Preparticipation Cardiovascular Screening of Athletes: Current Controversies and Challenges for the Future
Abstract
1. Introduction
2. Sudden Cardiac Death in Athletes
2.1. Epidemiology of SCD
2.2. Causes of SCD in Athletes
2.3. Controversies and Challenges for the Future
3. Preparticipation Cardiac Screening of Young Athletes
3.1. Clinical Evaluation
3.2. The Role of Electrocardiogram
3.3. Inclusion of Echocardiogram
3.4. Controversies and Challenges for the Future
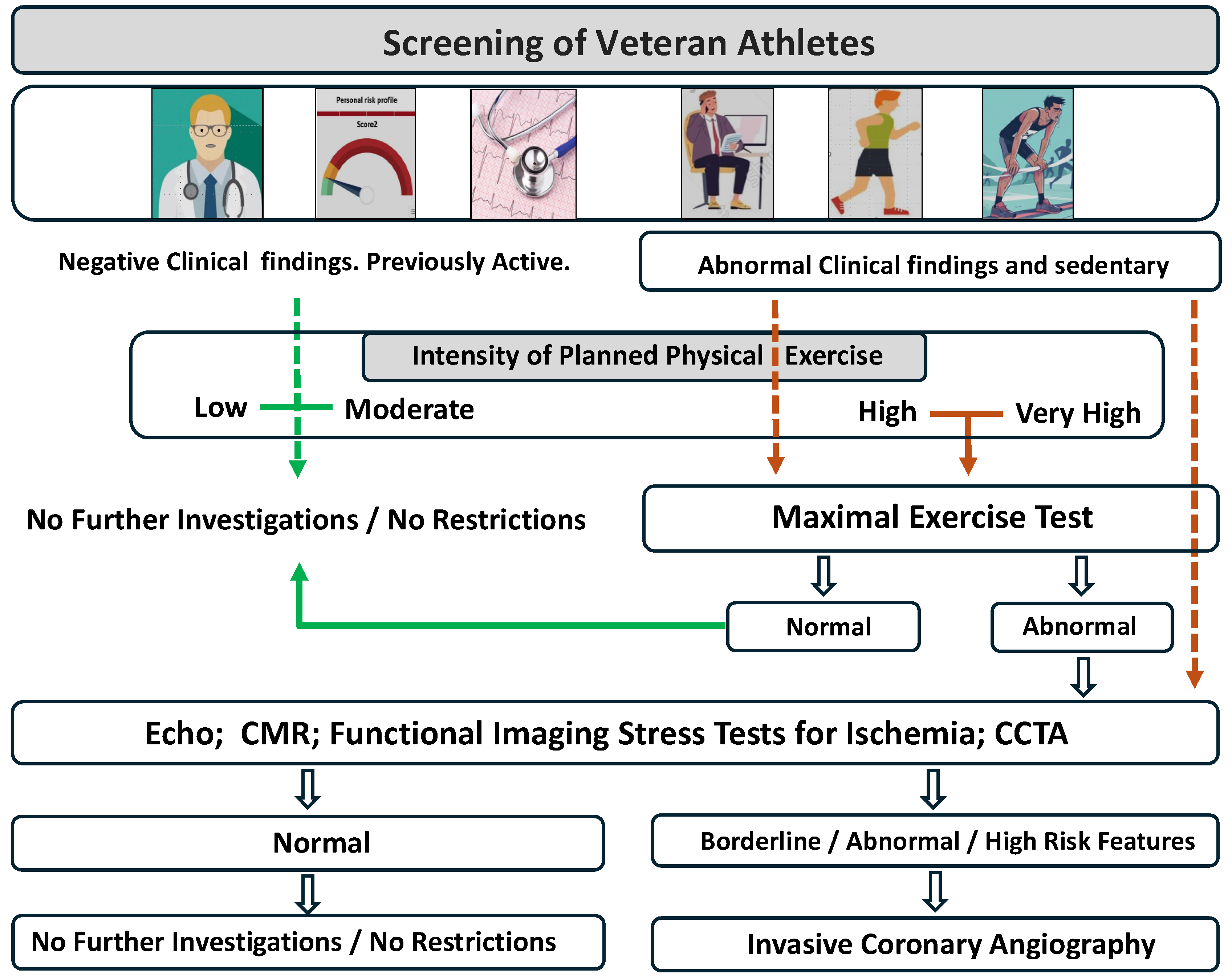
4. Screening of Veteran Athletes
4.1. Cardiovascular Risk Stratification of Veteran Athletes
4.2. The Role of Exercise Testing
4.3. Further Exams and Advanced Imaging Evaluation
4.4. Controversies and Challenges for the Future
5. Importance of Cardiopulmonary Resuscitation
5.1. Impact in Survival After Sudden Cardiac Arrest
5.2. Controversies and Challenges for the Future
6. The Role of Digital Technology
6.1. Where Are We?
6.2. Artificial Intelligence in Sports Cardiology
6.3. Controversies and Challenges for the Future
7. Conclusions
Funding
Conflicts of Interest
References
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M. SSedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Westaby, J.; Sheppard, M.N.; Papadakis, M.; Sharma, S. Sudden Cardiac Death in Young Athletes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Egger, F.; Scharhag, J.; Kästner, A.; Dvořák, J.; Bohm, P.; Meyer, T. FIFA Sudden Death Registry (FIFA-SDR): A prospective, observational study of sudden death in worldwide football from 2014 to 2018. Br. J. Sports Med. 2022, 56, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Maron, B.J.; Levine, B.D.; Washington, R.L.; Baggish, A.L.; Kovacs, R.J.; Maron, M.S. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 2, preparticipation screening for cardiovascular disease in competitive athletes. J. Am. Coll. Cardiol. 2015, 66, 2356–2361. [Google Scholar] [CrossRef]
- Drezner, J.A.; Rao, A.L.; Heistand, J.; Bloomingdale, M.K.; Harmon, K.G. Effectiveness of emergency response planning for sudden cardiac arrest in United States high schools with automated external defibrillators. Circulation 2009, 120, 518–525. [Google Scholar] [CrossRef]
- Palermi, S.; Vecchiato, M.; Saglietto, A.; Niederseer, D.; Oxborough, D.; Ortega-Martorell, S.; Olier, L.; Castelletti, S.; Baggish, A.; Maffessanti, F.; et al. Unlocking the potential of artificial intelligence in sports cardiology: Does it have a role in evaluating athlete’s heart? Eur. J. Prev. Cardiol. 2024, 31, 470–482. [Google Scholar] [CrossRef]
- Harmon, K.G.; Asif, I.M.; Maleszewski, J.J.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J.; et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic: Association athletes a decade in review. Circulation 2015, 132, 10–19. [Google Scholar] [CrossRef]
- Couper, K.; Putt, O.; Field, R.; Poole, K.; Bradlow, W.; Clarke, A.; Perkins, G.D.; Royle, P.; Yeung, J.; Taylor-Phillips, S. Incidence of sudden cardiac death in the young: A systematic review. BMJ Open 2020, 10, e040815. [Google Scholar] [CrossRef]
