Pre-Conception Androgen Levels and Obstetric Outcomes in Polycystic Ovary Syndrome: A Single-Center Retrospective Study
Abstract
1. Introduction
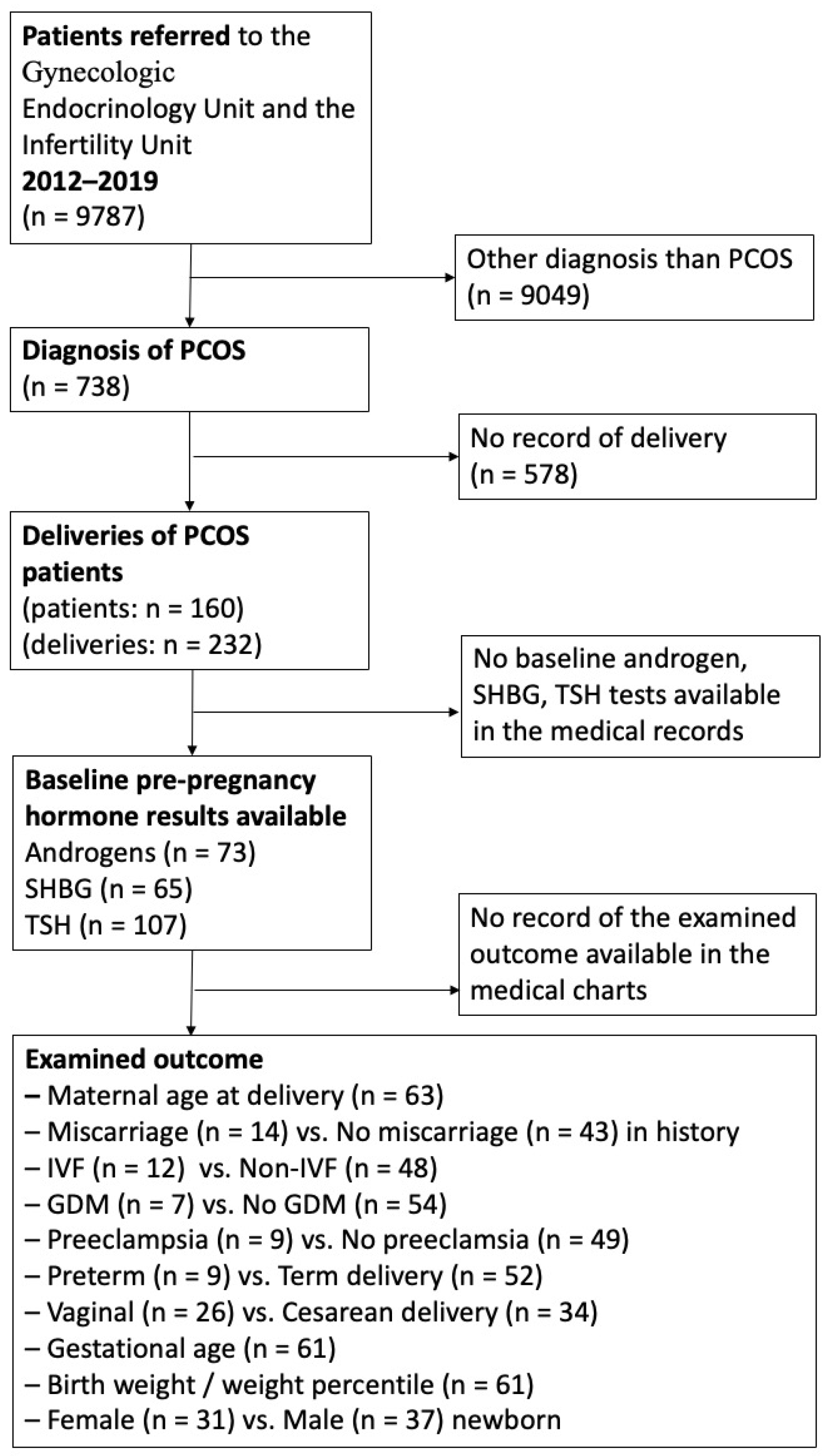
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Miscarriage
3.2. Mode of Conception—In Vitro Fertilization (IVF)
3.3. Gestational Diabetes Mellitus (GDM)
3.4. Preeclampsia
3.5. Fetal Gender
3.6. Gestational Age—Fetal Maturity
3.7. Mode of Delivery
3.8. Maternal Age at Delivery
3.9. Gestational Age
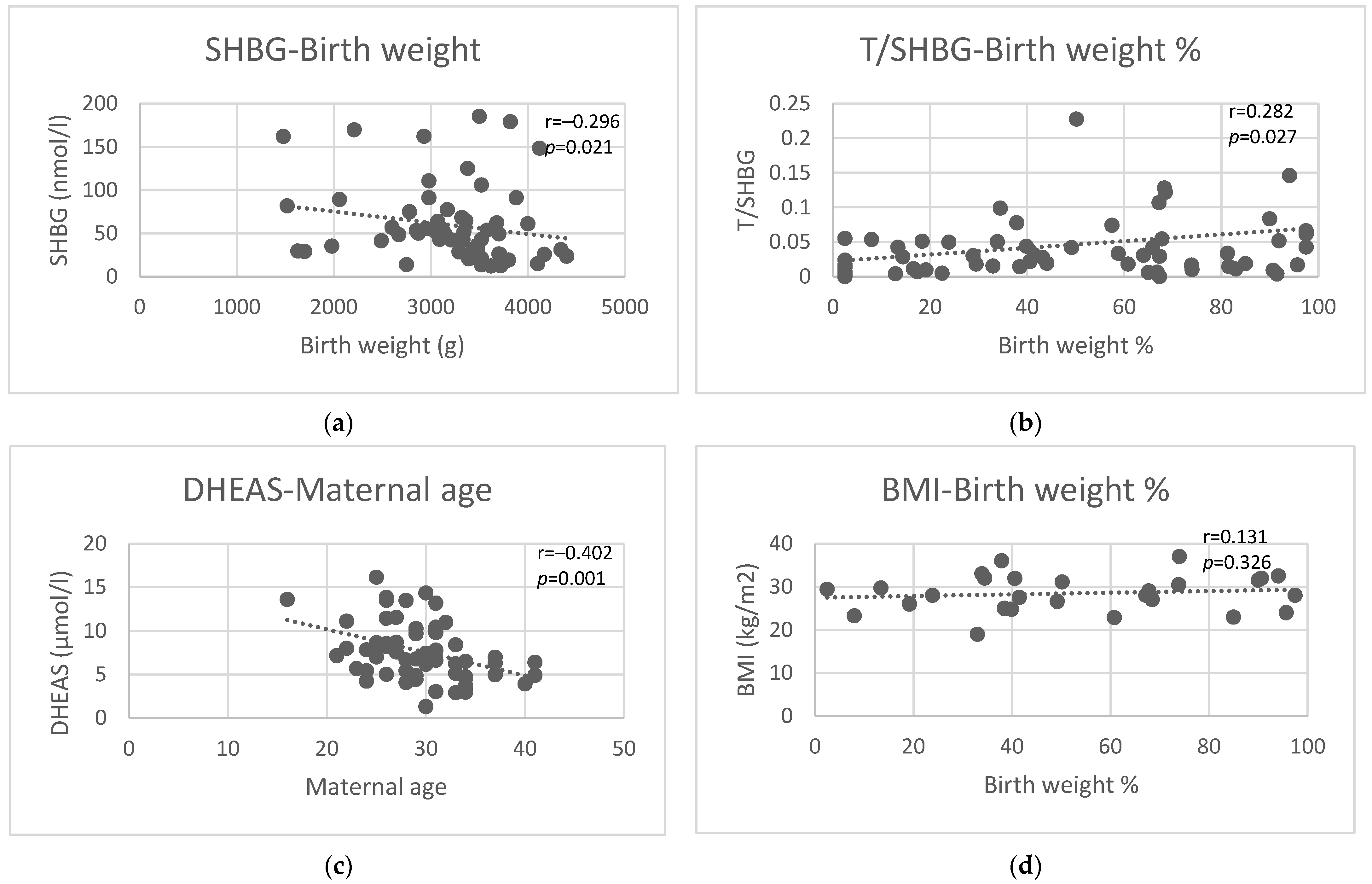
3.10. Birth Weight and Birth Weight Percentile
4. Discussion
- (1)
- Only the presence of PCOS as an “umbrella diagnosis” without attempting to differentiate according to the various manifestations [29];
- (2)
- (3)
- (4)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680, Erratum in Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.J.M.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Artini, P.G.; Obino, M.E.R.; Sergiampietri, C.; Pinelli, S.; Papini, F.; Casarosa, E.; Cela, V. PCOS and pregnancy: A review of available therapies to improve the outcome of pregnancy in women with polycystic ovary syndrome. Expert Rev. Endocrinol. Metab. 2018, 13, 87–98. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618, Erratum in Hum. Reprod. 2019, 34, 388. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Brzyski, R.G.; Diamond, M.P.; Coutifaris, C.; Schlaff, W.D.; Casson, P.; Christman, G.M.; Huang, H.; Yan, Q.; Alvero, R.; et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2014, 371, 119–129, Erratum in N. Engl. J. Med. 2014, 317, 1465. [Google Scholar] [CrossRef]
- Balen, A.H.; Tan, S.L.; MacDougall, J.; Jacobs, H.S. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with Buserelin. Hum. Reprod. 1993, 8, 959–964. [Google Scholar] [CrossRef]
- Glueck, C.J.; Wang, P.; Goldenberg, N.; Sieve-Smith, L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum. Reprod. 2002, 17, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Ranasinha, S.; Zoungas, S.; Moran, L.; Teede, H.J. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E447–E452. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.M.; Bouma, G.J. A Potential Role and Contribution of Androgens in Placental Development and Pregnancy. Life 2021, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; de Wilde, M.A.; Falbo, A.; Koster, M.P.H.; La Sala, G.B.; Fauser, B.C.J.M. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Nikbakht, R.; Zargar, M.; Moramezi, F.; Ziafat, M.; Tabesh, H.; Sattari, A.R.; Sattari, S.A. Insulin resistance and free androgen as predictors for ovarian hyperstimulation syndrome in non-PCOS women. Horm. Metab. Res. 2020, 52, 104–108. [Google Scholar] [CrossRef]
