Predictive Value of Diaphragm and Lung Ultrasonography for Weaning Failure in Critically Ill Patients with Acute Respiratory Failure Due to COVID-19 Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
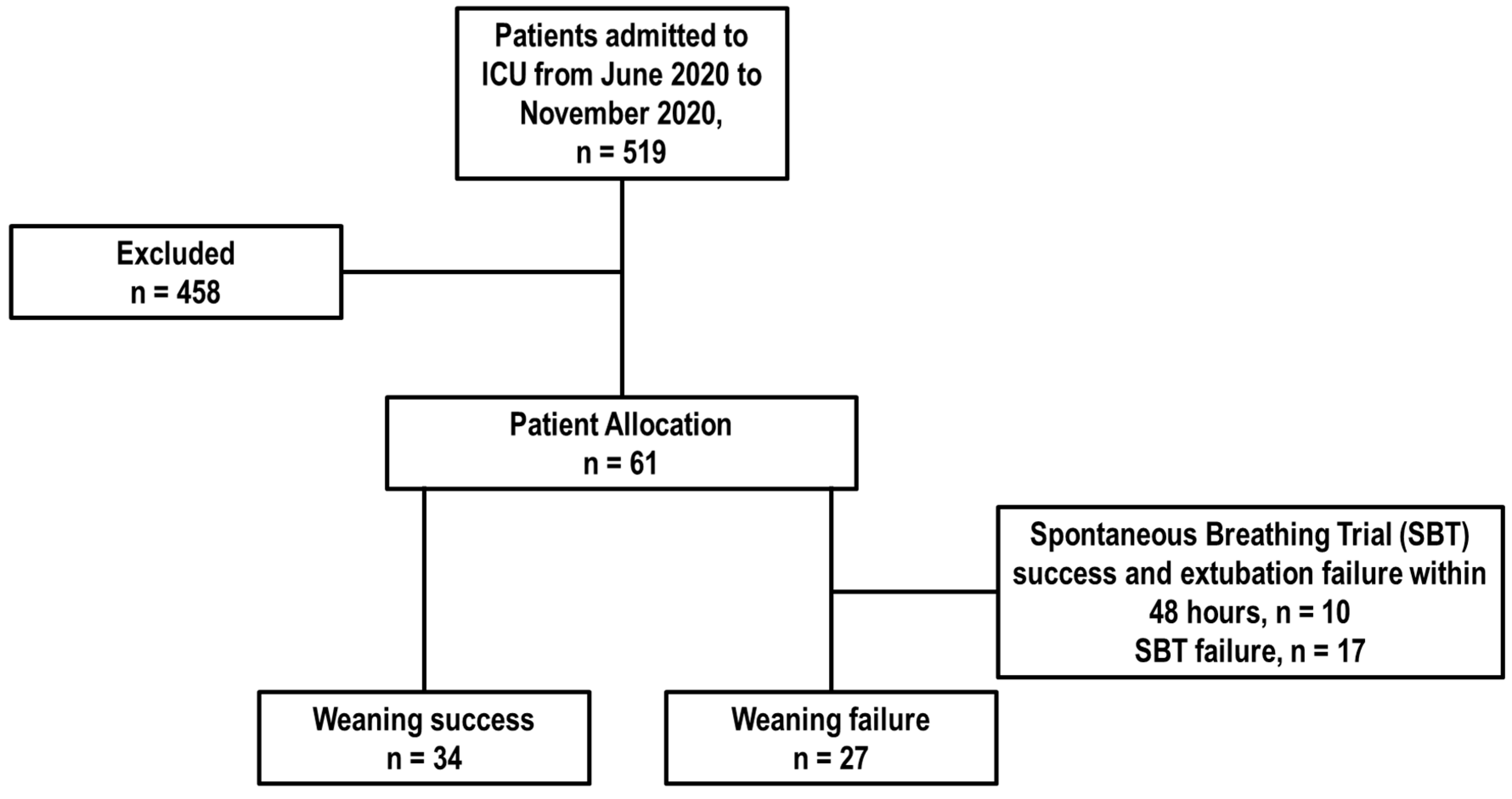
2.2. Participants
2.3. Data Collection
2.4. Definitions
2.5. Local Protocol for Weaning from Mechanical Ventilation
2.6. Ultrasound Evaluation
- 0 points: Normal aeration with the presence of A-lines or fewer than 2 isolated B-lines.
- 1 point: B1 pattern with more than 3 comet tails and less than 50% coalescence in the ultrasound window, indicating moderate loss of pulmonary aeration with multiple well-defined B-lines.