- MacLachlan, H.; Drezner, J.A. Cardiac evaluation of young athletes: Time for a risk-based approach? Clin. Cardiol. 2020, 43, 906–914. [Google Scholar] [CrossRef]
- Landry, C.H.; Allan, K.S.; Connelly, K.A.; Cunningham, K.; Morrison, L.J.; Dorian, P. Sudden Cardiac Arrest during Participation in Competitive Sports. N. Engl. J. Med. 2017, 377, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Rizzoli, G.; Schiavon, M.; Thiene, G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J. Am. Coll. Cardiol. 2003, 42, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.O.; Stovitz, S.D. Incidence of sudden cardiac death in Minnesota high school athletes 1993–2012 screened with a standardized preparticipation evaluation. J. Am. Coll. Cardiol. 2013, 62, 1298–1301. [Google Scholar] [CrossRef]
- Malhotra, A.; Dhutia, H.; Finocchiaro, G.; Gati, S.; Beasley, I.; Clift, P.; Cowie, C.; Kenny, A.; Mayet, J.; Oxborough, D.; et al. Outcomes of cardiac screening in adolescent soccer players. N. Engl. J. Med. 2018, 379, 524–534. [Google Scholar] [CrossRef]
- Risgaard, B.; Winkel, B.G.; Jabbari, R.; Behr, E.R.; Ingemann-Hansen, O.; Thomsen, J.L.; Ottesen, G.L.; Gislason, G.H.; Bundgaard, H.; Haunsø, S.; et al. Burden of sudden cardiac death in persons aged 1 to 49 years: Nationwide study in Denmark. Circ. Arrhythm. Electrophysiol. 2014, 7, 205–211. [Google Scholar] [CrossRef]
- Weizman, O.; Empana, J.-P.; Blom, M.; Tan, H.L.; Jonsson, M.; Narayanan, K.; Ringh, M.; Marijon, E.; Jouven, X. Incidence of cardiac arrest during sports among women in the European Union. J. Am. Coll. Cardiol. 2023, 81, 1021–1031. [Google Scholar] [CrossRef]
- Maron, B.J.; Haas, T.S.; Ahluwalia, A.; Murphy, C.J.; Garberich, R.F. Demographics and epidemiology of sudden deaths in young competitive athletes: From the United States National Registry. Am. J. Med. 2016, 129, 1170–1177. [Google Scholar] [CrossRef]
- Peterson, D.F.; Kucera, K.; Thomas, L.C.; Maleszewski, J.; Siebert, D.; Lopez-Anderson, M.; Zigman, M.; Schattenkerk, J.; Harmon, K.G.; A Drezner, J. Aetiology and incidence of sudden cardiac arrest and death in young competitive athletes in the USA: A 4-year prospective study. Br. J. Sports Med. 2021, 55, 1196–1203. [Google Scholar] [CrossRef]
- Sheppard, M.N.; Westaby, J.; Zullo, E.; E Fernandez, B.V.; Cox, S.; Cox, A. Sudden arrhythmic death and cardiomyopathy are important causes of sudden cardiac death in the UK: Results from a national coronial autopsy database. Histopathology 2023, 82, 1056–1066. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Radaelli, D.; D’errico, S.; Papadakis, M.; Behr, E.R.; Sharma, S.; Westaby, J.; Sheppard, M.N. Sudden cardiac death among adolescents in the United Kingdom. J. Am. Coll. Cardiol. 