- Cao, Z.; Lu, Y.; Cong, Y.; Liu, Y.; Li, Y.; Wang, H.; Zhang, Q.; Huang, W.; Liu, J.; Dong, Y.; et al. Simultaneous quantitation of four androgens and 17-hydroxyprogesterone in polycystic ovarian syndrome patients by LC-MS/MS. J. Clin. Lab. Anal. 2020, 34, e23539. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, Y.; Li, L.; Lu, Y.; Su, S.; Liu, Y.; Xu, Z.; Xin, M.; Zhang, Q.; Cao, Z. Divergent associations between serum androgens and ovarian reserve markers revealed in patients with polycystic ovary syndrome. Front. Endocrinol. 2022, 13, 881740. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Jiang, J.; Yang, N.; Liu, Y.; Cai, L.; Cui, Y.; Diao, F.; Han, X.; Liu, J.; et al. Follicular hyperandrogenism and insulin resistance in polycystic ovary syndrome patients with normal circulating testosterone levels. J. Biomed. Res. 2017, 32, 208–214. [Google Scholar] [CrossRef]
- Le, T.N.; Nestler, J.E.; Strauss, J.F., III; Wickham, E.P., III. Sex hormonebinding globulin and type 2 diabetes mellitus. Trends Endocrinol. Metab. 2012, 23, 32–40. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Chiossi, G.; Tolino, A.; Tucci, L.; La Sala, G.B.; Zullo, F. Early trophoblast invasion and placentation in women with different PCOS phenotypes. Reprod. Biomed. Online 2014, 29, 370–381. [Google Scholar] [CrossRef][Green Version]
- Sun, M.; Maliqueo, M.; Benrick, A.; Johansson, J.; Shao, R.; Hou, L.; Jansson, T.; Wu, X.; Stener-Victorin, E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1373–E1385. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H.M. Williams Textbook of Endocrinology, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Misichronis, G.; Georgopoulos, N.A.; Marioli, D.J.; Armeni, A.K.; Katsikis, I.; Piouka, A.D.; Saltamavros, A.D.; Roupas, N.D.; Panidis, D. The influence of obesity on androstenedione to testosterone ratio in women with polycystic ovary syndrome (PCOS) and hyperandrogenemia. Gynecol. Endocrinol. 2012, 28, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Münzker, J.; Lindheim, L.; Adaway, J.; Trummer, C.; Lerchbaum, E.; Pieber, T.R.; Keevil, B.; Obermayer-Pietsch, B. High salivary testosterone-to-androstenedione ratio and adverse metabolic phenotypes in women with polycystic ovary syndrome. Clin. Endocrinol. 