- 2 points: B2 pattern with more than 50% coalescence in the ultrasound window, indicating severe loss of pulmonary aeration with multiple coalescent B-lines.
- 3 points: Pattern C, representing complete loss of pulmonary aeration.
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Characteristics of Weaning Parameters in Patients Who Failed of Succeeded in Weaning
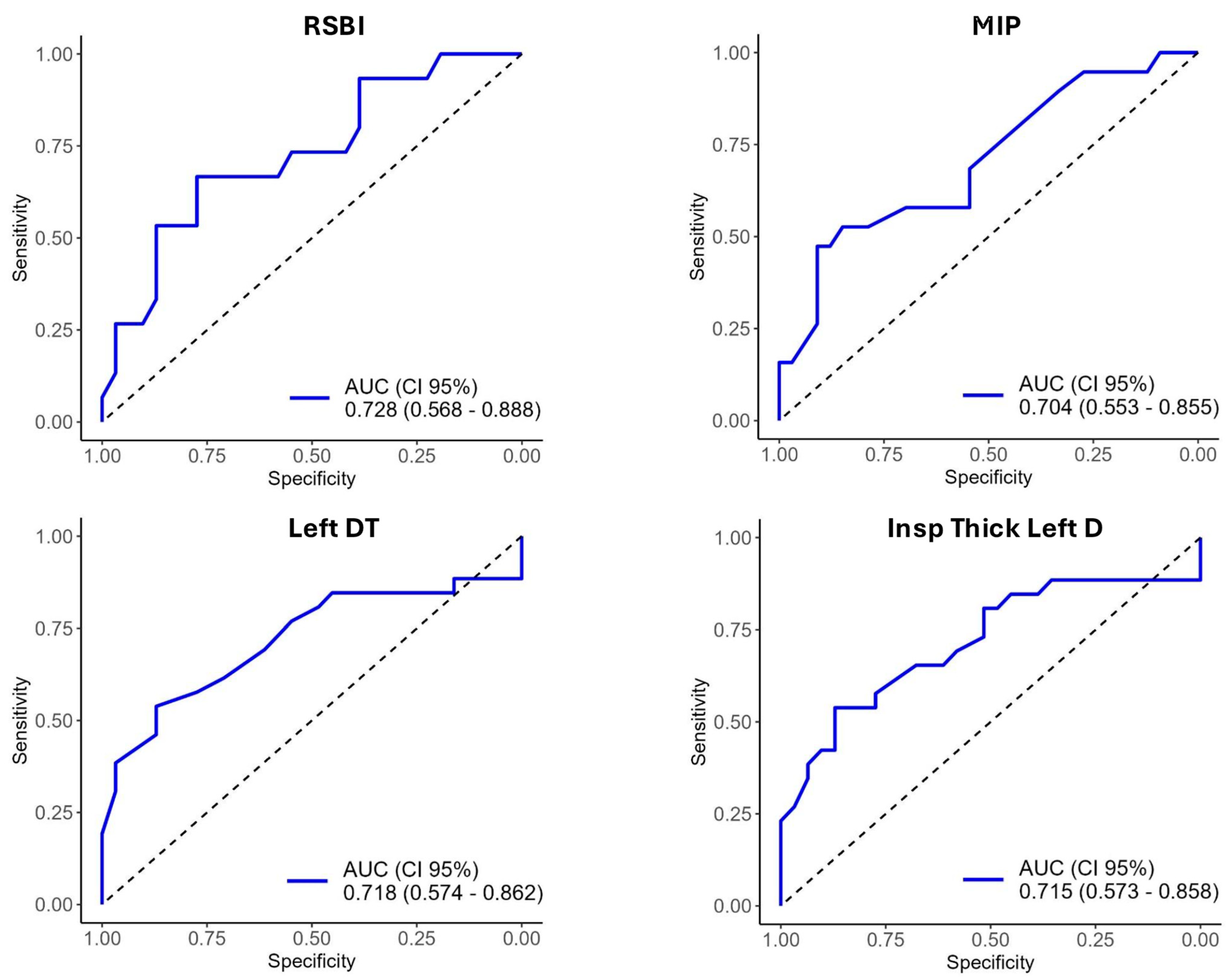
3.3. Diagnostic Accuracy of Weaning Parameters for Predicting Weaning Failure
3.4. Predictors of Weaning Failure
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gok, F.; Mercan, A.; Kilicaslan, A.; Sarkilar, G.; Yosunkaya, A. Diaphragm and Lung Ultrasonography During Weaning From Mechanical Ventilation in Critically Ill Patients. Cureus 2021, 13, e15057. [Google Scholar] [CrossRef] [PubMed]
- Al-Husinat, L.; Jouryyeh, B.; Rawashdeh, A.; Robba, C.; Silva, P.; Rocco, P.; Battaglini, D. The Role of Ultrasonography in the Process of Weaning from Mechanical Ventilation in Critically Ill Patients. Diagnostics 2024, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A. Characteristics and Outcomes in Adult Patients Receiving Mechanical VentilationA 28-Day International Study. JAMA 2002, 287, 345. [Google Scholar] [CrossRef]
- Béduneau, G.; Pham, T.; Schortgen, F.; Piquilloud, L.; Zogheib, E.; Jonas, M.; Grelon, F.; Runge, I.; Terzi, N.; Grangé, S.; et al. Epidemiology of Weaning Outcome According to a New Definition. The WIND Study. Am. J. Respir. Crit. Care Med. 2017, 195, 772–783. [Google Scholar] [CrossRef]
- Ely, E.W.; Baker, A.M.; Dunagan, D.P.; Burke, H.L.; Smith, A.C.; Kelly, P.T.; Johnson, M.M.; Browder, R.W.; Bowton, D.L.; Haponik, E.F. Effect on the Duration of Mechanical Ventilation of Identifying Patients Capable of Breathing Spontaneously. N. Engl. J. Med. 1996, 335, 1864–1869. [Google Scholar] [CrossRef]
- Vallverdú, I.; Calaf, N.; Subirana, M.; Net, A.; Benito, S.; Mancebo, J. Clinical Characteristics, Respiratory Functional Parameters, and Outcome of a Two-Hour T-Piece Trial in Patients Weaning from Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 1998, 158, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Alía, I.; Tobin, M.J.; Gil, A.; Gordo, F.; Vallverdú, I.; Blanch, L.; Bonet, A.; Vázquez, A.; de Pablo, R.; et al. Effect of Spontaneous Breathing Trial Duration on Outcome of Attempts to Discontinue Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 1999, 159, 512–518. [Google Scholar] [CrossRef]
- Nemer, S.N.; Barbas, C.S.V.; Caldeira, J.B.; Guimarães, B.; Azeredo, L.M.; Gago, R.; Souza, P.C.P. Evaluation of Maximal Inspiratory Pressure, Tracheal Airway Occlusion Pressure, and Its Ratio in the Weaning Outcome. J. Crit. Care 2009, 24, 441–446. [Google Scholar] [CrossRef]
- de Souza, L.C.; da Silva, C.T.; Almeida, J.R.; Lugon, J.R. Comparison of Maximal Inspiratory Pressure, Tracheal Airway Occlusion Pressure, and Its Ratio in the Prediction of Weaning Outcome: Impact of the Use of a Digital Vacuometer and the Unidirectional Valve. Respir. Care 2012, 57, 1285–1290. [Google Scholar] [CrossRef]
