Factors Influencing Background Parenchymal Enhancement in Contrast-Enhanced Mammography Images
Abstract
1. Background
2. Material and Methods
2.1. Inclusion Criteria
2.2. Imaging Technique
2.3. Image Analysis
2.4. Data Acquisition
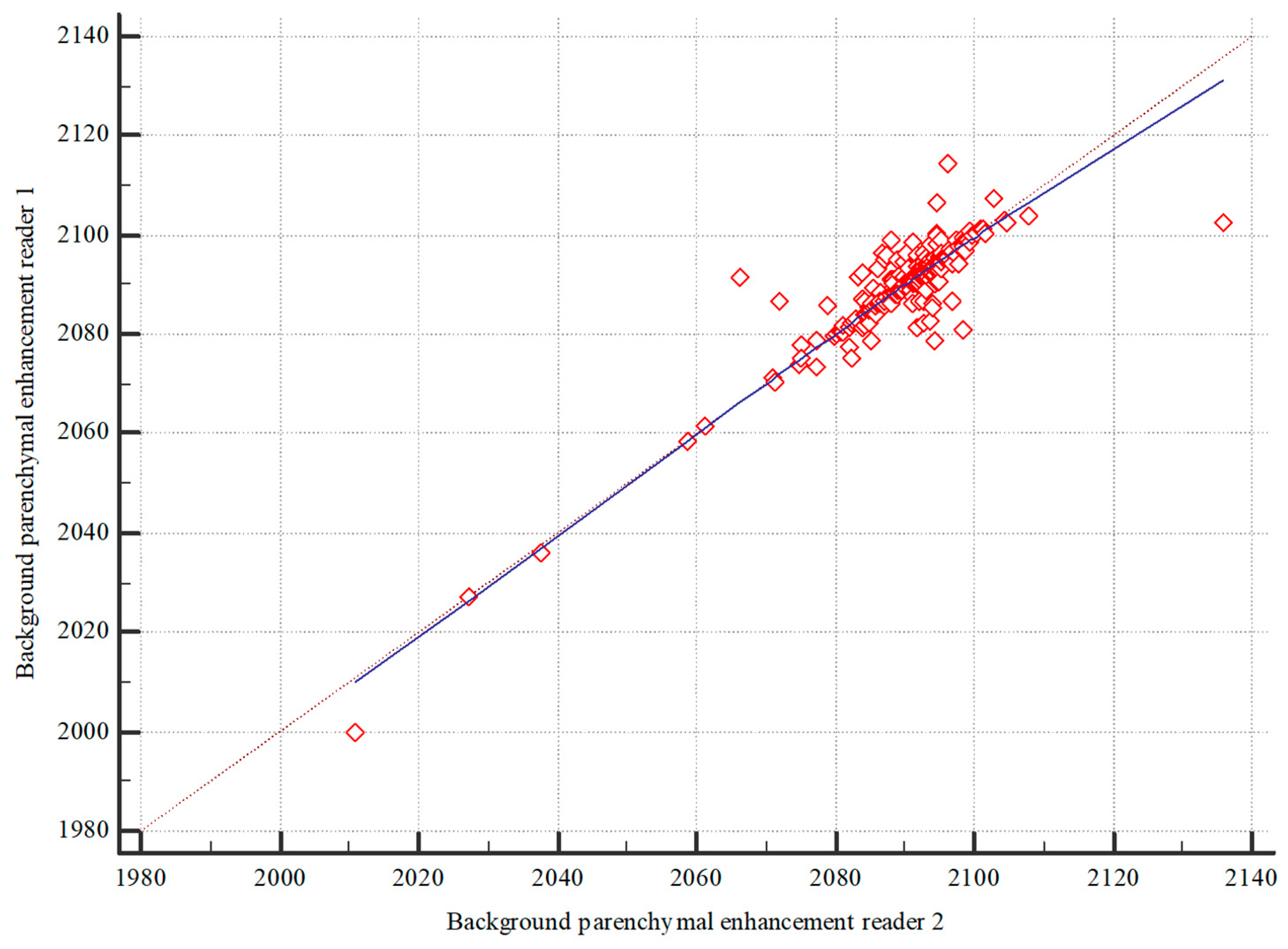
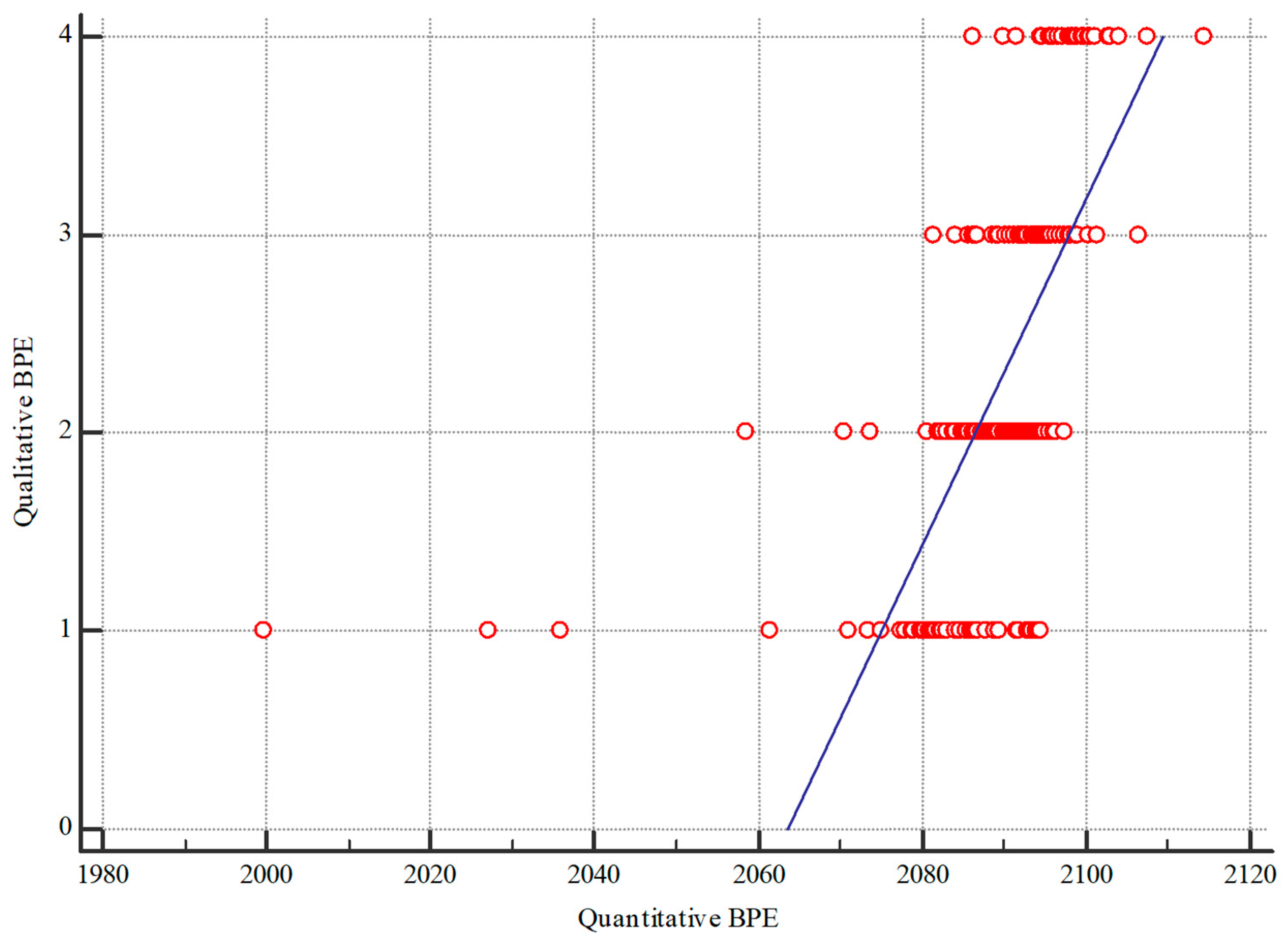
2.5. Statistical Analysis
3. Results
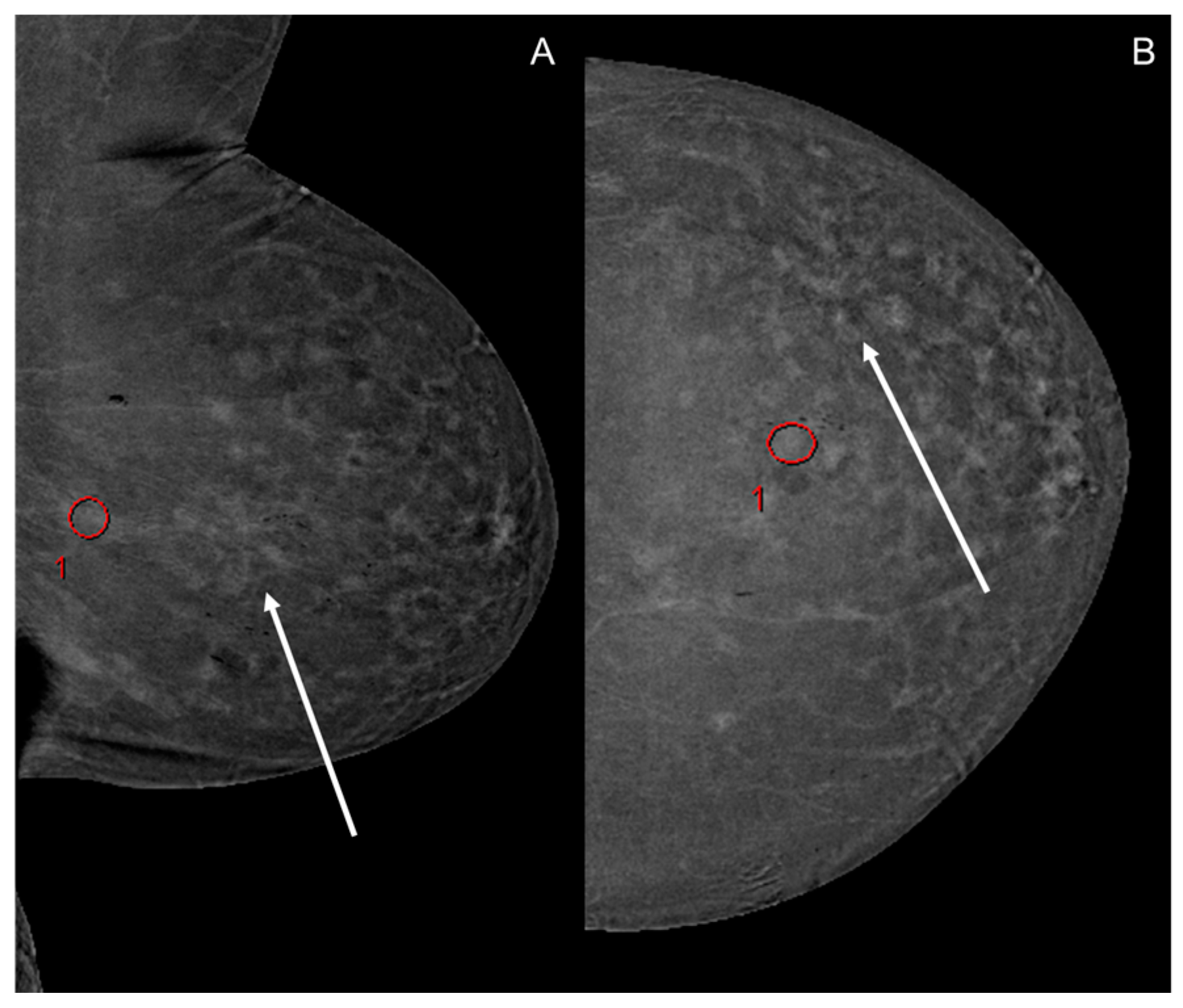
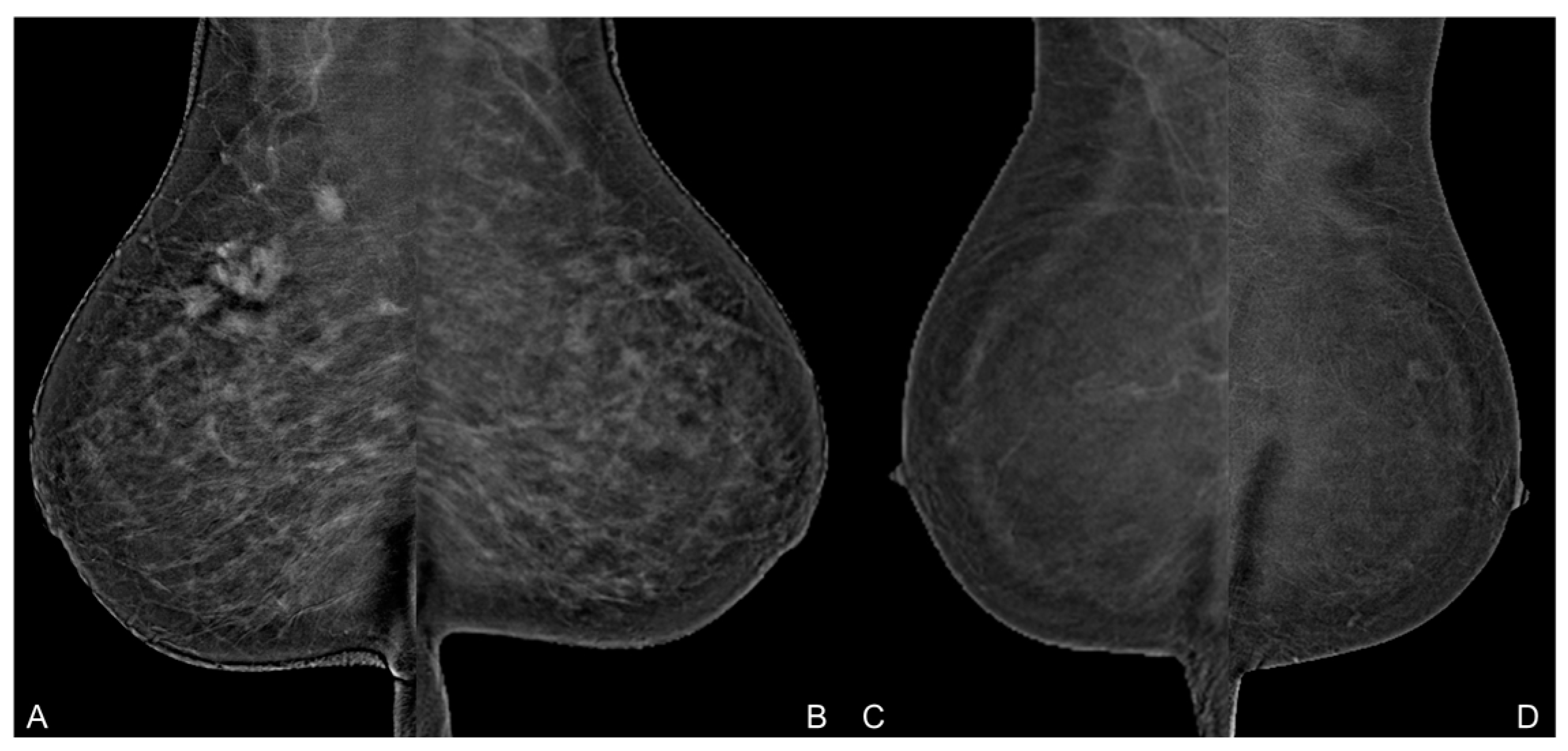
3.1. Image Analysis
3.2. Background Parenchymal Enhancement (BPE)
3.3. Quantitative BPE
3.4. Qualitative BPE
3.5. Hematologic Parameters
3.6. Cardiovascular Conditions
3.7. Breast Density
3.8. Body Mass Index (BMI) and Age
3.9. Age
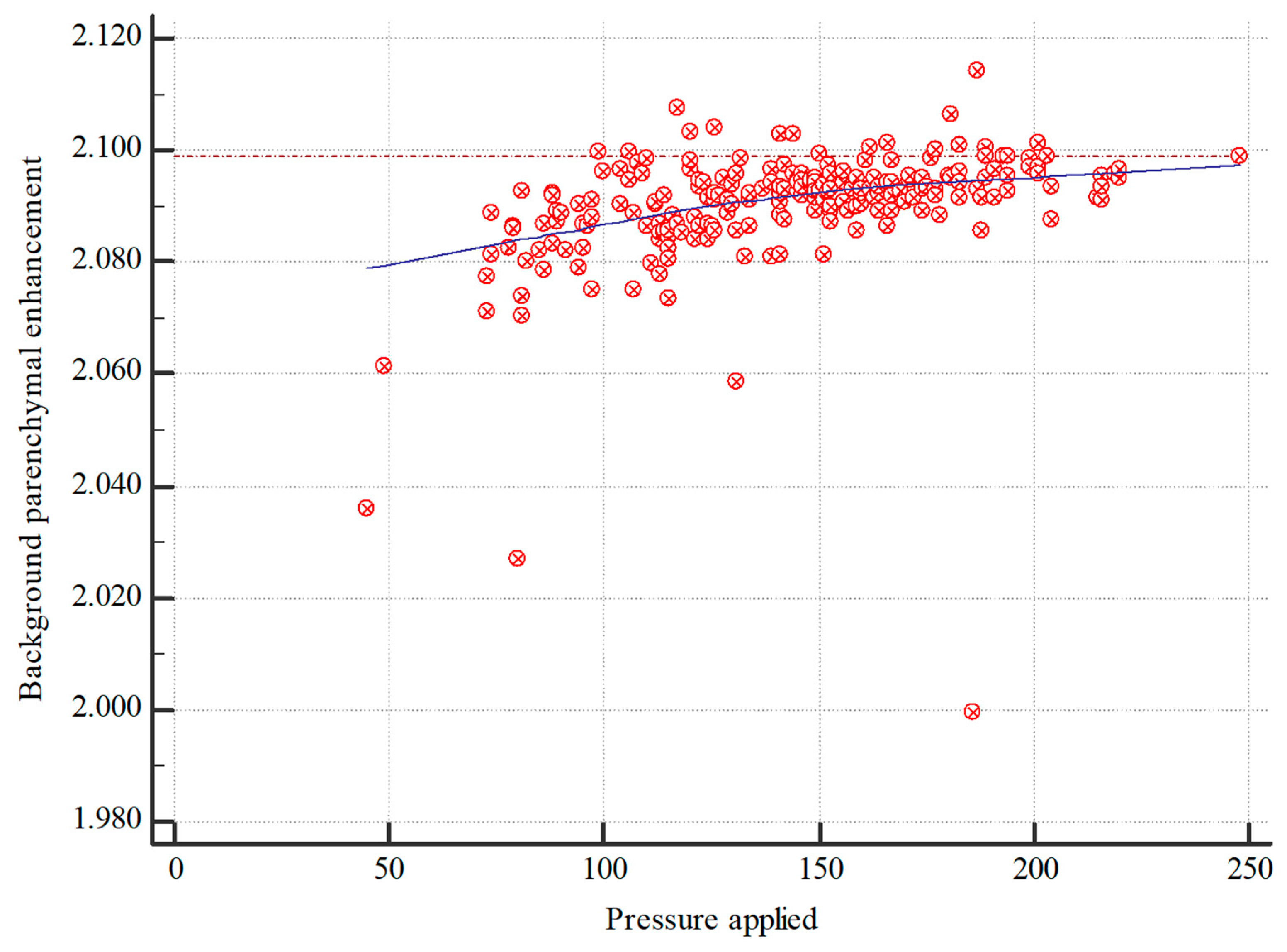
3.10. Applied Pressure
3.11. Menopausal Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPE | Background parenchymal enhancement |
| BMI | Body mass index |
| CESM | Contrast-enhanced spectral mammography |
References
- James, J.J.; Tennant, S.L. Contrast-enhanced spectral mammography (CESM). Clin. Radiol. 