2017, 86, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Tulberg, A.; McNamara, M.; Grogan, T.R.; Abbott, D.H.; Naik, R.; Lu, G.; Chazenbalk, G.D. Serum testosterone to androstenedione ratio predicts metabolic health in normal-weight polycystic ovary syndrome women. J. Endocr. Soc. 2021, 5, bvab158. [Google Scholar] [CrossRef] [PubMed]
- Faisal Ahmed, S.; Iqbal, A.; Hughes, I.A. The testosterone:androstenedione ratio in male undermasculinization. Clin. Endocrinol. 2000, 53, 697–702. [Google Scholar] [CrossRef]
- Mendonca, B.B.; Gomes, N.L.; Costa, E.M.F.; Inacio, M.; Martin, R.M.; Nishi, M.Y.; Carvalho, F.M.; Tibor, F.D.; Domenice, S. 46,XY disorder of sex development (DSD) due to 17β-hydroxysteroid dehydrogenase type 3 deficiency. J. Steroid Biochem. Mol. Biol. 2017, 165, 79–85. [Google Scholar] [CrossRef]
- Chuang, J.; Vallerie, A.; Breech, L.; Saal, H.M.; Alam, S.; Crawford, P.; Rutter, M.M. Complexities of gender assignment in 17β-hydroxysteroid dehydrogenase type 3 deficiency: Is there a role for early orchiectomy? Int. J. Pediatr. Endocrinol. 2013, 2013, 15. [Google Scholar] [CrossRef][Green Version]
- Subramanian, A.; Lee, S.I.; Phillips, K.; Toulis, K.A.; Kempegowda, P.; O’Reilly, M.W.; Adderley, N.J.; Thangaratinam, S.; Arlt, W.; Nirantharakumar, K. Polycystic ovary syndrome and risk of adverse obstetric outcomes: A retrospective population-based matched cohort study in England. BMC Med. 2022, 20, 298. [Google Scholar] [CrossRef]
- Naver, K.V.; Grinsted, J.; Larsen, S.O.; Hedley, P.L.; Jørgensen, F.S.; Christiansen, M.; Nilas, L. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG 2014, 121, 575–581. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Russo, T.; Tolino, A.; Orio, F.; Zullo, F. Pregnancy in women with polycystic ovary syndrome: The effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil. Steril. 2010, 94, 1805–1811. [Google Scholar] [CrossRef]
- de Wilde, M.A.; Lamain-de Ruiter, M.; Veltman-Verhulst, S.M.; Kwee, A.; Laven, J.S.; Lambalk, C.B.; Eijkemans, M.J.C.; Franx, A.; Fauser, B.C.J.M.; Koster, M.P.H. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil. Steril. 2017, 108, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Mumm, H.; Jensen, D.M.; Sørensen, J.A.; Andersen, L.L.T.; Ravn, P.; Andersen, M.; Glintborg, D. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet. Gynecol. Scand. 2015, 94, 204–211. [Google Scholar] [CrossRef] [PubMed]