- Haaksma, M.E.; Smit, J.M.; Boussuges, A.; Demoule, A.; Dres, M.; Ferrari, G.; Formenti, P.; Goligher, E.C.; Heunks, L.; Lim, E.H.T.; et al. EXpert Consensus On Diaphragm UltraSonography in the Critically Ill (EXODUS): A Delphi Consensus Statement on the Measurement of Diaphragm Ultrasound-Derived Parameters in a Critical Care Setting. Crit. Care 2022, 26, 99. [Google Scholar] [CrossRef]
- Song, J.; Qian, Z.; Zhang, H.; Wang, M.; Yu, Y.; Ye, C.; Hu, W.; Gong, S. Diaphragmatic Ultrasonography-Based Rapid Shallow Breathing Index for Predicting Weaning Outcome during a Pressure Support Ventilation Spontaneous Breathing Trial. BMC Pulm. Med. 2022, 22, 337. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- Black, L.F.; Hyatt, R.E. Maximal Respiratory Pressures: Normal Values and Relationship to Age and Sex. Am. Rev. Respir. Dis. 1969, 99, 696–702. [Google Scholar] [CrossRef]
- Tobin, M.J.; Perez, W.; Guenther, S.M.; Semmes, B.J.; Mador, M.J.; Allen, S.J.; Lodato, R.F.; Dantzker, D.R. The Pattern of Breathing during Successful and Unsuccessful Trials of Weaning from Mechanical Ventilation. Am. Rev. Respir. Dis. 1986, 134, 1111–1118. [Google Scholar] [CrossRef]
- Boles, J.-M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from Mechanical Ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Alhazzani, W.; Hylander Møller, M.; Arabi, Y.M.; Loeb, M.; Ng Gong, M.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 48, e440–e469. [Google Scholar] [CrossRef]
- Hernández Martínez, G.; Rodriguez, P.; Soto, J.; Caritg, O.; Castellví-Font, A.; Mariblanca, B.; García, A.M.; Colinas, L.; Añon, J.M.; Parrilla-Gomez, F.J.; et al. Effect of Aggressive vs Conservative Screening and Confirmatory Test on Time to Extubation among Patients at Low or Intermediate Risk: A Randomized Clinical Trial. Intensive Care Med. 2024, 50, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Frutos, F.; Tobin, M.J.; Alía, I.; Solsona, J.F.; Valverdu, V.; Fernández, R.; de la Cal, M.A.; Benito, S.; Tomás, R.; et al. A Comparison of Four Methods of Weaning Patients from Mechanical Ventilation. New Engl. J. Med. 1995, 332, 345–350. [Google Scholar] [CrossRef]
- Cohn, D.; Benditt, J.O.; Eveloff, S.; McCool, F.D. Diaphragm Thickening during Inspiration. J. Appl. Physiol. 1997, 83, 291–296. [Google Scholar] [CrossRef]
- Spadaro, S.; Grasso, S.; Mauri, T.; Dalla Corte, F.; Alvisi, V.; Ragazzi, R.; Cricca, V.; Biondi, G.; Di Mussi, R.; Marangoni, E.; et al. Can Diaphragmatic Ultrasonography Performed during the T-Tube Trial Predict Weaning Failure? The Role of Diaphragmatic Rapid Shallow Breathing Index. Crit. Care 2016, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Soummer, A.; Perbet, S.; Brisson, H.; Arbelot, C.; Constantin, J.-M.; Lu, Q.; Rouby, J.-J. Ultrasound Assessment of Lung Aeration Loss during a Successful Weaning Trial Predicts Postextubation Distress*. Crit. Care Med. 2012, 40, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; de Oliveira, R.A.; Friedman, G.; Moraes, R.B. Association of Biomarkers with Successful Ventilatory Weaning in COVID-19 Patients: An Observational Study. Crit. Care Sci. 2024, 36, e20240158en. [Google Scholar] [CrossRef] [PubMed]
