Extracellular Vesicle Characteristics in Local Fluid and Plasma Measured by Nanoparticle Tracking Analysis Can Help Differentiate High-Grade Serous Carcinoma from Benign Ovarian Pathology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. Sample Preparation and Storage
2.4. Sample Purification for Nanoparticle Tracking Analysis in Scatter Mode (S-NTA)
2.5. Quantification of EV Size and Concentration
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Correlation of EV Characteristics between Free Peritoneal Fluid and Plasma in BOP Patients
3.3. Correlation of EV Characteristics between FPF and Peritoneal Washing in BOP Patients
3.4. Correlation of EV Characteristics between Ascites and Plasma in HGSC Patients
3.5. Comparison of EV Concentration and Size Distribution in Local Fluid and Plasma between Patients with BOP and Patients with Advanced HGSC
3.6. Concentration and Size Distribution of EVs in Local Fluid and Plasma as Potential Biomarkers for HGSC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, N.; Sessa, C.; Du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ni, J.; Zhu, Y.; Pang, B.; Graham, P.; Zhang, H.; Li, Y. Liquid Biopsy in Ovarian Cancer: Recent Advances in Circulating Extracellular Vesicle Detection for Early Diagnosis and Monitoring Progression. Theranostics 2019, 9, 4130–4140. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Croft, P.K.; Sharma, S.; Godbole, N.; Rice, G.E.; Salomon, C. Ovarian-Cancer-Associated Extracellular Vesicles: Microenvironmental Regulation and Potential Clinical Applications. Cells 2021, 10, 2272. [Google Scholar] [CrossRef]
- Hergueta-Redondo, M.; Peinado, H. The Influence of Secreted Factors and Extracellular Vesicles in Ovarian Cancer Metastasis. Eur. J. Cancer Suppl. 2020, 15, 38–48. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F.; Nazarenko, I.; Ekström, K.; Murtaza, I.; Anees, M.; Sultan, A.; Neder, L.; Camussi, G.; Valadi, H.; et al. Extracellular Vesicles in Ovarian Cancer: Applications to Tumor Biology, Immunotherapy and Biomarker Discovery. Expert Rev. Proteom. 2016, 13, 395–409. [Google Scholar] [CrossRef]