- El Mahdi, E.; Fekry, N.; Ahmed, M.; Ghebremeskel, K. Testosterone, sex hormone-binding globulin and dehydroepiandrosterone levels and cervical length of Egyptian women with a history of recurrent miscarriages, polycystic ovary syndrome and without the conditions at three stages of pregnancy. J. Obstet. Gynaecol. 2023, 43, 2163625. [Google Scholar] [CrossRef] [PubMed]
- Sir-Petermann, T.; Maliqueo, M.; Angel, B.; Lara, H.E.; Pérez-Bravo, F.; Recabarren, S.E. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: Possible implications in prenatal androgenization. Hum. Reprod. 2002, 17, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, N.; Hidaka, Y.; Takano, T.; Shimaoka, Y.; Kobayashi, Y.; Amino, N. Serum concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate and their relation to cytokine production during and after normal pregnancy. Clin. Chim. Acta 2004, 340, 187–193. [Google Scholar] [CrossRef]
- Christ, J.P.; Gunning, M.N.; Meun, C.; Eijkemans, M.J.C.; van Rijn, B.B.; Bonsel, G.J.; Laven, J.S.E.; Fauser, B.C.J.M. Pre-conception characteristics predict obstetrical and neonatal outcomes in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 809–818. [Google Scholar] [CrossRef]
- Yusuf, A.N.M.; Amri, M.F.; Ugusman, A.; Hamid, A.A.; Wahab, N.A.; Mokhtar, M.H. Hyperandrogenism and its possible effects on endometrial receptivity: A review. Int. J. Mol. Sci. 2023, 24, 12026. [Google Scholar] [CrossRef]
- Tuckerman, E.M.; Okon, M.A.; Li, T.; Laird, S.M. Do androgens have a direct effect on endometrial function? An in vitro study. Fertil. Steril. 2000, 74, 771–779. [Google Scholar] [CrossRef]
- Bussen, S.; Sütterlin, M.; Steck, T. Endocrine abnormalities during the follicular phase in women with recurrent spontaneous abortion. Hum. Reprod. 1999, 14, 18–20. [Google Scholar] [CrossRef]
- Yang, W.; Yang, R.; Lin, M.; Yang, Y.; Song, X.; Zhang, J.; Yang, S.; Song, Y.; Li, J.; Pang, T.; et al. Body mass index and basal androstenedione are independent risk factors for miscarriage in polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2018, 16, 119. [Google Scholar] [CrossRef]
- Qin, J.C.; Fan, L.; Qin, A.P. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: Evidence from a meta-analysis. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 1–7. [Google Scholar] [CrossRef]