- Aldabayan, Y.S.; Tolba, A.A.; Alrajeh, A.M.; Ahmed, A.T.; Mahgoub, A.A.; Glalah, A.A.A.; Abdelhafez, A.I. Factors Affecting Mechanical Ventilator Weaning Success and 28-Day Survival Among Patients With Acute Respiratory Distress Syndrome Secondary to COVID-19. SAGE Open Nurs. 2023, 9, 23779608231187250. [Google Scholar] [CrossRef] [PubMed]
- Gerovasileiou, E.; Menis, A.-A.; Gavriilidis, G.; Magira, E.; Temperikidis, P.; Papoti, S.; Karavidas, N.; Spanos, M.; Zakynthinos, E.; Makris, D. Risk Factors for Weaning Failure in COVID-19 Patients. J. Crit. Care Med. (Targu Mures) 2023, 9, 170–177. [Google Scholar] [CrossRef]
- Battaglini, D.; Premraj, L.; White, N.; Sutt, A.-L.; Robba, C.; Cho, S.-M.; Di Giacinto, I.; Bressan, F.; Sorbello, M.; Cuthbertson, B.; et al. Tracheostomy Outcomes in Critically Ill COVID-19 Patients: A Systematic Review, Meta-Analysis, and Meta-Regression. Br. J. Anaesth. 2022, 129, 679–692. [Google Scholar] [CrossRef]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.M. Multiple Organ Dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef]
- Trudzinski, F.C.; Neetz, B.; Bornitz, F.; Müller, M.; Weis, A.; Kronsteiner, D.; Herth, F.J.F.; Sturm, N.; Gassmann, V.; Frerk, T.; et al. Risk Factors for Prolonged Mechanical Ventilation and Weaning Failure: A Systematic Review. Respiration 2022, 101, 959–969. [Google Scholar] [CrossRef]
- Panelli, A.; Grunow, J.J.; VERFUß, M.A.; Bartels, H.G.; Brass, Z.; Schaller, S.J. Outcomes in Critically Ill Patients after Diaphragmatic Stimulation on Ventilator-Induced Diaphragmatic Dysfunction: A Systematic Review. Eur. J. Phys. Rehabil. Med. 2023, 59, 772–781. [Google Scholar] [CrossRef]
- Pham, T.; Heunks, L.; Bellani, G.; Madotto, F.; Aragao, I.; Beduneau, G.; Goligher, E.C.; Grasselli, G.; Laake, J.H.; Mancebo, J.; et al. Weaning from Mechanical Ventilation in Intensive Care Units across 50 Countries (WEAN SAFE): A Multicentre, Prospective, Observational Cohort Study. Lancet Respir. Med. 2023, 11, 465–476. [Google Scholar] [CrossRef]
- Dorado, J.H.; Navarro, E.; Plotnikow, G.A.; Gogniat, E.; Accoce, M. Epidemiology of Weaning From Invasive Mechanical Ventilation in Subjects With COVID-19. Respir. Care 2023, 68, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Ohya, A.; Yamagata, H.; Iwashita, M.; Abe, T.; Takeuchi, I. Prolonged Mechanical Ventilation in Patients with Severe COVID-19 Is Associated with Serial Modified-Lung Ultrasound Scores: A Single-Centre Cohort Study. PLoS ONE 2022, 17, e0271391. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.E.A.; Raptis, S.; Nisenbaum, R.; Rizvi, L.; Jones, A.; Bakshi, J.; Tan, W.; Meret, A.; Cook, D.J.; Lellouche, F.; et al. International Practice Variation in Weaning Critically Ill Adults from Invasive Mechanical Ventilation. Ann. Am. Thorac. Soc. 2018, 15, 494–502. [Google Scholar] [CrossRef]
