Impact of a New Preoperative Stratification Based on Cardiac Structural Compromise in Patients with Severe Aortic Stenosis Undergoing Valve Replacement Surgery †
Abstract
:1. Introduction
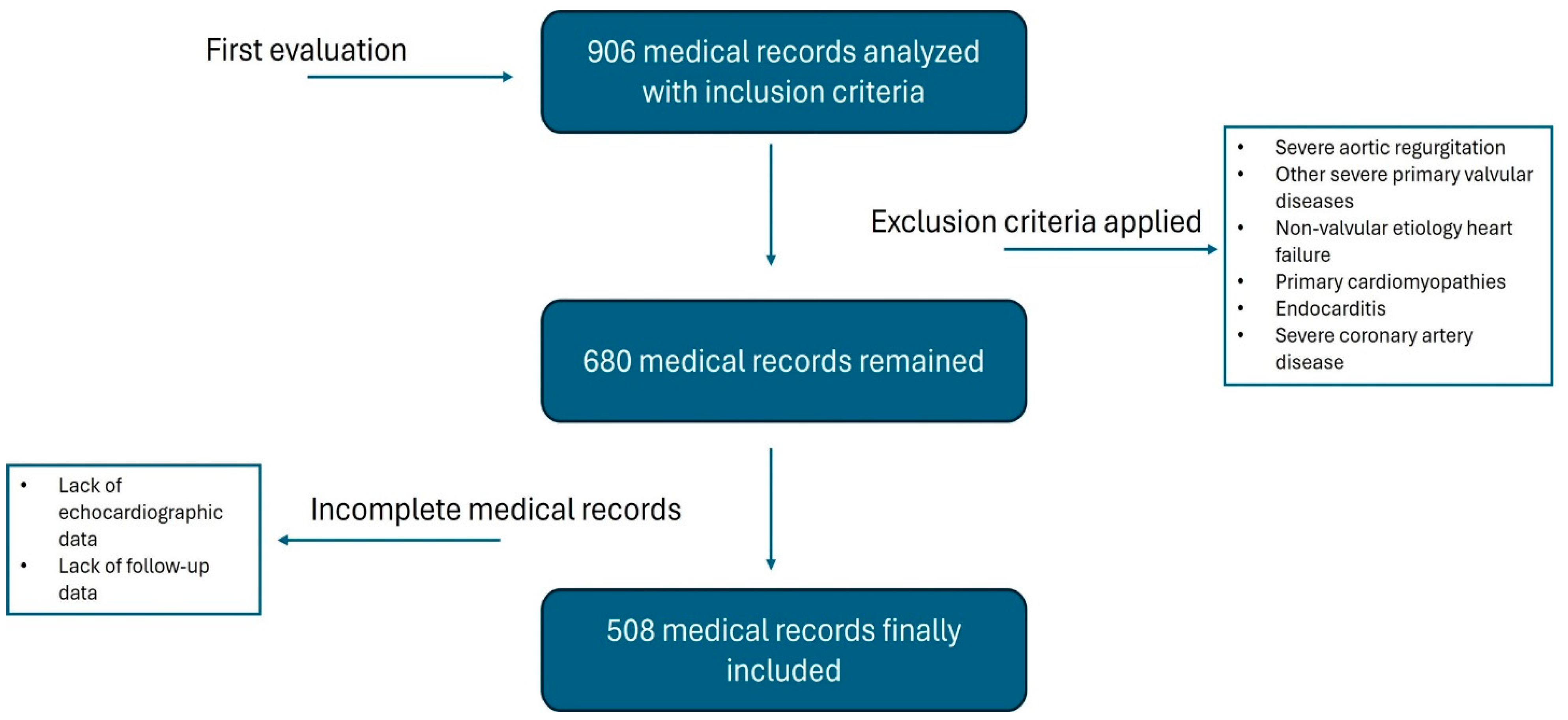
2. Materials and Methods
- -
- Group 1: no or minimal cardiac damage (characterized by left ventricular [LV] impact with LVH [LV mass index > 95 g/m2 for women and >115 g/m2 for men]), DD (E/e′ > 14), or systolic dysfunction (LV ejection fraction [LVEF] < 50%);
- -
- Group 2: secondary/functional mitral valve dysfunction or LA dilation (defined by increased indexed volume [>34 mL/m2]), presence of atrial fibrillation (AF), or moderate to severe mitral regurgitation;
- -
- Group 3: pulmonary hypertension (PH), defined as systolic pulmonary artery pressure (SPAP) ≥ 60 mmHg, moderate to severe tricuspid regurgitation, or involvement of the right ventricle (RV) with moderate to severe dysfunction (tricuspid annular plane systolic excursion [TAPSE] <11 mm, Fractional area change [FAC] <25%).