- Montoya-Botero, P.; Rodriguez-Purata, J.; Polyzos, N.P. Androgen supplementation in assisted reproduction: Where are we in 2019? Curr. Opin. Obstet. Gynecol. 2019, 31, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Hossein Rashidi, B.; Hormoz, B.; Shahrokh Tehraninejad, E.; Shariat, M.; Mahdavi, A. Testosterone and dehydroepiandrosterone sulphate levels and IVF/ICSI results. Gynecol. Endocrinol. 2009, 25, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.M.; Xu, F.; Darbinian, J.A.; Quesenberry, C.P.; Sridhar, S.; Kim, C.; Gunderson, E.P.; Ferrara, A. Prepregnancy SHBG concentrations and risk for subsequently developing gestational diabetes mellitus. Diabetes Care 2014, 37, 1296–1303. [Google Scholar] [CrossRef]
- Luo, X.; Cai, W.Y.; Song, J.Y.; Duan, C.C.; Wu, W.; Man, Y.J.; Wu, X.K.; Xu, J. Predictive value of circulating sex hormone-binding globulin for gestational diabetes: A meta-analysis. Biomark. Med. 2021, 15, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Faal, S.; Abedi, P.; Jahanfar, S.; Ndeke, J.M.; Mohaghegh, Z.; Sharifipour, F.; Zahedian, M. Sex hormone binding globulin for prediction of gestational diabetes mellitus in pre-conception and pregnancy: A systematic review. Diabetes Res. Clin. Pract. 2019, 152, 39–52. [Google Scholar] [CrossRef]
- Veltman-Verhulst, S.M.; van Haeften, T.W.; Eijkemans, M.J.; de Valk, H.W.; Fauser, B.C.; Goverde, A.J. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum. Reprod. 2010, 25, 3123–3128. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Sikaris, K.; Ly, L.P. Estimating age-specific trends in circulating testosterone and sex hormone-binding globulin in males and females across the lifespan. Ann. Clin. Biochem. 2016, 53, 377–384. [Google Scholar] [CrossRef]
- Spencer, K.; Yu, C.K.H.; Rembouskos, G.; Bindra, R.; Nicolaides, K.H. First trimester sex hormone-binding globulin and subsequent development of preeclampsia or other adverse pregnancy outcomes. Hypertens. Pregnancy 2005, 24, 303–311. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, R.; Sun, X.; Wang, L.; Zhang, W. Risk factors for preeclampsia in infertile Chinese women with polycystic ovary syndrome: A prospective cohort study. J. Clin. Hypertens. 2017, 19, 504–509. [Google Scholar] [CrossRef]
- Rahmanian, M.; Salari, Z.; Mirmohammadkhani, M.; Ghorbani, R. Is the sex hormone binding globulin related to preeclampsia independent of insulin resistance? J. Pak. Med. Assoc. 2014, 64, 640–643. [Google Scholar] [PubMed]