- Burns, K.E.A.; Khan, J.; Phoophiboon, V.; Trivedi, V.; Gomez-Builes, J.C.; Giammarioli, B.; Lewis, K.; Chaudhuri, D.; Desai, K.; Friedrich, J.O. Spontaneous Breathing Trial Techniques for Extubating Adults and Children Who Are Critically Ill. JAMA Netw. Open 2024, 7, e2356794. [Google Scholar] [CrossRef]
- Thille, A.W.; Gacouin, A.; Coudroy, R.; Ehrmann, S.; Quenot, J.-P.; Nay, M.-A.; Guitton, C.; Contou, D.; Labro, G.; Reignier, J.; et al. Spontaneous-Breathing Trials with Pressure-Support Ventilation or a T-Piece. N. Engl. J. Med. 2022, 387, 1843–1854. [Google Scholar] [CrossRef]
- Cohen, J.D.; Shapiro, M.; Grozovski, E.; Lev, S.; Fisher, H.; Singer, P. Extubation Outcome Following a Spontaneous Breathing Trial with Automatic Tube Compensation versus Continuous Positive Airway Pressure. Crit. Care Med. 2006, 34, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, V.; Chaudhuri, D.; Jinah, R.; Piticaru, J.; Agarwal, A.; Liu, K.; McArthur, E.; Sklar, M.C.; Friedrich, J.O.; Rochwerg, B.; et al. The Usefulness of the Rapid Shallow Breathing Index in Predicting Successful Extubation. Chest 2022, 161, 97–111. [Google Scholar] [CrossRef]
- Varón-Vega, F.; Tuta-Quintero, E.; Robayo-Amortegui, H.; Rincón, A.; Giraldo-Cadavid, L.F.; Palacios, J.; Crevoisier, S.; Duarte, D.C.; Poveda, M.; Cucunubo, L.; et al. Clinical Utility of Rapid Shallow Breathing Index in Predicting Successful Weaning: Secondary Analysis of the COBRE-US Trial. Med. Intensiv. (Engl. Ed.) 2024. [Google Scholar] [CrossRef]
- Terzi, N.; Lofaso, F.; Masson, R.; Beuret, P.; Normand, H.; Dumanowski, E.; Falaize, L.; Sauneuf, B.; Daubin, C.; Brunet, J.; et al. Physiological Predictors of Respiratory and Cough Assistance Needs after Extubation. Ann. Intensive Care 2018, 8, 18. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, Y.; Zhang, F.; Yao, Y.-Y.; Chen, Y.-X.; Wu, C.-M.; Wang, R.-Y.; Yan, M. Lung Ultrasound Score as a Predictor of Failure to Wean COVID-19 Elderly Patients off Mechanical Ventilation: A Prospective Observational Study. Clin. Interv. Aging 2024, 19, 313–322. [Google Scholar] [CrossRef]
- Vetrugno, L.; Orso, D.; Corradi, F.; Zani, G.; Spadaro, S.; Meroi, F.; D’Andrea, N.; Bove, T.; Cammarota, G.; De Robertis, E.; et al. Diaphragm Ultrasound Evaluation during Weaning from Mechanical Ventilation in COVID-19 Patients: A Pragmatic, Cross-Section, Multicenter Study. Respir. Res. 2022, 23, 210. [Google Scholar] [CrossRef] [PubMed]