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group, 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Claessen, B.E.; Tang, G.H.L.; Kini, A.S.; Sharma, S.K. Considerations for Optimal Device Selection in Transcatheter Aortic Valve Replacement: A Review. JAMA Cardiol. 2021, 6, 102–112. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744, discussion 744–745. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Feng, L.; He, X.; Xian, Y.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C., Jr.; Lobdell, K.W.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-Statistical Methods and Results. Ann. Thorac. Surg. 2018, 105, 1419–1428. [Google Scholar] [CrossRef]
- Alnajar, A.; Chatterjee, S.; Chou, B.P.; Khabsa, M.; Rippstein, M.; Lee, V.V.; La Pietra, A.; Lamelas, J. Current Surgical Risk Scores Overestimate Risk in Minimally Invasive Aortic Valve Replacement. Innovations 2021, 16, 43–51. [Google Scholar] [CrossRef]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; A Jaber, W.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Glenn, K. Report Finds TAVR is Dominant Form of Aortic Valve Replacement, Outcomes Steadily Improving in the United States. Data from the STS/ACC TVT Registry Provides Insights on ‘State of TAVR in the United States’. 16 November 2020. Available online: https://www.acc.org/about-acc/press-releases/2020/11/16/18/53/report-finds-tavr-is-dominant-form-of-aortic-valve-replacement-outcomes-steadily-improving-in-the-us (accessed on 16 November 2020).
- Fukui, M.; Gupta, A.; Abdelkarim, I.; Sharbaugh, M.S.; Althouse, A.D.; Elzomor, H.; Mulukutla, S.; Lee, J.S.; Schindler, J.T.; Gleason, T.G.; et al. Association of Structural and Functional Cardiac Changes with Transcatheter Aortic Valve Replacement Outcomes in Patients with Aortic Stenosis. JAMA Cardiol. 2019, 4, 215–222. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1.e14–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Shvartz, V.; Sokolskaya, M.; Petrosyan, A.; Ispiryan, A.; Donakanyan, S.; Bockeria, L.; Bockeria, O. Predictors of Mortality Following Aortic Valve Replacement in Aortic Stenosis Patients. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2022, 29, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Tsai, Y.C.; Niranjan, N.; Bouchard, D.; Carrel, T.P.; E Dapunt, O.; Eichstaedt, H.C.; Fischlein, T.; Gersak, B.; Glauber, M.; et al. Sutureless aortic valve replacement: A systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2015, 4, 100–111. [Google Scholar] [CrossRef]