2023, 81, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Emery, M.S.; Kovacs, R.J. Sudden Cardiac Death in Athletes. JACC Heart Fail 2018, 6, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, P.; Pozza, R.D.; Ehringer-Schetitska, D.; Jokinen, E.; Herceg, V.; Hidvegi, E.; Oberhoffer, R.; Petropoulos, A. European Paediatric Cardiology Working Group Cardiovascular Prevention. Cardiovascular pre-participation screening in young athletes: Recommendations of the Association of European Paediatric Cardiology. Cardiol. Young. 2017, 27, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Pelliccia, A.; Bjornstad, H.H.; Vanhees, L.; Biffi, A.; Borjesson, M.; Panhuyzen-Goedkoop, N.; Deligiannis, A.; Solberg, E.; Dugmore, D.; et al. Cardiovascular Pre-Participation Screening of Young Competitive Athletes for Prevention of Sudden Death: Proposal for a Common European Protocol (Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology). Eur. Heart J. 2005, 26, 516–524. [Google Scholar]
- Drezner, J.A.; O’Connor, F.G.; Harmon, K.G.; Fields, K.B.; Asplund, C.A.; Asif, I.M.; Price, D.E.; Dimeff, R.J.; Bernhardt, D.T.; Roberts, W.O. Position Statement on Cardiovascular Preparticipation Screening in Athletes: Current Evidence, Knowledge Gaps, Recommendations, and Future Directions. Clin. J. Sport Med. 2016, 26, 347–361. [Google Scholar] [CrossRef]
- Raukar, N.; Arciero, E.; Noyes, A.; Drezner, J.; Weiss, J. Cardiovascular pre-participation screening in the young athlete: Addressing concerns. Phys. Sportsmed. 2017, 45, 365–369. [Google Scholar] [CrossRef][Green Version]
- Corrado, D.; Basso, C.; Pavei, A.; Michieli, P.; Schiavon, M.; Thiene, G. Trends in sudden cardiovascular death in young competitive athletes after implementation of preparticipation screening program. JAMA 2006, 296, 1593–1601. [Google Scholar] [CrossRef]
- Drezner, A.J.; Sharma, S.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Criteria for electrocardiographic interpretation in athletes: Consensus statement. Br. J. Sports Med. 2017, 51, 704–731. [Google Scholar] [CrossRef]
- Petek, B.J.; Drezner, J.A.; Churchill, T.W. The International Criteria for Electrocardiogram Interpretation in Athletes: Common Pitfalls and Future Directions. Cardiol. Clin. 2023, 41, 35–49. [Google Scholar] [CrossRef]
- Cheyenne, M.B.; Rachel, L. Optimizing pre-participation screening to prevent tragedy in young athletes: Moving from if to how. Eur. Heart J. 2023, 14, 1093–1095. [Google Scholar]
- Sarto, P.; Zorzi, A.; Merlo, L.; Vessella, T.; Pegoraro, C.; Giorgiano, F.; Graziano, F.; Basso, C.; A Drezner, J.; Corrado, D. Value of screening for the risk of sudden cardiac death in young competitive athletes. Eur. Heart J. 2023, 44, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Donati, F.; Guicciardi, C.; Lodi, E.; Fernando, F.; Palermi, S.; Modena, M.G.; Biffi, A. Echocardiography in the preparticipation screening: An old topic revisited. J. Cardiovasc. Med. 2023, 24, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kleiven, Ø.; Edvardsen, T.; Ørn, S. Echocardiography in the pre-participation evaluation of asymptomatic athletes: The never-ending story. Eur. J. Prev. Cardiol. 