- Vieira Santana, P.; Zumpano Cardenas, L.; Pereira de Albuquerque, A.L.; Ribeiro de Carvalho, C.R.; Caruso, P. Diaphragmatic Ultrasound: A Review of Its Methodological Aspects and Clinical Uses. J. Bras. De Pneumol. 2020, 46, e20200064. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, D.; Battaglini, D.; Robba, C.; Viganò, M.; Bergamaschi, G.; Mignatti, T.; Radice, M.L.; Lapolla, A.; Turtulici, G.; Pelosi, P. Coronavirus Disease 2019 Phenotypes, Lung Ultrasound, Chest Computed Tomography and Clinical Features in Critically Ill Mechanically Ventilated Patients. Ultrasound Med. Biol. 2021, 47, 3323–3332. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 61) | Weaning Failure (n = 27) | Weaning Success (n = 34) | p-Value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 63 (9.7) | 64 (9.6) | 62 (9.8) | 0.351 |
| Gender, n (%) | ||||
| Female | 22 (36%) | 12 (44.44%) | 10 (29.41%) | 0.344 |
| Male | 39 (64%) | 15 (55.55%) | 24 (70.59%) | |
| Comorbidities, n (%) | ||||
| Hypertension | 34 (56%) | 15 (56%) | 19 (56%) | >0.99 |
| Diabetes mellitus | 27 (44%) | 15 (56%) | 12 (35%) | 0.186 |
| Renal failure | 5 (8%) | 2 (7%) | 3 (9%) | >0.99 |
| Pulmonary disease | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Neurological disease | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Malignancy | 0 (0%) | 0 (0%) | 0 (0%) | - |
| SOFA score, points, median (IQR) | 6 (2–12) | 9 (2–12) | 3.5 (2–11) | <0.001 |
| History of symptoms | ||||
| Days of illness before hospital admission, median (IQR) | 6.4 (1–26) | 5.5 (2–11) | 7.0 (1–26) | 0.215 |
| Days of illness before ICU admission, median (IQR) | 8.9 (1–33) | 7.6 (3–22) | 10 (1–33) | 0.086 |
| Days of illness before IMV, median (IQR) | 10.7 (3–35) | 8.9 (3–28) | 12 (3–35) | 0.040 |
| Treatment before IMV, n (%) | ||||
| COT | 22 (36%) | 17 (63%) | 5 (15%) | 0.000 |
| NIV | 2 (3%) | 1 (4%) | 1 (3%) | |
| HFOT | 37 (61%) | 9 (33%) | 28 (82%) | |
| Treatment after IMV, n (%) | n = 44 | n = 10 | n = 34 | |
| COT | 5 (11%) | 0 (0%) | 5 (15%) | 0.418 |
| NIV | 21 (48%) | 5 (50%) | 13 (38%) | |
| HFOT | 18 (41%) | 5 (50%) | 16 (47%) | |
| Prone vigilant before the IMV, n (%) | 24 (39%) | 10 (37%) | 14 (41%) | 0.742 |
| Prone during the IMV, n (%) | 32 (52%) | 12 (44%) | 20 (59%) | 0.003 |
| Organic dysfunction during ICU stay, n (%) | ||||
| Renal | 9 (15%) | 5 (19%) | 4 (12%) | 0.460 |
| Cardiovascular | 6 (10%) | 4 (15%) | 2 (6%) | 0.245 |
| Hepatic | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Hematology | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Neurological | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Outcomes | ||||
| IMV duration, days, median (IQR) | 10 (2–65) | 29 (5–65) | 5 (2–15) | <0.001 |
| IMV duration before SBT, days, median (IQR) | 7 (2–36) | 14 (2–36) | 5 (2–15) | <0.001 |
| COT duration, days, median (IQR) | 0 (0–6) | 0 (0–4) | 1.5 (0–6) | 0.030 |
| NIV duration, days, median (IQR) | 0 (0–7) | 0 (0–7) | 0 (0–7) | 0.864 |
| HFOT duration, days, median (IQR) | 1 (0–6) | 0 (0–4) | 0 (0–6) | 0.008 |