- Samuel, P.; Mulcahy, L.A.; Furlong, F.; McCarthy, H.O.; Brooks, S.A.; Fabbri, M.; Pink, R.C.; Carter, D.R.F. Cisplatin Induces the Release of Extracellular Vesicles from Ovarian Cancer Cells That Can Induce Invasiveness and Drug Resistance in Bystander Cells. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170065. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-Free Detection and Molecular Profiling of Exosomes with a Nano-Plasmonic Sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peng, P.; Ou, X.; Shen, K.; Wu, X. Ovarian Cancer Circulating Extracelluar Vesicles Promote Coagulation and Have a Potential in Diagnosis: An iTRAQ Based Proteomic Analysis. BMC Cancer 2019, 19, 1095. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Zeng, Y. Ultrasensitive Microfluidic Analysis of Circulating Exosomes Using a Nanostructured Graphene Oxide/Polydopamine Coating. Lab. Chip 2016, 16, 3033–3042. [Google Scholar] [CrossRef]
- Yamamoto, C.M.; Oakes, M.L.; Murakami, T.; Muto, M.G.; Berkowitz, R.S.; Ng, S.-W. Comparison of Benign Peritoneal Fluid- and Ovarian Cancer Ascites-Derived Extracellular Vesicle RNA Biomarkers. J. Ovarian Res. 2018, 11, 20. [Google Scholar] [CrossRef]
- Lucidi, A.; Buca, D.; Ronsini, C.; Tinari, S.; Bologna, G.; Buca, D.; Leombroni, M.; Liberati, M.; D’Antonio, F.; Scambia, G.; et al. Role of Extracellular Vesicles in Epithelial Ovarian Cancer: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 8762. [Google Scholar] [CrossRef]
- Herzog, M.; Verdenik, I.; Kobal, B.; Černe, K. Higher EpCAM-Positive Extracellular Vesicle Concentration in Ascites Is Associated with Shorter Progression-Free Survival of Patients with Advanced High-Grade Serous Carcinoma. Int. J. Mol. Sci. 2024, 25, 6780. [Google Scholar] [CrossRef]
- Zhai, C.; Xie, F.; Xu, J.; Yang, Y.; Zheng, W.; Hu, H.; Ding, X.; Yu, H. Correlation between Membrane Proteins and Sizes of Extracellular Vesicles and Particles: A Potential Signature for Cancer Diagnosis. J. Extracell. Vesicles 2023, 12, 12391. [Google Scholar] [CrossRef]
- Guan, S.; Yu, H.; Yan, G.; Gao, M.; Sun, W.; Zhang, X. Size-Dependent Sub-Proteome Analysis of Urinary Exosomes. Anal. Bioanal. Chem. 2019, 411, 4141–4149. [Google Scholar] [CrossRef]
- Zheng, H.; Guan, S.; Wang, X.; Zhao, J.; Gao, M.; Zhang, X. Deconstruction of Heterogeneity of Size-Dependent Exosome Subpopulations from Human Urine by Profiling N-Glycoproteomics and Phosphoproteomics Simultaneously. Anal. Chem. 2020, 92, 9239–9246. [Google Scholar] [CrossRef]
- Badovinac, D.; Goričar, K.; Lavrin, T.; Zavrtanik, H.; Dolžan, V.; Lenassi, M.; Tomažič, A. Plasma Extracellular Vesicle Characteristics as Biomarkers of Resectability and Radicality of Surgical Resection in Pancreatic Cancer—A Prospective Cohort Study. Cancers 2023, 15, 605. [Google Scholar] [CrossRef] [PubMed]