- Jonsdottir, F.; Nilas, L.; Andreasen, K.R.; Grinsted, J.; Christiansen, M.; Hedley, P.L.; Naver, K.V. Obstetrical complications in dichorionic twin pregnancies in women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2017, 96, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Rainey, W.E.; Carr, B.R.; Sasano, H.; Suzuki, T.; Mason, J.I. Dissecting human adrenal androgen production. Trends Endocrinol. Metab. 2002, 13, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Rizvi, S.A.; Shahid, R.; Manzoor, R. Dehydroepiandrosterone Sulfate (DHEAS) Levels in Polycystic Ovarian Syndrome (PCOS). J. Coll. Physicians Surg. Pak. 2021, 31, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, S.M.; Jacobsen, G.; Romundstad, P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur. J. Endocrinol. 2006, 155, 365–370. [Google Scholar] [CrossRef]
- Palm, C.V.B.; Dreyer, A.F.; Boye, H.; Jørgensen, J.S.; Wu, C.; Højsager, F.D.; Jensen, T.K.; Glintborg, D.; Andersen, M.S. Higher free testosterone in the third trimester was associated with lower abdominal circumference at birth in boys: Odense child cohort. BJOG 2024, 131, 36–45. [Google Scholar] [CrossRef]
- Morisset, A.S.; Dubé, M.C.; Drolet, R.; Robitaille, J.; Weisnagel, S.J.; Tchernof, A. Sex hormone-binding globulin levels and obesity in women with gestational diabetes: Relationship with infant birthweight. Gynecol. Endocrinol. 2011, 27, 905–909. [Google Scholar] [CrossRef]
| Miscarriage in Medical History | Mode of Conception | Gestational Diabetes | Preeclampsia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | IVF | Non-IVF | p | Yes | No | p | Yes | No | p | |
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | |||||
| n = 14 | n = 43 | n = 12 | n = 48 | n = 7 | n = 54 | n = 9 | n = 49 | |||||
| Testosterone (nmol/L) | 1.47 ± 0.47 | 1.64 ± 0.98 | 0.56 | 1.44 ± 1.31 | 1.59 ± 0.75 | 0.60 | 1.84 ± 0.59 | 1.5 ± 0.90 | 0.37 | 1.86 ± 0.99 | 1.50 ± 0.88 | 0.28 |
| Androstenedione (ug/L) | 3.60 ± 1.97 | 2.68 ± 1.22 | 0.04 | 2.31 ± 1.09 | 2.93 ± 1.41 | 0.16 | 2.81 ± 1.54 | 2.77 ± 1.36 | 0.95 | 3.29 ± 1.47 | 2.73 ± 1.37 | 0.29 |
| DHEAS (µmol/L) | 8.40 ± 3.71 | 7.85 ± 3.29 | 0.60 | 6.45 ± 3.01 | 8.05 ± 3.30 | 0.13 | 6.77 ± 2.21 | 7.81 ± 3.38 | 0.43 | 9.19 ± 3.32 | 7.48 ± 3.22 | 0.17 |
| SHBG (nmol/L) | 69.40 ± 54.20 | 57.77 ± 41.35 | 0.42 | 72.01 ± 54.40 | 54.73 ± 37.85 | 0.20 | 38.51 ± 22.58 | 61.86 ± 44.43 | 0.18 | 44.47 ± 21.68 | 63.08 ± 46.41 | 0.25 |