2021, 28, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Anselmi, F.; Mondillo, S.; Finocchiaro, G.; Caselli, S.; Garza, M.S.; Schmied, C.; Adami, P.E.; Galderisi, M.; Adler, Y. The use of cardiac imaging in the evaluation of athletes in the clinical practice: A survey by the Sports Cardiology and Exercise Section of the European Association of Preventive Cardiology and University of Siena, in collaboration with the European Association of Cardiovascular Imaging, the European Heart Rhythm Association and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Prev. Cardiol. 2021, 28, 1071–1077. [Google Scholar]
- Oxborough, D.; Augustine, D.; Gati, S.; George, K.; Harkness, A.; Mathew, T.; Papadakis, M.; Ring, L.; Robinson, S.; Sandoval, J.; et al. A guideline update for the practice of echocardiography in the cardiac screening of sports participants: A joint policy statement from the British Society of Echocardiography and Cardiac Risk in the Young. Echo Res. Pract. 2018, 5, G1–G10. [Google Scholar] [CrossRef]
- Churchill, T.W.; Baggish, A.L. Cardiovascular care of masters athletes. J. Cardiovasc. Transl. Res. 2020, 13, 313–321. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Mosterd, A.; Braber, T.L.; Prakken, N.H.; Doevendans, P.A.; Grobbee, D.E.; Thompson, P.D.; Eijsvogels, T.M.; Velthuis, B.K. The relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 2017, 136, 138–148. [Google Scholar] [CrossRef]
- Merghani, A.; Maestrini, V.; Rosmini, S.; Cox, A.T.; Dhutia, H.; Bastiaenan, R.; David, S.; Yeo, T.J.; Narain, R.; Malhotra, A.; et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef]
- Dores, H.; de Araújo Goncalves, P.; Monge, J.; Costa, R.; Tata, L.; Malhotra, A.; Sharma, S.; Cardim, N.; Neuparth, N. Subclinical coronary artery disease in veteran athletes: Is a new preparticipation methodology required? Br. J. Sports Med. 2018, 54, 349–353. [Google Scholar] [CrossRef]
- Farooq, M.; Brown, L.A.E.; Fitzpatrick, A.; Broadbent, D.A.; Wahab, A.; Klassen, J.R.L.; Farley, J.; Saunderson, C.E.D.; Das, A.; Craven, T.; et al. Identification of non-ischaemic fibrosis in male veteran endurance athletes, mechanisms and association with premature ventricular beats. Sci. Rep. 2023, 13, 14640. [Google Scholar] [CrossRef]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s recommendations for exercise preparticipation health screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Ermolao, A.; Gasperetti, A.; Rigon, A.; Patti, A.; Battista, F.; Frigo, A.C.; Duregon, F.; Zaccaria, M.; Bergamin, M.; Neunhaeuserer, D. Comparison of cardiovascular screening guidelines for middle-aged/older adults. Scand. J. Med. Sci. Sports 2019, 29, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, R.; Dausin, C.; Claus, P.; Bogaert, J.; Dymarkowski, S.; Goetschalckx, K.; Ghekiere, O.; Van De Heyning, C.M.; Van Herck, P.; Paelinck, B.; et al. Lifelong endurance exercise and its relation with coronary atherosclerosis. Eur. Heart J. 2023, 44, 2388–2399. [Google Scholar] [CrossRef]