- Černe, K.; Kelhar, N.; Resnik, N.; Herzog, M.; Vodnik, L.; Veranič, P.; Kobal, B. Characteristics of Extracellular Vesicles from a High-Grade Serous Ovarian Cancer Cell Line Derived from a Platinum-Resistant Patient as a Potential Tool for Aiding the Prediction of Responses to Chemotherapy. Pharmaceuticals 2023, 16, 907. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; Van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Yu, J.; Sane, S.; Kim, J.-E.; Yun, S.; Kim, H.-J.; Jo, K.B.; Wright, J.P.; Khoshdoozmasouleh, N.; Lee, K.; Oh, H.T.; et al. Biogenesis and Delivery of Extracellular Vesicles: Harnessing the Power of EVs for Diagnostics and Therapeutics. Front. Mol. Biosci. 2024, 10, 1330400. [Google Scholar] [CrossRef]
- Van Der Pol, E.; Coumans, F.A.W.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; Van Leeuwen, T.G.; Nieuwland, R. Particle Size Distribution of Exosomes and Microvesicles Determined by Transmission Electron Microscopy, Flow Cytometry, Nanoparticle Tracking Analysis, and Resistive Pulse Sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Gercel-Taylor, C.; Atay, S.; Tullis, R.H.; Kesimer, M.; Taylor, D.D. Nanoparticle Analysis of Circulating Cell-Derived Vesicles in Ovarian Cancer Patients. Anal. Biochem. 2012, 428, 44–53. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Jerman, K.G.; Kobal, B.; Jakimovska, M.; Verdenik, I.; Cerne, K. Control Values of Ovarian Cancer Tumor Markers and Standardisation of a Protocol for Sampling Peritoneal Fluid and Performing Washing during Laparoscopy. World J. Surg. Oncol. 2014, 12, 278. [Google Scholar] [CrossRef][Green Version]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Sterbenc, N.; Kosec, M.; Bollwein, H.; Klinc, P. The Effect of Equex STM in Freezing Media on Post Thaw Motility, Viability and Dna Integrity of Frozen—Thawed Ram Spermatozoa. Slov. Vet. Res. 2014, 51, 35–42. [Google Scholar] [CrossRef]