| Testosterone/SHBG | 0.05 ± 0.09 | 0.05 ± 0.05 | 0.80 | 0.06 ± 0.10 | 0.04 ± 0.0.4 | 0.49 | 0.05 ± 0.05 | 0.04 ± 0.06 | 0.66 | 0.06 ± 0.04 | 0.04 ± 0.06 | 0.57 |
| Androstenedione/SHBG | 0.07 ± 0.07 | 0.09 ± 0.11 | 0.55 | 0.67 ± 0.08 | 0.09 ± 0.10 | 0.48 | 0.12 ± 0.15 | 0.08 ± 0.09 | 0.33 | 0.11 ± 0.13 | 0.08 ± 0.09 | 0.45 |
| DHEAS/SHBG | 0.22 ± 0.25 | 0.22 ± 0.24 | 0.99 | 0.20 ± 0.28 | 0.23 ± 0.23 | 0.66 | 0.27 ± 0.26 | 0.21 ± 0.23 | 0.56 | 0.25 ± 0.23 | 0.21 ± 0.24 | 0.61 |
| T/AD | 0.57 ± 0.26 | 0.56 ± 0.32 | 0.93 | 0.58 ± 0.28 | 0.59 ± 0.38 | 0.96 | 0.66 ± 0.66 | 0.58 ± 0.31 | 0.58 | 0.65 ± 0.22 | 0.58 ± 0.39 | 0.64 |
| TSH | 2.11 ± 0.83 | 2.19 ± 1.37 | 0.81 | 2.05 ± 0.75 | 2.00 ± 0.99 | 0.86 | 2.50 ± 0.92 | 1.96 ± 0.92 | 0.18 | 2.39 ± 0.74 | 1.98 ± 0.98 | 0.26 |
| Age (year) | 29.44 ± 3.70 | 29.18 ± 5.09 | 0.84 | 32.00 ± 5.26 | 28.83 ± 4.44 | 0.027 | 32.22 ± 4.06 | 28.85 ± 4.70 | 0.044 | 28.00 ± 6.22 | 29.69 ± 4.52 | 0.32 |
| BMI (kg/m2) | 28.59 ± 6.46 | 28.11 ± 4.65 | 0.82 | 30.17 ± 7.35 | 28.03 ± 4.79 | 0.48 | 28.68 ± 4.09 | 28.13 ± 5.10 | 0.82 | 32.12 ± 3.05 | 27.66 ± 4.58 | 0.046 |
| Gender of Newborn | Maturity | Mode of Delivery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | p | Preterm Delivery | Term Delivery | p | Vaginal | Cesarean | p | |
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||||
| n = 31 | n = 37 | n = 9 | n = 52 | n = 26 | n = 34 | ||||
| Testosterone (nmol/L) | 1.56 ± 0.67 | 1.57 ± 1.04 | 0.97 | 1.57 ± 1.37 | 1.55 ± 0.81 | 0.95 | 1.69 ± 1.00 | 1.50 ± 0.80 | 0.41 |
| Androstenedione (ug/L) | 3.57 ± 1.96 | 2.58 ± 1.12 | 0.01 | 2.37 ± 1.04 | 2.91 ± 1.44 | 0.31 | 2.85 ± 1.48 | 2.81 ± 1.31 | 0.91 |
| DHEAS (µmol/L) | 7.50 ± 2.96 | 8.08 ± 3.59 | 0.51 | 7.34 ± 3.08 | 7.88 ± 3.40 | 0.67 | 7.86 ± 3.37 | 7.78 ± 3.30 | 0.93 |
| SHBG (nmol/L) | 55.84 ± 40.28 | 62.41 ± 46.84 | 0.57 | 77.17 ± 56.51 | 57.11 ± 41.53 | 0.21 | 50.08 ± 30.84 | 63.89 ± 48.78 | 0.21 |
| Testosterone/SHBG | 0.05 ± 0.04 | 0.05 ± 0.07 | 0.97 | 0.06 ± 0.11 | 0.04 ± 0.05 | 0.41 | 0.06 ± 0.08 | 0.04 ± 0.04 | 0.29 |
| Androstenedione/SHBG | 0.11 ± 0.12 | 0.07 ± 0.07 | 0.16 | 0.06 ± 0.08 | 0.09 ± 0.10 | 0.43 | 0.10 ± 0.12 | 0.07 ± 0.07 | 0.19 |
| DHEAS/SHBG | 0.23 ± 0.21 | 0.22 ± 0.26 | 0.81 | 0.22 ± 0.32 | 0.22 ± 0.23 | 0.91 | 0.27 ± 0.26 | 0.20 ± 0.22 | 0.29 |
| T/AD | 0.50 ± 0.21 | 0.66 ± 0.45 | 0.09 | 0.82 ± 0.61 | 0.55 ± 0.31 | 0.052 | 0.60 ± 0.39 | 0.58 ± 0.35 | 0.81 |
| TSH | 1.97 ± 0.79 | 2.09 ± 1.05 | 0.63 | 2.15 ± 0.85 | 1.98 ± 0.95 | 0.61 | 1.77 ± 0.67 | 2.21 ± 1.08 | 0.09 |