- Martinsson, A.; Nielsen, S.J.; Milojevic, M.; Redfors, B.; Omerovic, E.; Tønnessen, T.; Gudbjartsson, T.; Dellgren, G.; Jeppsson, A. Life Expectancy After Surgical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 78, 2147–2157. [Google Scholar] [CrossRef]
- De Almeida, A.S.; Picon, P.D.; Belmonte Wender, O.C. Resultados de pacientes submetidos à cirurgia de substituição valvar aórtica usando próteses mecânicas ou biológicas. Braz. J. Cardiovasc. Surg. 2011, 26, 326–337. [Google Scholar] [CrossRef]
- Gogbashian, A.; Sedrakyan, A.; Treasure, T. EuroSCORE: A systematic review of international performance. Eur. J. Cardiothorac. Surg. 2004, 25, 695–700. [Google Scholar] [CrossRef]
- Kunt, A.G.; Kurtcephe, M.; Hidiroglu, M.; Cetin, L.; Kucuker, A.; Bakuy, V.; Akar, A.R.; Sener, E. Comparison of original EuroSCORE, EuroSCORE II and STS risk models in a Turkish cardiac surgical cohort. Interact. CardioVascular Thorac. Surg. 2013, 16, 625–629. [Google Scholar] [CrossRef]
- Khan, A.A.; Murtaza, G.; Khalid, M.F.; Khattak, F. Risk Stratification for Transcatheter Aortic Valve Replacement. Cardiol. Res. 2019, 10, 323–330. [Google Scholar] [CrossRef]
- Marzec, K.; Jaworska-Wilczyńska, M.; Kowalik, I.; Nieznańska, M.; Witkowski, A.; Dąbrowski, M.; Kuśmierski, K.; Hryniewiecki, T. Comparison of the long-term outcomes and risk factors of aortic stenosis treatment in patients undergoing transcatheter aortic valve implantation and surgical aortic valve replacement. Kardiol. Pol. 2022, 80, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Turina, J.; Stark, T.; Seifert, B.; Turina, M. Predictors of the Long-Term Outcome After Combined Aortic and Mitral Valve Surgery. Circulation 1999, 100, II-48–II-53. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with aself-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016, 374, 1609–1620.8. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with aself-expanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Tastet, L.; Tribouilloy, C.; Maréchaux, S.; Vollema, E.M.; Delgado, V.; Salaun, E.; Shen, M.; Capoulade, R.; Clavel, M.-A.; Arsenault, M.; et al. Staging Cardiac Damage in Patients With Asymptomatic Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2019, 74, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Avvedimento, M.; Franzone, A.; Leone, A.; Piccolo, R.; Castiello, D.S.; Ilardi, F.; Mariani, A.; Esposito, R.; Iapicca, C.; Angellotti, D.; et al. Extent of Cardiac Damage and Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2021, 10, 4563. [Google Scholar] [CrossRef]