- Sedej, I.; Tušek Žnidarič, M.; Dolžan, V.; Lenassi, M.; Arnol, M. Optimization of Isolation Protocol and Characterization of Urinary Extracellular Vesicles as Biomarkers of Kidney Allograft Injury. Clin. Nephrol. 2021, 96, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Wei, S.; Zhou, C.; Yu, J.; Wang, G.; Wang, W.; Zhao, L. Extracellular Vesicles Isolated by Size-Exclusion Chromatography Present Suitability for RNomics Analysis in Plasma. J. Transl. Med. 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Kurtjak, M.; Kereïche, S.; Klepac, D.; Križan, H.; Perčić, M.; Krušić Alić, V.; Lavrin, T.; Lenassi, M.; Wechtersbach, K.; Kojc, N.; et al. Unveiling the Native Morphology of Extracellular Vesicles from Human Cerebrospinal Fluid by Atomic Force and Cryogenic Electron Microscopy. Biomedicines 2022, 10, 1251. [Google Scholar] [CrossRef]
- György, B.; Pálóczi, K.; Balbisi, M.; Turiák, L.; Drahos, L.; Visnovitz, T.; Koltai, E.; Radák, Z. Effect of the 35 Nm and 70 Nm Size Exclusion Chromatography (SEC) Column and Plasma Storage Time on Separated Extracellular Vesicles. Curr. Issues Mol. Biol. 2024, 46, 4337–4357. [Google Scholar] [CrossRef]
- Englisz, A.; Smycz-Kubańska, M.; Mielczarek-Palacz, A. Sensitivity and Specificity of Selected Biomarkers and Their Combinations in the Diagnosis of Ovarian Cancer. Diagnostics 2024, 14, 949. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, P.; Kuang, Y.; Yang, J.; Cao, D.; You, Y.; Shen, K. Characterization of Exosomes Derived from Ovarian Cancer Cells and Normal Ovarian Epithelial Cells by Nanoparticle Tracking Analysis. Tumor Biol. 2016, 37, 4213–4221. [Google Scholar] [CrossRef]

| Study Group | n | |
|---|---|---|
| HGSC | FIGO stage | 37 |
| IIIA, n (%) IIIB, n (%) IIIC, n (%) IVA, n (%) IVB, n (%) | 1 (2.7%) 2 (5.4%) 30 (81%) 1 (2.7%) 3 (8.1%) | |
| Ascites | 37 (100%) | |
| BOP | HP | 40 |
| Endometriotic cyst | 9 (22.5%) | |
| Mucinous cystadenoma | 8 (20%) | |
| Cystic teratoma | 7 (17.5%) | |
| Follicular cyst | 5 (12.5%) | |
| Fibroma | 5 (12.5%) | |
| Paraovarian cyst | 2 (5%) | |
| Corpus luteum cyst | 2 (5%) | |
| Serous cystadenoma | 2 (5%) | |
| FPF | 35 (87.5%) | |
| Peritoneal Washing | 14 (35%) |
| Variables | HGSC | BOP | p-Value | |
|---|---|---|---|---|
| Median (25–75%) | Median (25–75%) | |||
| Age | Years | 65 (59–74) | 35 (29–50) | <0.001 * |
| Preoperative CA125 level | 758 (460–1825) | 20 (13–28) | <0.001 * |
| BOP Patients (Control Group) | ||||
|---|---|---|---|---|
| Variables | FPF | Plasma | p-Value | |
| n = 40 | n = 40 | |||
| Median (25–75%) | Median (25–75%) | |||
| Concentration | EVs/mL | 7.88 × 109 (5.80 × 109–1.03 × 1010) | 3.26 × 1010 (2.03 × 1010–3.86 × 1010) | 0.103 |
| Mean | nm | 131.2 (109.4–159.9) | 89.6 (81.7–96.2) | 0.107 |