| Age (year) | 28.77 ± 3.75 | 30.05 ± 5.34 | 0.26 | 31.20 ± 5.96 | 29.15 ± 4.50 | 0.21 | 28.52 ± 3.98 | 30.34 ± 5.19 | 0.10 |
| BMI (kg/m2) | 28.02 ± 3.92 | 28.97 ± 4.54 | 0.55 | 26.36 ± 6.88 | 28.62 ± 4.43 | 0.28 | 28.53 ± 4.37 | 28.77 ± 4.33 | 0.87 |
| Maternal Age at Delivery | Gestational Age | Birth Weight | Birth Weight Percentile | |||||
|---|---|---|---|---|---|---|---|---|
| n = 63 | n = 61 | n = 61 | n = 61 | |||||
| r | p | r | p | r | p | r | p | |
| Testosterone (nmol/L) | −0.14 | 0.28 | 0.11 | 0.42 | 0.19 | 0.15 | 0.18 | 0.18 |
| Androstenedione (ug/L) | −0.19 | 0.16 | 0.11 | 0.40 | 0.18 | 0.18 | 0.18 | 0.18 |
| DHEAS (µmol/L) | −0.40 | 0.001 | 0.15 | 0.25 | 0.13 | 0.32 | 0.15 | 0.28 |
| SHBG (nmol/L) | 0.13 | 0.30 | −0.19 | 0.14 | −0.30 | 0.02 | −0.27 | 0.04 |
| Testosterone/SHBG | −0.09 | 0.48 | 0.07 | 0.59 | 0.28 | 0.03 | 0.28 | 0.03 |
| Androstendione/SHBG | −0.22 | 0.08 | 0.11 | 0.39 | 0.24 | 0.06 | 0.25 | 0.05 |
| DHEAS/SHBG | −0.32 | 0.01 | 0.15 | 0.27 | 0.26 | 0.04 | 0.27 | 0.03 |
| T/AD | 0.03 | 0.84 | −0.06 | 0.68 | −0.12 | 0.37 | 0.11 | 0.43 |
| TSH | 0.06 | 0.66 | 0.02 | 0.89 | 0.07 | 0.60 | 0.03 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orosz, M.; Borics, F.; Rátonyi, D.; Vida, B.; Csehely, S.; Jakab, A.; Lukács, L.; Lampé, R.; Deli, T. Pre-Conception Androgen Levels and Obstetric Outcomes in Polycystic Ovary Syndrome: A Single-Center Retrospective Study. Diagnostics 2024, 14, 2241. https://doi.org/10.3390/diagnostics14192241
Orosz M, Borics F, Rátonyi D, Vida B, Csehely S, Jakab A, Lukács L, Lampé R, Deli T. Pre-Conception Androgen Levels and Obstetric Outcomes in Polycystic Ovary Syndrome: A Single-Center Retrospective Study. Diagnostics. 2024; 14(19):2241. https://doi.org/10.3390/diagnostics14192241
Chicago/Turabian StyleOrosz, Mónika, Fanni Borics, Dávid Rátonyi, Beáta Vida, Szilvia Csehely, Attila Jakab, Luca Lukács, Rudolf Lampé, and Tamás Deli. 2024. "Pre-Conception Androgen Levels and Obstetric Outcomes in Polycystic Ovary Syndrome: A Single-Center Retrospective Study" Diagnostics 14, no. 19: 2241. https://doi.org/10.3390/diagnostics14192241
APA StyleOrosz, M., Borics, F., Rátonyi, D., Vida, B., Csehely, S., Jakab, A., Lukács, L., Lampé, R., & Deli, T. (2024). Pre-Conception Androgen Levels and Obstetric Outcomes in Polycystic Ovary Syndrome: A Single-Center Retrospective Study. Diagnostics, 14(19), 2241. https://doi.org/10.3390/diagnostics14192241