2018, 73, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, J.M.; Zhang, K.; Xia, L.J.; Feng, L.; Yang, P.; Zhang, M.Y.; Xiao, W.; Lin, H.X.; Yu, Y.H. Diagnostic Value of Contrast-Enhanced Spectral Mammography for Screening Breast Cancer: Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e985–e995. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.B.; Pesapane, F.; Agazzi, G.M.; Gagliardi, T.; Nigro, O.; Bozzini, A.; Priolo, F.; Penco, S.; Cassano, E.; Chini, C.; et al. Diagnostic accuracy of contrast-enhanced spectral mammography for breast lesions: A systematic review and meta-analysis. Breast 2020, 53, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Akashi-Tanaka, S.; Suzuki, S.; Daniels, M.I.; Watanabe, C.; Hirose, M.; Nakamura, S. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2017, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, S.; Huang, J.; Zhang, X.; Qin, Y.; Zhong, H.; Yu, J. Quantitative Analysis of Enhancement Intensity and Patterns on Contrast-enhanced Spectral Mammography. Sci. Rep. 2020, 10, 9807. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.P.; Miller, K.D. Angiogenesis of breast cancer. J. Clin. Oncol. 2005, 23, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Lobbes, M.B.; Lalji, U.; Houwers, J.; Nijssen, E.C.; Nelemans, P.J.; van Roozendaal, L.; Smidt, M.L.; Heuts, E.; Wildberger, J.E. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur. Radiol. 2014, 24, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Travieso-Aja, M.D.M.; Naranjo-Santana, P.; Fernández-Ruiz, C.; Severino-Rondón, W.; Maldonado-Saluzzi, D.; Rodríguez Rodríguez, M.; Vega-Benítez, V.; Luzardo, O.P. Factors affecting the precision of lesion sizing with contrast-enhanced spectral mammography. Clin. Radiol. 2018, 73, 296–303. [Google Scholar] [CrossRef]
- Deng, C.Y.; Juan, Y.H.; Cheung, Y.C.; Lin, Y.C.; Lo, Y.F.; Lin, G.; Chen, S.C.; Ng, S.H. Quantitative analysis of enhanced malignant and benign lesions on contrast-enhanced spectral mammography. Br. J. Radiol. 2018, 91, 20170605. [Google Scholar] [CrossRef]
- Rudnicki, W.; Heinze, S.; Niemiec, J.; Kojs, Z.; Sas-Korczynska, B.; Hendrick, E.; Luczynska, E. Correlation between quantitative assessment of contrast enhancement in contrast-enhanced spectral mammography (CESM) and histopathology-preliminary results. Eur. Radiol. 2019, 29, 6220–6226. [Google Scholar] [CrossRef] [PubMed]
- Sorin, V.; Yagil, Y.; Shalmon, A.; Gotlieb, M.; Faermann, R.; Halshtok-Neiman, O.; Sklair-Levy, M. Background Parenchymal Enhancement at Contrast-Enhanced Spectral Mammography (CESM) as a Breast Cancer Risk Factor. Acad. Radiol. 2020, 27, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, X.; Zhong, H.; Qin, Y.; Li, Y.; Song, B.; Huang, J.; Yu, J. Background Parenchymal Enhancement on Contrast-Enhanced Spectral Mammography: Influence of Age, Breast Density, Menstruation Status, and Menstrual Cycle Timing. Sci. Rep. 2020, 10, 8608. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Phillips, J.; Slanetz, P.; Lotfi, P.; Dialani, V.; Karimova, J.; Mehta, T. Factors Associated With Background Parenchymal Enhancement on Contrast-Enhanced Mammography. AJR Am. J. Roentgenol. 2021, 216, 340–348. [Google Scholar] [CrossRef]
- Savaridas, S.L.; Taylor, D.B.; Gunawardana, D.; Phillips, M. Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? Correlation with known radiology risk factors. Clin. Radiol. 2017, 72, e1081–e1085. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, M.; Lin, F.; Zhang, K.; Xie, H.; Lv, W.; Ji, H.; Mao, N. Qualitative assessments of density and background parenchymal enhancement on contrast-enhanced spectral mammography associated with breast cancer risk in high-risk women. Br. J. Radiol. 2023, 96, 20220051. [Google Scholar] [CrossRef]
- Watt, G.P.; Sung, J.; Morris, E.A.; Buys, S.S.; Bradbury, A.R.; Brooks, J.D.; Conant, E.F.; Weinstein, S.P.; Kontos, D.; Woods, M.; et al. Association of breast cancer with MRI background parenchymal enhancement: The IMAGINE case-control study. Breast Cancer Res. 2020, 22, 138. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, M.J.; Yoen, H.; Lee, Y.; Kim, Y.S.; Park, A.R.; Ha, S.M.; Kim, S.Y.; Chang, J.M.; Cho, N.; et al. Background Parenchymal Enhancement at Postoperative Surveillance Breast MRI: Association with Future Second Breast Cancer Risk. Radiology 2023, 306, 90–99. [Google Scholar] [CrossRef]
- Wessling, D.; Männlin, S.; Schwarz, R.; Hagen, F.; Brendlin, A.; Olthof, S.C.; Hattermann, V.; Gassenmaier, S.; Herrmann, J.; Preibsch, H. Background enhancement in contrast-enhanced spectral mammography (CESM): Are there qualitative and quantitative differences between imaging systems? Eur. Radiol. 2023, 33, 2945–2953. [Google Scholar] [CrossRef]