- Quere, J.P.; Monin, J.L.; Levy, F.; Petit, H.; Baleynaud, S.; Chauvel, C.; Pop, C.; Ohlmann, P.; Lelguen, C.; Dehant, P.; et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation 2006, 113, 1738–1744. [Google Scholar] [CrossRef]
- Clavel, M.A.; Fuchs, C.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Baumgartner, H.; Bergler-Klein, J.; Beanlands, R.S.; Mathieu, P.; Magne, J.; et al. Predictors of outcomes in low-flow, low-gradient aortic stenosis: Results of the multicenter TOPAS Study. Circulation 2008, 118 (Suppl. S14), S234–S242. [Google Scholar] [CrossRef]
- Awtry, E.; Davidoff, R. Low-Flow/Low-Gradient Aortic Stenosis. Circulation 2011, 124, e739–e741. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Magne, J.; Pibarot, P. Low-gradient aortic stenosis. Eur. Heart J. 2016, 37, 2645–2657. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Annabi, M.S.; Rosa, V.E.E.; Ribeiro, H.B.; Stanberry, L.I.; Clavel, M.A.; Rodés-Cabau, J.; Tarasoutchi, F.; Schelbert, E.B.; Bergler-Klein, J.; et al. Comprehensive myocardial characterization using cardiac magnetic resonance associates with outcomes in low gradient severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.E.E.; Ribeiro, H.B.; Sampaio, R.O.; Morais, T.C.; Rosa, M.E.E.; Pires, L.J.T.; Vieira, M.L.; Mathias, W.; Rochitte, C.E.; de Santis, A.S.; et al. Myocardial Fibrosis in Classical Low-Flow, Low-Gradient Aortic Stenosis. Circ. Cardiovasc. Imaging 2019, 12, e008353. [Google Scholar] [CrossRef]
- Iliuta, L.; Rac-Albu, M.; Rac-Albu, M.E.; Andronesi, A. Impact of Pulmonary Hypertension on Mortality after Surgery for Aortic Stenosis. Medicina 2022, 58, 1231. [Google Scholar] [CrossRef]
- Denault, A.; Deschamps, A.; Tardif, J.C.; Lambert, J.; Perrault, L. Pulmonary hypertension in cardiac surgery. Curr. Cardiol. Rev. 2010, 6, 1–14. [Google Scholar] [CrossRef]
- Eleid, M.F.; Padang, R.; Pislaru, S.V.; Greason, K.L.; Crestanello, J.; Nkomo, V.T.; Pellikka, P.A.; Jentzer, J.C.; Gulati, R.; Sandhu, G.S.; et al. Effect of transcatheter aortic valve replacement on right ventricular-pulmonary artery coupling. J. Am. Coll. Cardiol. 2019, 12, 2145–2154. [Google Scholar] [CrossRef]