| ICU—length of stay, days, median (IQR) | 17 (3–65) | 10 (3–51) | 30 (7–65) | <0.001 |
| ICU—mortality, n (%) | 6.0 (9.8%) | 6.0 (22.2%) | 0 (0%) | 0.014 |
| Overall (n = 61) | Weaning Failure (n = 27) | Weaning Success (n = 34) | p-Value | |
|---|---|---|---|---|
| Ventilatory setting before SBT | ||||
| Pressure support, cmH2O, mean (SD) | 10.2 (2.5) | 11.2 (2.3) | 9.4 (2.5) | 0.003 |
| PEEP, cmH2O, mean (SD) | 6.7 (1.5) | 6.8 (1.4) | 6.6 (1.6) | 0.764 |
| FiO2, %, mean (SD) | 0.3 (0.5) | 0.3 (0.5) | 0.3 (0.5) | 0.094 |
| D-dimer, mean (SD) | 1197 (1293) | 1240 (1343) | 1162 (1270) | 0.835 |
| Blood gas analysis before SBT | ||||
| pHa, mean (SD) | 7.41 (0.04) | 7.40 (0.05) | 7.41 (0.04) | 0.560 |
| PaO2, mmHg, mean (SD) | 77.5 (9.3) | 76.5 (10.8) | 78.3 (8.1) | 0.309 |
| PaO2/FiO2, mmHg, mean (SD) | 248.4 (45.4) | 235.6 (45.1) | 258.5 (43.7) | 0.058 |
| PaCO2, mmHg, mean (SD) | 41.4 (6.3) | 42.0 (8.1) | 40.9 (4.5) | 0.850 |
| HCO3−, mmol/L, mean (SD) | 25.6 (3.1) | 25.7 (4.0) | 25.4 (2.1) | 0.850 |
| SaO2, %, mean (SD) | 94.9 (1.5) | 94.5 (1.7) | 95.3 (1.2) | 0.035 |
| Overall (n = 61) | Weaning Failure (n = 27) | Weaning Success (n = 34) | p-Value | |
|---|---|---|---|---|
| RSBI | 55.10 (24.93) | 69.53 (26.90) | 48.11 (20.97) | 0.013 |
| MIP | 42.10 (12.27) | 36.32 (12.09) | 45.42 (11.24) | 0.015 |
| PFE | 99.13 (27.32) | 103.64 (31.07) | 97.21 (25.85) | 0.500 |
| Right DTF | 0.21 (0.25) | 0.23 (0.31) | 0.18 (0.19) | 0.500 |
| Left DTF | 0.33 (0.36) | 0.30 (0.46) | 0.36 (0.26) | 0.061 |
| Right DE | 1.75 (0.77) | 1.73 (0.77) | 1.77 (0.78) | 0.900 |
| Left DE | 1.76 (0.72) | 1.80 (0.85) | 1.72 (0.60) | 0.700 |
| Right D-RSBI | 1.83 (1.58) | 2.27 (2.12) | 1.49 (0.83) | 0.300 |
| Left D-RSBI | 1.54 (0.88) | 1.62 (0.96) | 1.48 (0.83) | 0.800 |
| Right DT | 0.22 (0.07) | 0.20 (0.08) | 0.23 (0.06) | 0.017 |
| Left DT | 0.21 (0.08) | 0.19 (0.11) | 0.22 (0.05) | 0.005 |
| Inspiration thickness of the right diaphragm | 0.26 (0.08) | 0.24 (0.09) | 0.27 (0.08) | 0.160 |
| Inspiration thickness of the left diaphragm | 0.28 (0.17) | 0.26 (0.22) | 0.30 (0.1) | 0.006 |
| LUS before SBT | 15.05 (4.81) | 16.67 (4.74) | 13.76 (4.54) | 0.019 |
| LUS after SBT | 17.61 (4.86) | 19.50 (2.53) | 16.78 (5.41) | 0.046 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| RSBI | 1.04 | 1.01–1.07 | 0.010 |
| MIP | 0.92 | 0.86–0.99 | 0.018 |
| PFE | 1.01 | 0.99–1.03 | 0.458 |
| Right DTF | 2.37 | 0.29–19.18 | 0.418 |
| Left DTF | 0.58 | 0.12–2.73 | 0.494 |
| Right DE | 0.92 | 0.46–1.85 | 0.825 |
| Left DE | 1.18 | 0.48–2.89 | 0.720 |
| Right D-RSBI | 1.43 | 0.94–2.19 | 0.098 |
| Left D-RSBI | 1.20 | 0.57–2.55 | 0.631 |
| Right DT | 0.001 | <0.001–5.11 | 0.116 |
| Left DT | 0.003 | <0.001–6.14 | 0.136 |
| Inspiration thickness of the right diaphragm | 0.01 | <0.001–8.63 | 0.190 |
| Inspiration thickness of the left diaphragm | 0.15 | 0.004–6.29 | 0.318 |
| LUS before SBT | 1.16 | 1.02–1.31 | 0.025 |
| LUS after SBT | 1.15 | 0.98–1.35 | 0.096 |