| Mode | nm | 78.4 (68.4–87.7) | 69.2 (65.0–76.7) | 0.058 |
| D10 | nm | 74.2 (63.5–81.3) | 60.9 (56.1–68.3) | 0.406 |
| D50 | nm | 104.0 (86.1–141.6) | 77.4 (71.7–86.2) | 0.126 |
| D90 | nm | 229.9 (180.6–275.1) | 131.2 (118.2–146.7) | 0.081 |
| BOP Patients (Control Group) | ||||
|---|---|---|---|---|
| Variables | FPF | Peritoneal Washing | p-Value | |
| n = 14 | n = 14 | |||
| Median (25–75%) | Median (25–75%) | |||
| Concentration | EVs/mL | 9.11 × 109 (5.05 × 109–1.04 × 1010) | 1.68 × 109 (1.5 × 109–2.09 × 109) | 0.007 * |
| Mean | nm | 129.1 (107.9–151.6) | 135.6 (119.4–154.0) | 0.009 * |
| Mode | nm | 76.1 (67.7–83.5) | 76.0 (70.3–82.3) | 0.875 |
| D10 | nm | 74.4 (64.4–78.0) | 72.1 (65.8–81.2) | 0.045 * |
| D50 | nm | 101.1 (86.8–124.9) | 115.3 (87.6–130.4) | 0.001 * |
| D90 | nm | 206.9 (179.5–248.5) | 228.3 (219.1–258.9) | 0.001 * |
| HGSC Patients (Study Group) | ||||
|---|---|---|---|---|
| Variables | Ascites | Plasma | p-Value | |
| n = 37 | n = 37 | |||
| Median (25–75%) | Median (25–75%) | |||
| Concentration | EVs/mL | 1.41 × 1010 (9.03 × 109–2.49 × 1010) | 2.64 × 1010 (1.92 × 1010–3.65 × 1010) | 0.163 |
| Mean | nm | 166.0 (148.1–184.6) | 83.6 (76.9–91.5) | 0.015 * |
| Mode | nm | 101.6 (93.2–128.0) | 61.7 (59.2–66.3) | 0.769 |
| D10 | nm | 95.9 (84.6–103.9) | 54.9 (51.4–58.7) | 0.079 |
| D50 | nm | 150.0 (128.5–166.4) | 69.0 (65.3–80.0) | 0.125 |
| D90 | nm | 243.8 (214.2–280.4) | 141.1 (122.5–156.4) | 0.007 * |
| EV Characteristics Measured by NTA | BOP | HGSC | p |
|---|---|---|---|
| Local fluid (FPF/ascites) | Median (25–75%) | Median (25–75%) | |
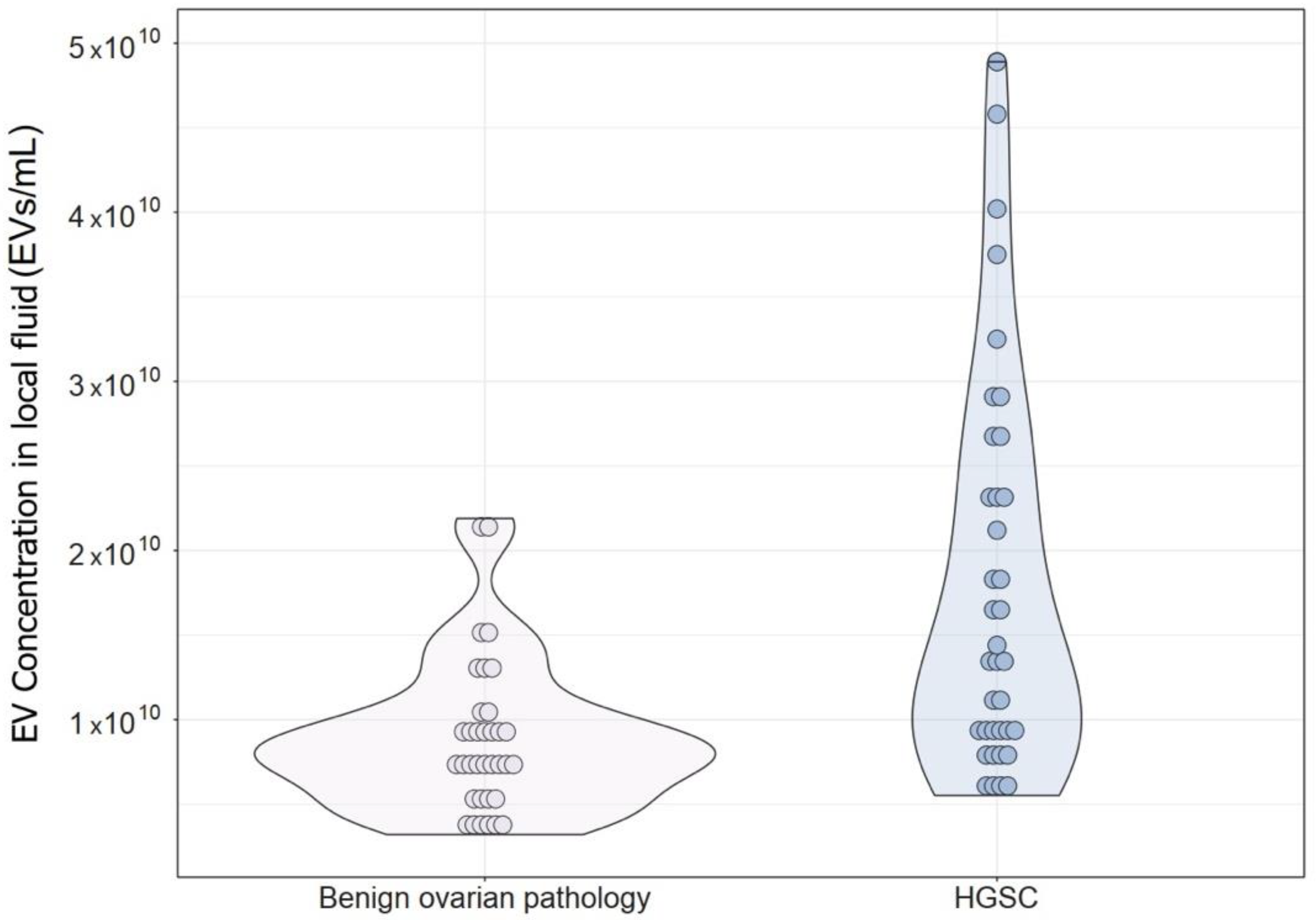
| Concentration (EV/mL) | 7.88 × 109 (5.80 × 109–1.03 × 1010) | 1.41 × 1010 (9.03 × 109–2.49 × 1010) | <0.001 * |
| Mean EV size (nm) | 131.2 (109.4–159.9) | 166.0 (148.1–184.6) | <0.001 * |
| Modal EV size (nm) | 78.4 (68.4–87.7) | 101.6 (93.2–128.0) | <0.001 * |
| D10 value (nm) | 74.2 (63.5–81.3) | 95.9 (84.6–103.9) | <0.001 * |
| D50 value (nm) | 104.0 (86.1–141.6) | 150.0 (128.5–166.4) | <0.001 * |
| D90 value (nm) | 229.9 (180.6–275.1) | 243.8 (214.2–280.4) | 0.058 |
| Plasma | |||
| Concentration (particles/mL) | 3.26 × 1010 (2.03 × 1010–3.86 × 1010) | 2.64 × 1010 (1.92 × 1010–3.65 × 1010) | 0.289 |
| Mean EV size (nm) | 89.6 (81.7–96.2) | 83.6 (76.9–91.5) | 0.085 |
| Modal EV size (nm) | 69.2 (65.0–76.7) | 61.7 (59.2–66.3) | <0.001 * |