- Schewel, J.; Schmidt, T.; Kuck, K.-H.; Frerker, C.; Schewel, D. Impact of pulmonary hypertension hemodynamic on long-term outcome after trans-catheter aortic valve replacement. J. Am. Coll. Car.-Diol. 2019, 12, 2155–2168. [Google Scholar]
- Cremer, P.C.; Zhang, Y.; Alu, M.; Rodriguez, L.L.; Lindman, B.R.; Zajarias, A.; Hahn, R.T.; Lerakis, S.; Malaisrie, S.C.; Douglas, P.S.; et al. The incidence and prognostic implications of worsening right ventricular function after surgical or transcatheter aortic valve replacement: Insights from PARTNER IIA. Eur. Heart J. 2018, 39, 2659–2667. [Google Scholar] [CrossRef]
- Blackstone, E.H.; Pande, A.; Rajeswaran, J. Commentary: Enhancing risk assessment by incorporating more of what we know. J. Thorac. Cardiovasc. Surg. 2022, 163, 1388–1390.e3. [Google Scholar] [CrossRef]

| Variable | Stage 1 (N = 235) | Stage 2 (N = 206) | Stage 3 (N = 67) | p |
|---|---|---|---|---|
| Clinical data | ||||
| Age, years | 62 (55–70) | 67 (61–72) | 67 (60–73) | <0.001 †‡ |
| Male sex | 139 (59.1) | 110 (53.4) | 41 (61.2) | 0.365 |
| Diabetes Mellitus | 66 (28.1) | 77 (37.4) | 24 (35.8) | 0.100 |
| Arterial hypertension | 155 (66.0) | 158 (76.7) | 49 (73.1) | 0.042 † |
| Dyslipidemia | 151 (64.3) | 138 (67.0) | 40 (59.7) | 0.542 |
| Chronic kidney disease | 21 (8.9) | 20 (9.7) | 14 (20.9) | 0.017 ഠ|
| Atrial fibrillation | 1 (0.4) | 22 (10.7) | 17 (25.4) | <0.001 †‡¥ |
| Coronary artery disease | 0.346 | |||
| One vessel | 42 (17.9) | 30 (14.6) | 12 (17.9) | |
| Two vessels | 15 (6.4) | 24 (11.7) | 9 (13.4) | |
| Rheumatic etiology | 13 (5.5) | 17 (8.3) | 5 (7.5) | 0.158 |
| Bicuspid etiology | 37 (15.7) | 18 (8.7) | 6 (9.0) | 0.157 |
| Diuretic use | 144 (61.3) | 161 (78.2) | 58 (86.6) | <0.001 †‡ |
| ACE-I use | 61 (26.0) | 53 (25.7) | 33 (49.3) | <0.001 †‡ |
| ARB use | 77 (32.8) | 78 (37.9) | 13 (19.4) | 0.020 ¥ |
| B-blocker use | 35 (14.9) | 55 (26.7) | 29 (43.3) | <0.001 †‡¥ |
| Statin use | 153 (65.1) | 137 (66.5) | 40 (59.7) | 0.597 |
| EuroSCOREII, % | 0.99 (0.80–1.30) | 1.43 (1.03–1.87) | 2.75 (1.63–3.81) | <0.001 †‡¥ |
| STS score, % | 1.54 (1.44–1.64) | 2.00 (1.85–2.16) | 2.62 (2.25–2.99) | <0.001 †‡¥ |
| Symptoms | ||||
| NYHA | <0.001 | |||
| II | 129 (54.9) | 90 (43.7) | 6 (9.0) | ഠ|
| III | 79 (33.6) | 95 (46.1) | 48 (71.6) | †‡¥ |
| IV | 7 (3.0) | 10 (4.9) | 13 (19.4) | ഠ|
| Laboratory data | ||||
| Glomerular filtration rate, mL/min 2,* | 72 (62–87) | 65 (56–80) | 58 (48–70) | <0.001 †‡¥ |