| Variables | Mod. 1 p-Value | Mod. 2 p-Value | Mod. 3 p-Value | Mod. 4 p-Value | Mod. 5 p-Value | Mod. 6 p-Value | Mod. 7 p-Value | Mod. 8 p-Value | Mod. 9 p-Value | Mod. 10 p-Value | Mod. 11 p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Intercept) | 1.000 | 0.997 | 0.997 | 0.996 | 0.997 | 0.996 | 0.994 | 0.995 | 0.002 | 0.002 | 0.001 |
| RSBI (>57.50) | 1.000 | 0.701 | 0.701 | 0.597 | 0.876 | 0.945 | 0.509 | 0.569 | 0.190 | 0.174 | |
| MIP (>36.00) | 1.000 | 0.999 | 0.999 | 0.998 | |||||||
| PFE (>117.50) | 1.000 | 0.997 | 0.997 | 0.996 | 0.997 | 0.996 | 0.996 | 0.070 | 0.089 | 0.061 | 0.051 |
| Right DTF (>0.305) | 1.000 | 0.999 | 0.999 | ||||||||
| Left DTF (>0.194) | 1.000 | ||||||||||
| Right D-RSBI (>1.765) | 1.000 | 0.917 | 0.917 | 0.792 | 0.876 | 0.806 | 0.934 | 0.944 | 0.458 | ||
| Right DT (>0.195) | 1.000 | 0.997 | 0.997 | 0.996 | 0.997 | 0.996 | 0.996 | ||||
| Left DT (>0.195) | 1.000 | 0.997 | 0.997 | 0.997 | 0.997 | 0.997 | |||||
| inspiration thickness of the left diaphragm (>0.205) | 1.000 | 1.000 | |||||||||
| LUS before SBT (>15.50) | 1.000 | 0.998 | 0.998 | 0.998 | 0.998 | ||||||
| LUS after SBT (>15.50) | 1.000 | 0.997 | 0.997 | 0.997 | 0.997 | 0.996 | 0.995 | 0.995 | |||
| AIC | 24.00 | 32.99 | 30.99 | 29.49 | 28.48 | 27.52 | 30.06 | 30.39 | 32.26 | 30.80 | 30.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, C.; Novoa, C.; Aguayo, M.; Arriagada, R.; Alvarado, C.; Pedreros, C.; Kraunik, D.; Martins, C.M.; Rocco, P.R.M.; Battaglini, D. Predictive Value of Diaphragm and Lung Ultrasonography for Weaning Failure in Critically Ill Patients with Acute Respiratory Failure Due to COVID-19 Pneumonia. Diagnostics 2024, 14, 2263. https://doi.org/10.3390/diagnostics14202263
Fonseca C, Novoa C, Aguayo M, Arriagada R, Alvarado C, Pedreros C, Kraunik D, Martins CM, Rocco PRM, Battaglini D. Predictive Value of Diaphragm and Lung Ultrasonography for Weaning Failure in Critically Ill Patients with Acute Respiratory Failure Due to COVID-19 Pneumonia. Diagnostics. 2024; 14(20):2263. https://doi.org/10.3390/diagnostics14202263
Chicago/Turabian StyleFonseca, Camila, Claudio Novoa, Matias Aguayo, Ricardo Arriagada, Cristóbal Alvarado, César Pedreros, David Kraunik, Camila M. Martins, Patricia R. M. Rocco, and Denise Battaglini. 2024. "Predictive Value of Diaphragm and Lung Ultrasonography for Weaning Failure in Critically Ill Patients with Acute Respiratory Failure Due to COVID-19 Pneumonia" Diagnostics 14, no. 20: 2263. https://doi.org/10.3390/diagnostics14202263
APA StyleFonseca, C., Novoa, C., Aguayo, M., Arriagada, R., Alvarado, C., Pedreros, C., Kraunik, D., Martins, C. M., Rocco, P. R. M., & Battaglini, D. (2024). Predictive Value of Diaphragm and Lung Ultrasonography for Weaning Failure in Critically Ill Patients with Acute Respiratory Failure Due to COVID-19 Pneumonia. Diagnostics, 14(20), 2263. https://doi.org/10.3390/diagnostics14202263







