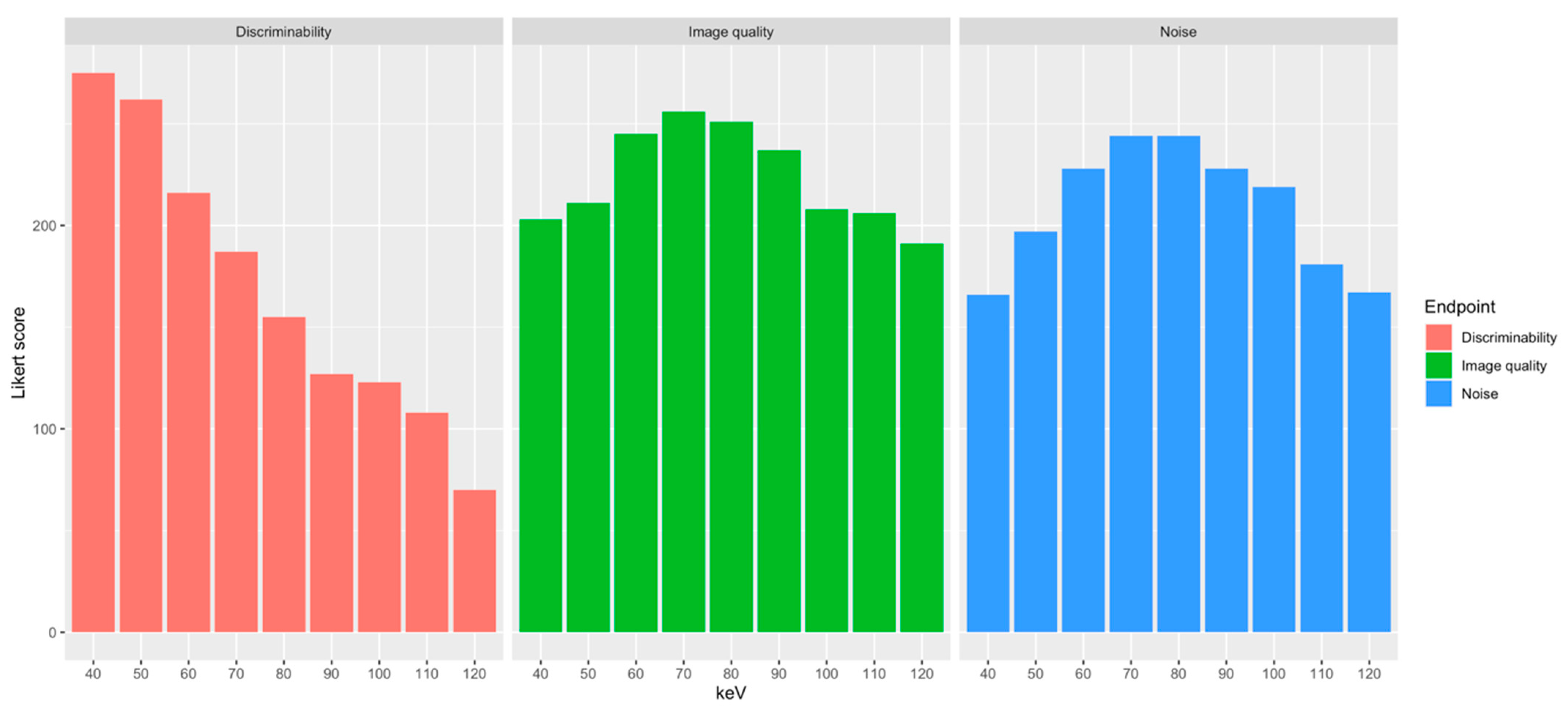
| Energy Level | Discriminability | Noise | Image Quality | Overall Score |
| 40 keV | 4.66 ± 0.69 | 2.81 ± 0.6 | 3.44 ± 0.6 | 644 |
| 50 keV | 4.44 ± 0.56 | 3.34 ± 0.61 | 3.58 ± 0.53 | 670 |
| 60 keV | 3.66 ± 0.69 | 3.86 ± 0.47 | 4.15 ± 0.48 | 689 |
| 70 keV | 3.17 ± 0.77 | 4.14 ± 0.39 | 4.34 ± 0.48 | 687 |
| 80 keV | 2.63 ± 0.79 | 4.14 ± 0.47 | 4.25 ± 0.51 | 650 |
| 90 keV | 2.15 ± 0.66 | 3.86 ± 0.73 | 4.02 ± 0.68 | 592 |
| 100 keV | 2.08 ± 0.92 | 3.71 ± 0.85 | 3.53 ± 0.8 | 550 |
| 110 keV | 1.83 ± 0.91 | 3.07 ± 1.27 | 3.49 ± 0.8 | 495 |
| 120 keV | 1.18 ± 0.43 | 2.83 ± 1.29 | 3.24 ± 0.82 | 428 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaatsch, H.L.; Völlmecke, M.F.; Becker, B.V.; Dillinger, D.; Kubitscheck, L.; Wöhler, A.; Schaaf, S.; Piechotka, J.; Schreyer, C.; Schwab, R.; et al. Improved Discriminability of Severe Lung Injury and Atelectasis in Thoracic Trauma at Low keV Virtual Monoenergetic Images from Photon-Counting Detector CT. Diagnostics 2024, 14, 2231. https://doi.org/10.3390/diagnostics14192231
Kaatsch HL, Völlmecke MF, Becker BV, Dillinger D, Kubitscheck L, Wöhler A, Schaaf S, Piechotka J, Schreyer C, Schwab R, et al. Improved Discriminability of Severe Lung Injury and Atelectasis in Thoracic Trauma at Low keV Virtual Monoenergetic Images from Photon-Counting Detector CT. Diagnostics. 2024; 14(19):2231. https://doi.org/10.3390/diagnostics14192231
Chicago/Turabian StyleKaatsch, Hanns Leonhard, Maximilian Franz Völlmecke, Benjamin V. Becker, Daniel Dillinger, Laura Kubitscheck, Aliona Wöhler, Sebastian Schaaf, Joel Piechotka, Christof Schreyer, Robert Schwab, and et al. 2024. "Improved Discriminability of Severe Lung Injury and Atelectasis in Thoracic Trauma at Low keV Virtual Monoenergetic Images from Photon-Counting Detector CT" Diagnostics 14, no. 19: 2231. https://doi.org/10.3390/diagnostics14192231
APA StyleKaatsch, H. L., Völlmecke, M. F., Becker, B. V., Dillinger, D., Kubitscheck, L., Wöhler, A., Schaaf, S., Piechotka, J., Schreyer, C., Schwab, R., Overhoff, D., & Waldeck, S. (2024). Improved Discriminability of Severe Lung Injury and Atelectasis in Thoracic Trauma at Low keV Virtual Monoenergetic Images from Photon-Counting Detector CT. Diagnostics, 14(19), 2231. https://doi.org/10.3390/diagnostics14192231