| Hemoglobin, g/dL 1,* | 13.3 (12.3–14.5) | 13.0 (11.7–14.2) | 13.0 (11.8–14.2) | 0.033 † |
| BNP, pg/mL 96,* | 79 (35–226) | 155 (81–331) | 920 (446–2740) | <0.001 †‡¥ |
| Echocardiographic data | ||||
| Aortic regurgitation (mild or moderate) 2,* | 110 (47.0) | 136 (66.3) | 58 (86.6) | <0.001 †‡ |
| Left Ventricular Hypertrophy 2,* | 165 (70.2) | 191 (92.7) | 60 (89.6) | <0.001 †‡ |
| Diastolic dysfunction | 146 (62.1) | 182 (88.3) | 43 (64.2) | <0.001 †¥ |
| Ejection fraction < 50% | 10 (4.3) | 29 (14.1) | 41 (61.2) | <0.001 †‡¥ |
| Enlargement left atrium | 10 (4.3) | 200 (97.1) | 67 (100.0) | <0.001 †‡ |
| Mitral regurgitation (moderate/severe) | 2 (0.9) | 45 (21.8) | 32 (47.8) | <0.001 †‡¥ |
| Pulmonary hypertension 107,* | 0 (0.0) | 0 (0.0) | 34 (50.7) | <0.001 ഠ|
| Tricuspid regurgitation moderate/severe | 1 (0.4) | 0 (0.0) | 43 (64.2) | <0.001 ഠ|
| RV dysfunction (moderate/severe) | 0 (0.0) | 0 (0.0) | 12 (17.9) | <0.001 ഠ|
| Mass 2,*, g/m2 | 120 (100–140) | 130 (114–154) | 142 (124–179) | <0.001 †‡¥ |
| Mean gradient aortic, mmhg | 52 (43–63) | 53 (45–66) | 50 (40–60) | 0.023 ¥ |
| Aortic valvular area 1,*, cm2 | 0.75 (0.60–0.85) | 0.7 (0.6–0.8) | 0.7 (0.60–0.85) | 0.202 |
| Pulmonary systolic pressure, mmhg 107,* | 30 (26–36) | 35 (30–42) | 60 (50–65) | <0.001 †‡¥ |
| Ejection fraction, % | 63 (60–66) | 62 (57–66) | 45 (30–60) | <0.001 ‡¥ |
| Postoperative findings | ||||
| Postoperative AF | 44 (18.7) | 52 (25.2) | 28 (41.8) | 0.001 ഠ|
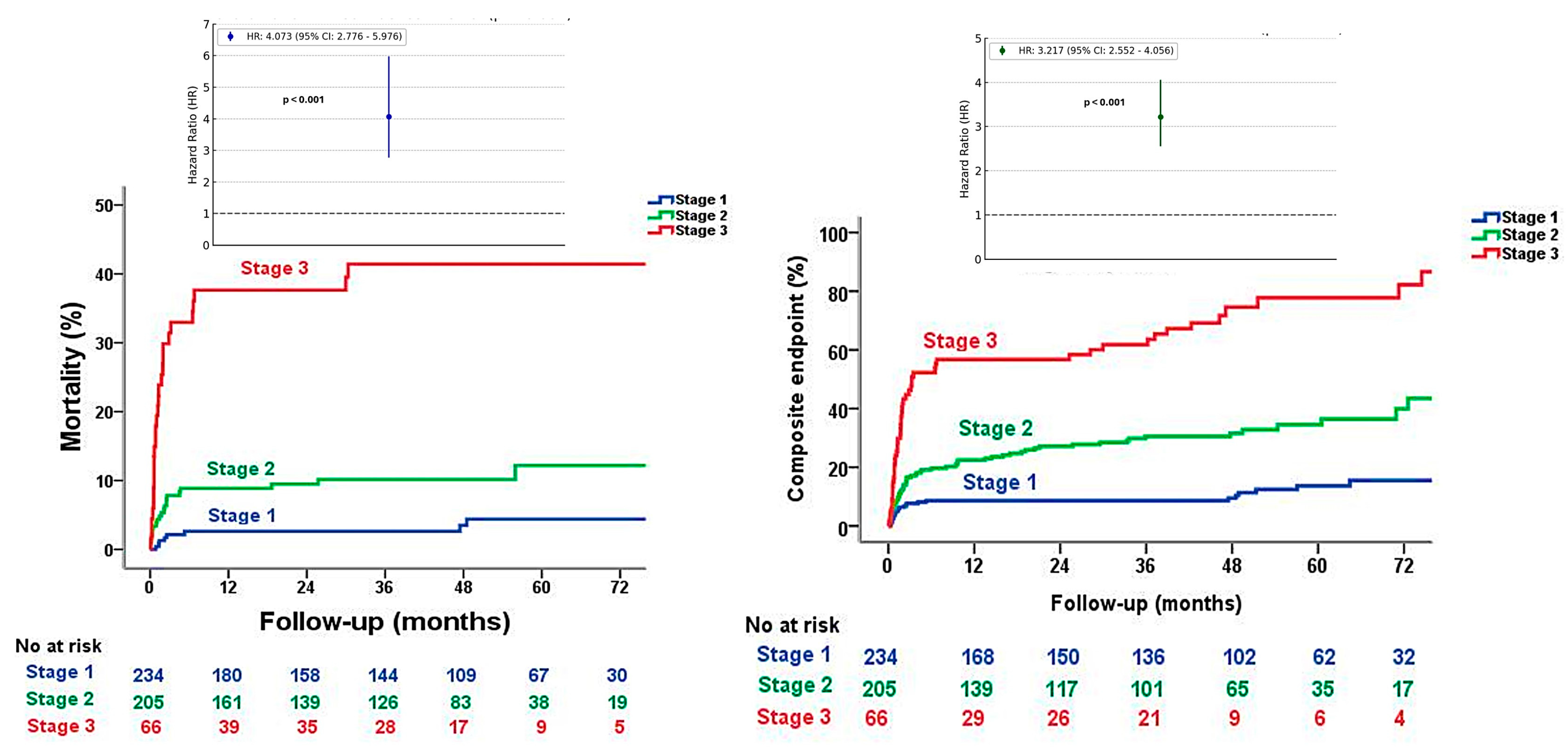
| Long-term all-cause mortality | 8 (3.4) | 21 (10.2) | 27 (40.3) | <0.001 †‡¥ |
| Long-term all-cause mortality and cardiovascular readmissions | 26 (11.1) | 65 (31.6) | 51 (77.3) | <0.001 †‡¥ |
| 30-day mortality | 1 (0.4) | 10 (4.9) | 15 (22.7) | <0.001 †‡¥ |
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (CI 95%) | p | HR (CI 95%) | p | |
| Proposed stage classification | 4.07 (2.77–5.97) | <0.001 | 3.22 (1.44–7.20) | 0.004 |
| Mean transaortic gradient, mmHg | 0.97 (0.95–0.98) | 0.003 | 0.96 (0.92–0.99) | 0.021 |
| Glomerular filtration rate, mL/min | 0.96 (0.95–0.97) | <0.001 | 0.99 (0.96–1.01) | 0.431 |
| BNP, pg/mL | 1.000 (1.000–1.001) | 0.001 | 1.000 (1.000–1.001) | 0.172 |
| Age, years | 1.04 (1.01–1.07) | 0.003 | 1.03 (0.98–1.09) | 0.168 |
| NYHA stages | 2.72 (1.83–4.05) | <0.001 | 0.86 (0.40–1.82) | 0.701 |
| Univariable Analysis | Multivariable Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Original Classification | |||||||||