- Morrison, B.N.; Isserow, S.; Taunton, J.; Oxborough, D.; Moulson, N.; Warburton, D.E.R.; McKinney, J. Masters athlete screening study (MASS): Incidence of cardiovascular disease and major adverse cardiac events and efficacy of screening over five years. Eur. J. Prev. Cardiol. 2023, 30, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Laily, I.; Wiggers, T.G.; van Steijn, N.; Bijsterveld, N.; Bakermans, A.J.; Froeling, M.; Berg-Faay, S.v.D.; de Haan, F.H.; de Bruin-Bon, R.H.; Boekholdt, S.M.; et al. Pre-participation screenings frequently miss occult cardiovascular conditions in apparently healthy male middle-aged first-time marathon runners. Cardiology 2024, 149, 255–263. [Google Scholar] [CrossRef]
- Semsarian, C.; Sweeting, J.; Ackerman, M.J. Sudden cardiac death in athletes. BMJ 2015, 350, h1218. [Google Scholar] [CrossRef]
- Robles, A.G.; Palamà, Z.; Nesti, M.; Tunzi, R.M.; Delise, P.; Cavarretta, E.; Penco, M.; Romano, S.; Sciarra, L. Sport Related Sudden Death: The Importance of Primary and Secondary Prevention. J. Clin. Med. 2022, 11, 4683. [Google Scholar] [CrossRef]
- Drezner, J.A.; O’Connor, F.G.; Harmon, K.G.; Fields, K.B.; Asplund, C.A.; Asif, I.M.; Price, D.E.; Dimeff, R.J.; Bernhardt, D.T.; Roberts, W.O. AMSSM Position Statement on Cardiovascular Preparticipation Screening in Athletes: Current evidence, knowledge gaps, recommendations and future directions. Br. J. Sports Med. 2017, 51, 153–167. [Google Scholar] [CrossRef]
- Casa, D.J.; Almquist, J.; Anderson, S.A.; Baker, L.; Bergeron, M.F.; Biagioli, B.; Boden, B.; Brenner, J.S.; Carroll, M.; Colgate, B.; et al. The Inter-Association Task Force for Preventing Sudden Death in Secondary School Athletics Programs: Best-Practices Recommendations. J. Athl. Train. 2013, 48, 546–553. [Google Scholar] [CrossRef]
- Corrado, D.; Cipriani, A.; Zorzi, A. Shocking insights on resuscitation after sports-related cardiac arrest. Eur. Heart J. 2023, 44, 193–195. [Google Scholar] [CrossRef]
- Besenius, E.; Cabri, J.; Delagardelle, C.; Stammet, P.; Urhausen, A. Five years-results of a nationwide database on sudden cardiac events in sports practice in luxembourg. Dtsch Z Sportmed. 2022, 73, 24–29. [Google Scholar] [CrossRef]
- Karam, N.; Pechmajou, L.; Narayanan, K.; Bougouin, W.; Sharifzadehgan, A.; Anys, S.; Weizman, O.; Perrot, D.; Waldmann, V.; Beganton, F.; et al. Evolution of Incidence, Management, and Outcomes Over Time in Sports-Related Sudden Cardiac Arrest. J. Am. Coll. Cardiol. 2022, 79, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Michelland, L.; Murad, M.H.; Bougouin, W.; Van Der Broek, M.; Prokop, L.J.; Anys, S.; Perier, M.-C.; Cariou, A.; Empana, J.P.; Marijon, E.; et al. Association between basic life support and survival in sports-related sudden cardiac arrest: A meta-analysis. Eur. Heart J. 2023, 44, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Chukumerije, M.; Truglio, T.S.; Dadekian, G.A.; Toft, L.E.B. The fallen athlete: Fellow athletes are not performing cardiopulmonary resuscitation when a teammate suffers sudden cardiac arrest. Am. J. Emerg. Med. 2023, 73, 209–211. [Google Scholar] [CrossRef]