- Bae, K.T.; Seeck, B.A.; Hildebolt, C.F.; Tao, C.; Zhu, F.; Kanematsu, M.; Woodard, P.K. Contrast enhancement in cardiovascular MDCT: Effect of body weight, height, body surface area, body mass index, and obesity. AJR Am. J. Roentgenol. 2008, 190, 777–784. [Google Scholar] [CrossRef]
- Brown, J.C.; Ligibel, J.A.; Crane, T.E.; Kontos, D.; Yang, S.; Conant, E.F.; Mack, J.A.; Ahima, R.S.; Schmitz, K.H. Obesity and metabolic dysfunction correlate with background parenchymal enhancement in premenopausal women. Obesity 2023, 31, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Löbe, S.; Leuthäusser, C.; Pölkow, A.; Hilbert, S.; Sommer, P.; Bollmann, A.; Hindricks, G.; Paetsch, I.; Jahnke, C. Optimal timing of contrast-enhanced three-dimensional magnetic resonance left atrial angiography before pulmonary vein ablation. Cardiol. J. 2021, 28, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Brink, J.A. Contrast optimization and scan timing for single and multidetector-row computed tomography. J. Comput. Assist. Tomogr. 2003, 27 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, P.H.; Muller, M.; Vincken, K.L.; Westerink, J.; Mali, W.P.; van der Graaf, Y.; Geerlings, M.I. Hemoglobin, hematocrit, and changes in cerebral blood flow: The Second Manifestations of ARTerial disease-Magnetic Resonance study. Neurobiol. Aging 2015, 36, 1417–1423. [Google Scholar] [CrossRef]
- Thomas, D.J.; Marshall, J.; Russell, R.W.; Wetherley-Mein, G.; du Boulay, G.H.; Pearson, T.C.; Symon, L.; Zilkha, E. Effect of haematocrit on cerebral blood-flow in man. Lancet 1977, 2, 941–943. [Google Scholar] [CrossRef]
- Meucci, R.; Pistolese, C.A.; Perretta, T.; Vanni, G.; Beninati, E.; Di Tosto, F.; Serio, M.L.; Caliandro, A.; Materazzo, M.; Pellicciaro, M.; et al. Background Parenchymal Enhancement in Contrast-enhanced Spectral Mammography: A Retrospective Analysis and a Pictorial Review of Clinical Cases. In Vivo 2022, 36, 853–858. [Google Scholar] [CrossRef]
- Lee, C.; Phillips, J.; Sung, J.; Lewin, J.; Newell, M. ACR BI-RADS® ATLAS-MAMMOGRAPHY CONTRAST ENHANCED MAMMOGRAPHY (CEM). A Supplement to ACR BI-RADS® Mammography 2013, 2022. Available online: https://www.acr.org/-/media/ACR/Files/RADS/BI-RADS/BIRADS_CEM_2022.pdf (accessed on 28 August 2024).