| Variables | HR | CI, 95% | p | Variables | HR | CI, 95% | p | ||
| Proposed staged classification | 5.655 | 3.034 | 10.539 | <0.001 | BNP | 1.000 | 1.000 | 1.000 | 0.629 |
| EuroSCORE II | 1.004 | 0.677 | 1.490 | 0.983 | |||||
| Original classification | 3.247 | 2.165 | 4.870 | <0.001 | STS score | 0.953 | 0.602 | 1.509 | 0.839 |
| Creatinine clearance | 0.985 | 0.949 | 1.022 | 0.431 | |||||
| BNP | 1.000 | 1.000 | 1.001 | 0.004 | Original classification | 2.632 | 1.211 | 5.717 | 0.015 |
| NYHA stages | 0.551 | 0.178 | 1.705 | 0.301 | |||||
| EuroSCORE II | 1.278 | 1.127 | 1.450 | <0.001 | Proposed staged classification | ||||
| BNP | 1.000 | 1.000 | 1.001 | 0.533 | |||||
| STS score | 1.274 | 1.006 | 1.613 | 0.045 | EuroSCORE II | 1.019 | 0.678 | 1.531 | 0.929 |
| STS score | 0.901 | 0.558 | 1.456 | 0.671 | |||||
| Creatinine clearance | 0.965 | 0.945 | 0.985 | 0.001 | Creatinine clearance | 0.986 | 0.950 | 1.024 | 0.470 |
| NYHA stages | 0.474 | 0.150 | 1.493 | 0.202 | |||||
| NYHA stages | 2.638 | 1.501 | 4.637 | 0.001 | Proposed classification | 5.019 | 1.596 | 15.784 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza Romero, C.; Rosa, V.E.E.; Octavio Kormann, S.; Nicolalde, B.; de Santis Andrade Lopes, A.S.; Sobreira Spina, G.; Cordeiro Fernandes, J.R.; Tarasoutchi, F.; Sampaio, R.O. Impact of a New Preoperative Stratification Based on Cardiac Structural Compromise in Patients with Severe Aortic Stenosis Undergoing Valve Replacement Surgery. Diagnostics 2024, 14, 2250. https://doi.org/10.3390/diagnostics14192250
Espinoza Romero C, Rosa VEE, Octavio Kormann S, Nicolalde B, de Santis Andrade Lopes AS, Sobreira Spina G, Cordeiro Fernandes JR, Tarasoutchi F, Sampaio RO. Impact of a New Preoperative Stratification Based on Cardiac Structural Compromise in Patients with Severe Aortic Stenosis Undergoing Valve Replacement Surgery. Diagnostics. 2024; 14(19):2250. https://doi.org/10.3390/diagnostics14192250
Chicago/Turabian StyleEspinoza Romero, Cristhian, Vitor Emer Egypto Rosa, Sérgio Octavio Kormann, Bryan Nicolalde, Antonio Sérgio de Santis Andrade Lopes, Guilherme Sobreira Spina, João Ricardo Cordeiro Fernandes, Flavio Tarasoutchi, and Roney Orismar Sampaio. 2024. "Impact of a New Preoperative Stratification Based on Cardiac Structural Compromise in Patients with Severe Aortic Stenosis Undergoing Valve Replacement Surgery" Diagnostics 14, no. 19: 2250. https://doi.org/10.3390/diagnostics14192250