- Gräsner, J.T.; Lefering, R.; Koster, R.W.; Masterson, S.; Böttiger, B.W.; Herlitz, J.; Wnent, J.; Tjelmeland, I.B.; Ortiz, F.R.; Maurer, H.; et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry. Resuscitation 2016, 105, 188–195. [Google Scholar] [CrossRef]
- Alexander, T.D.; McGovern, S.K.; Leary, M.; Abella, B.S.; Blewer, A.L. Association of state-level CPR training initiatives with layperson CPR knowledge in the United States. Resuscitation 2019, 140, 9–15. [Google Scholar] [CrossRef]
- Seneviratne, M.G.; Connolly, S.B.; Martin, S.S.; Parakh, K. Grains of Sand to Clinical Pearls: Realizing the Potential of Wearable Data. Am. J. Med. 2023, 136, 136–142. [Google Scholar] [CrossRef]
- D’andrea, A.; Sperlongano, S.; Russo, V.; D’ascenzi, F.; Benfari, G.; Renon, F.; Palermi, S.; Ilardi, F.; Giallauria, F.; Limongelli, G.; et al. The Role of Multimodality Imaging in Athlete’s Heart Diagnosis: Current Status and Future Directions. J. Clin. Med. 2021, 10, 5126. [Google Scholar] [CrossRef]
- Petek, B.J.; Al-Alusi, M.A.; Moulson, N.; Grant, A.J.; Besson, C.; Guseh, J.S.; Wasfy, M.M.; Gremeaux, V.; Churchill, T.W.; Baggish, A.L. Consumer Wearable Health and Fitness Technology in Cardiovascular Medicine. J. Am. Coll. Cardiol. 2023, 82, 245–264. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Bellfield, R.A.A.; Ortega-Martorell, S.; Lip, G.Y.H.; Oxborough, D.; Olier, I. The Athlete’s Heart and Machine Learning: A Review of Current Implementations and Gaps for Future Research. J. Cardiovasc. Dev. Dis. 2022, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Itchhaporia, D. Artificial intelligence in cardiology. Trends Cardiovasc. Med. 2022, 32, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Benaroia, M.; Elinson, R.; Zarnke, K. Patient-directed intelligent and interactive computer medical history-gathering systems: A utility and feasibility study in the emergency department. Int. J. Med. Inform. 2007, 76, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Chorba, J.S.; Shapiro, A.M.; Le, L.; Maidens, J.; Prince, J.; Pham, S.; Kanzawa, M.M.; Barbosa, D.N.; Currie, C.; Brooks, C.; et al. Deep Learning Algorithm for Automated Cardiac Murmur Detection via a Digital Stethoscope Platform. J. Am. Heart Assoc. 2021, 10, e019905. [Google Scholar] [CrossRef]
- Smaranda, A.M.; Drăgoiu, T.S.; Caramoci, A.; Afetelor, A.A.; Ionescu, A.M.; Bădărău, I.A. Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review. Sports 2024, 12, 144. [Google Scholar] [CrossRef]
- Fiorina, L.; Maupain, C.; Gardella, C.; Manenti, V.; Salerno, F.; Socie, P.; Li, J.; Henry, C.; Plesse, A.; Narayanan, K.; et al. Evaluation of an Ambulatory ECG Analysis Platform Using Deep Neural Networks in Routine Clinical Practice. J. Am. Heart Assoc. 2022, 11, e026196. [Google Scholar] [CrossRef]