| D10 value (nm) | 60.9 (56.1–68.3) | 54.9 (51.4–58.7) | <0.001 * |
| D50 value (nm) | 77.4 (71.7–86.2) | 54.9 (51.4–58.7) | 0.003 * |
| D90 value (nm) | 131.2 (118.2–146.7) | 141.1 (122.5–156.4) | 0.167 |
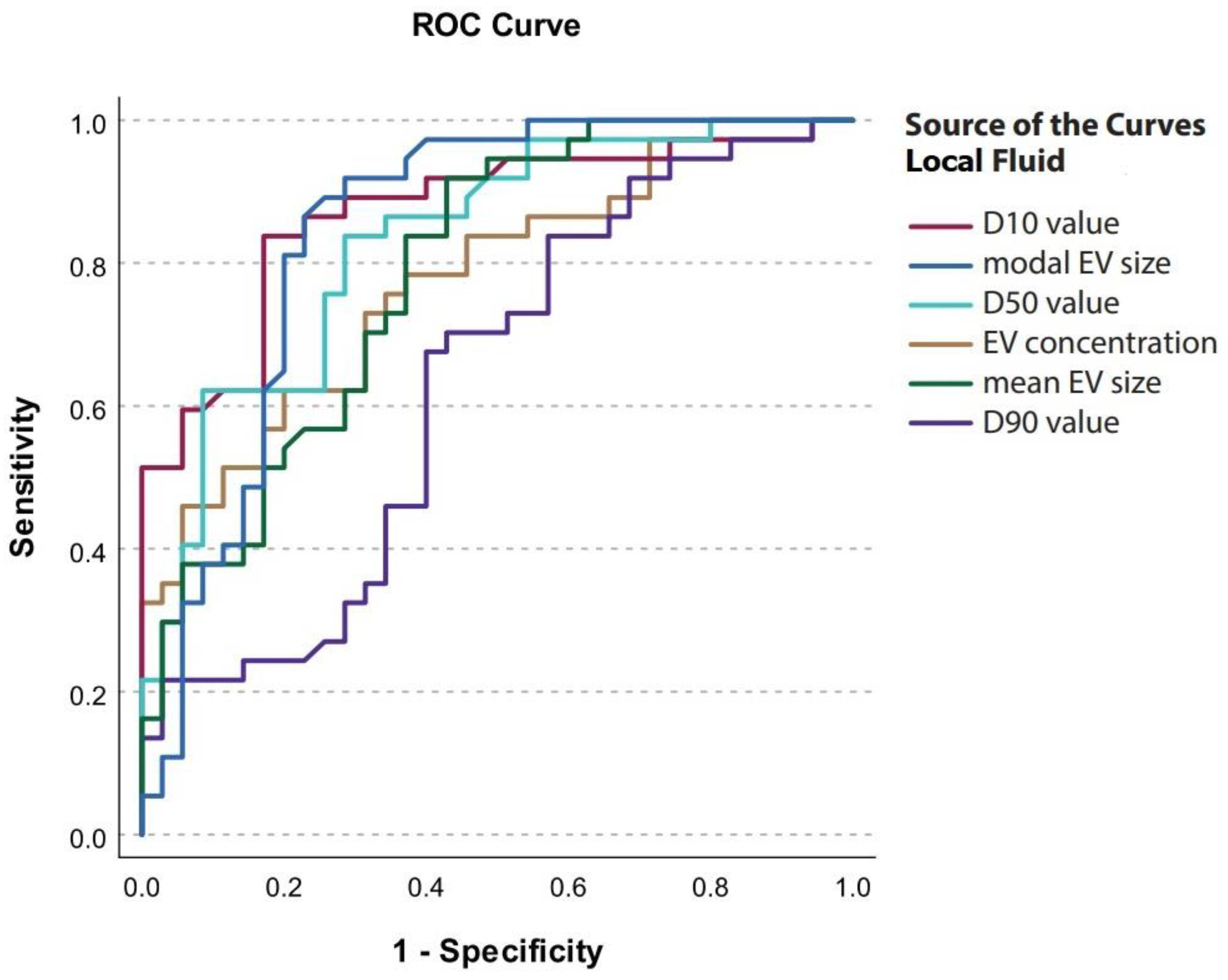
| EV Characteristic in Local Fluid | Cut-Off Value | Sensitivity | Specificity | AUC | 95% CI |
|---|---|---|---|---|---|
| Concentration (EVs/mL) | 1.07 × 1010 | 0.622 | 0.800 | 0.777 | 0.672–0.882 |
| Mean (nm) | 133.3 | 0.919 | 0.571 | 0.786 | 0.682–0.891 |
| Mode (nm) | 87.8 | 0.865 | 0.771 | 0.844 | 0.748–0.941 |
| D10 (nm) | 83.9 | 0.838 | 0.800 | 0.872 | 0.789–0.955 |
| D50 (nm) | 122.7 | 0.838 | 0.571 | 0.830 | 0.736–0.923 |
| D90 (nm) | 234.0 | 0.676 | 0.600 | 0.630 | 0.499–0.760 |
| EV Characteristic in Plasma | Cut-Off Value | Sensitivity | Specificity | AUC | 95% CI |
|---|---|---|---|---|---|
| Mode (nm) | 63.7 | 0.872 | 0.622 | 0.783 | 0.678–0.888 |
| D10 (nm) | 57.6 | 0.718 | 0.676 | 0.736 | 0.619–0.852 |
| D50 (nm) | 71.5 | 0.795 | 0.622 | 0.698 | 0.578–0.818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzog, M.; Verdenik, I.; Černe, K.; Kobal, B. Extracellular Vesicle Characteristics in Local Fluid and Plasma Measured by Nanoparticle Tracking Analysis Can Help Differentiate High-Grade Serous Carcinoma from Benign Ovarian Pathology. Diagnostics 2024, 14, 2235. https://doi.org/10.3390/diagnostics14192235
Herzog M, Verdenik I, Černe K, Kobal B. Extracellular Vesicle Characteristics in Local Fluid and Plasma Measured by Nanoparticle Tracking Analysis Can Help Differentiate High-Grade Serous Carcinoma from Benign Ovarian Pathology. Diagnostics. 2024; 14(19):2235. https://doi.org/10.3390/diagnostics14192235
Chicago/Turabian StyleHerzog, Maruša, Ivan Verdenik, Katarina Černe, and Borut Kobal. 2024. "Extracellular Vesicle Characteristics in Local Fluid and Plasma Measured by Nanoparticle Tracking Analysis Can Help Differentiate High-Grade Serous Carcinoma from Benign Ovarian Pathology" Diagnostics 14, no. 19: 2235. https://doi.org/10.3390/diagnostics14192235
APA StyleHerzog, M., Verdenik, I., Černe, K., & Kobal, B. (2024). Extracellular Vesicle Characteristics in Local Fluid and Plasma Measured by Nanoparticle Tracking Analysis Can Help Differentiate High-Grade Serous Carcinoma from Benign Ovarian Pathology. Diagnostics, 14(19), 2235. https://doi.org/10.3390/diagnostics14192235