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Wong, C.Y.Y.; Lee, S.Y.S.; Mahmood, R.D. Contrast-enhanced spectral mammography. Singap. Med. J. 2024, 65, 195–201. [Google Scholar] [CrossRef]
- Sahoo, P.; Gupta, P.K.; Awasthi, A.; Pandey, C.M.; Patir, R.; Vaishya, S.; Saha, I.; Gupta, R.K. Comparison of actual with default hematocrit value in dynamic contrast enhanced MR perfusion quantification in grading of human glioma. Magn. Reson. Imaging 2016, 34, 1071–1077. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Xing, D.; Gong, P.; Chen, Q.; Lv, Y. Background parenchymal enhancement on contrast-enhanced spectral mammography does not represent an influencing factor for breast cancer: A preliminary study. Medicine 2020, 99, e23857. [Google Scholar] [CrossRef] [PubMed]
- Youn, I.; Choi, S.; Choi, Y.J.; Moon, J.H.; Park, H.J.; Ham, S.Y.; Park, C.H.; Kim, E.Y.; Kook, S.H. Contrast enhanced digital mammography versus magnetic resonance imaging for accurate measurement of the size of breast cancer. Br. J. Radiol. 2019, 92, 20180929. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.W. Contrast-enhanced breast MRI: Factors affecting sensitivity and specificity. Eur. Radiol. 1997, 7 (Suppl. S5), 281–288. [Google Scholar] [CrossRef] [PubMed]
- Dontchos, B.N.; Rahbar, H.; Partridge, S.C.; Lehman, C.D.; DeMartini, W.B. Influence of Menstrual Cycle Timing on Screening Breast MRI Background Parenchymal Enhancement and Diagnostic Performance in Premenopausal Women. J. Breast Imaging 2019, 1, 205–211. [Google Scholar] [CrossRef]
| 100.0% | Maximum | 2114.3 |
|---|---|---|
| 99.5% | 2113.076 | |
| 97.5% | 2102.73 | |
| 90.0% | 2098.48 | |
| 75.0% | Quartile | 2094.8 |
| 50.0% | Median | 2092 |
| 25.0% | Quartile | 2086.6 |
| 10.0% | 2081.36 | |
| 2.5% | 2069.59 | |
| 0.5% | 2004.632 | |
| 0.0% | Minimum | 1999.7 |
| BPE | n | % |
|---|---|---|
| 1 | 46 | 19.83 |
| 2 | 105 | 44.30 |
| 3 | 52 | 21.94 |
| 4 | 32 | 13.92 |
| All | 235 |
| Age | n | % | ACR | n | % |
|---|---|---|---|---|---|
| 20–30 | 0 | 0 | A | 2 | 3.23 |
| 30–40 | 4 | 6.45 | B | 30 | 48.39 |
| 40–50 | 9 | 14.52 | C | 24 | 38.71 |
| 50–60 | 17 | 27.42 | D | 6 | 9.68 |
| 60–70 | 11 | 17.74 | |||
| 70–80 | 17 | 27.42 | |||
| 80–90 | 4 | 6.45 | |||
| All | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wessling, D.; Männlin, S.; Schwarz, R.; Hagen, F.; Brendlin, A.; Gassenmaier, S.; Preibsch, H. Factors Influencing Background Parenchymal Enhancement in Contrast-Enhanced Mammography Images. Diagnostics 2024, 14, 2239. https://doi.org/10.3390/diagnostics14192239
Wessling D, Männlin S, Schwarz R, Hagen F, Brendlin A, Gassenmaier S, Preibsch H. Factors Influencing Background Parenchymal Enhancement in Contrast-Enhanced Mammography Images. Diagnostics. 2024; 14(19):2239. https://doi.org/10.3390/diagnostics14192239
Chicago/Turabian StyleWessling, Daniel, Simon Männlin, Ricarda Schwarz, Florian Hagen, Andreas Brendlin, Sebastian Gassenmaier, and Heike Preibsch. 2024. "Factors Influencing Background Parenchymal Enhancement in Contrast-Enhanced Mammography Images" Diagnostics 14, no. 19: 2239. https://doi.org/10.3390/diagnostics14192239
APA StyleWessling, D., Männlin, S., Schwarz, R., Hagen, F., Brendlin, A., Gassenmaier, S., & Preibsch, H. (2024). Factors Influencing Background Parenchymal Enhancement in Contrast-Enhanced Mammography Images. Diagnostics, 14(19), 2239. https://doi.org/10.3390/diagnostics14192239