- Alsharqi, M.; Woodward, W.J.; Mumith, J.A.; Markham, D.C.; Upton, R.; Leeson, P. Artificial intelligence and echocardiography. Echo Res. Pract. 2018, 5, R115–R125. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Choi, E.; Kaplin, S.; Venner, E.; Murugan, M.; Wang, Z.; Glicksberg, B.S.; Amos, C.I.; Schatz, M.C.; et al. Artificial Intelligence and Cardiovascular Genetics. Life 2022, 12, 279. [Google Scholar] [CrossRef]

| ATHLETE’S SPECIFIC CHARACTERISTICS | |
|---|---|
| Geographic origin and race | Black athlete repolarization pattern (J-point elevation and TWI in V1–V4) is considered a normal finding; however, it shows heterogeneous patterns of benignity in different geographic regions or African population. |
| Age and gender | More research and normative ECG data on age and gender is needed. Some ECG parameters are dynamic and change with age (QT interval, QRS voltage). TWI in V1–V3 is more prevalent in female athletes (benign pattern?). |
| SPORTS SPECIFIC CHARACTERISTICS | |
| Type of sport/Modality | Cardiac remodeling, both structural and electrical, is dependent on the type of sports. Endurance athletes have predominantly eccentric remodeling and strength athletes a concentric type. |
| Intensity | Elite athletes show more marked physiological cardiac adaptations. Future research should be focus on normative ECG data according to exercise-related characteristics. |
| SPECIFIC ECG FINDINGS | |
| Low QRS voltage | Causes of SCD such as arrhythmogenic cardiomyopathy, myocarditis, infiltrative cardiac diseases and nonischemic left ventricle scar may demonstrate low QRS voltage. In athletes this is infrequent. |
| QRS fragmentation | In lead V1 it may represent a sign of right ventricle remodeling. More research is needed to understand its clinical value. |
| Premature Ventricular Contractions | Beyond the PVC burden, the morphology and relationship with exercise matters. PVC from concerning origins (e.g., Left bundle branch block with superior or intermediate axis) warrant an indepth investigation. |
| ST-segment depression morphology | Upsloping ST-segment depression can have a different value (benign), than a horizontal or downsloping ST-depression morphology. Research is needed. |
| Key Measure | Description |
|---|---|
| Improved Screening Strategies | Enhance CV PPS to better identify at-risk athletes. |
| Emergency Action Plans | Implement well-rehearsed EAPs in sports venues, ensuring fast and efficient response. |
| Bystander Training | Provide widespread CPR and AED training to coaches, medical staff, and spectators to ensure rapid response. |
| Athlete CPR Training | Encourage CPR training for athletes to improve immediate intervention. |
| Mandatory AED Availability | Enforce the presence of AEDs in both competitive and recreational sports settings. |
| Legislative and Tax Incentives | Promote AED accessibility laws and provide tax incentives for deployment and training. |
| Education in Schools | Incorporate basic life support and AED training into school curricula to increase awareness. |
| History and Physical Examination | Anamnestic Data Collection: AI-powered chatbots facilitate the collection of medical history by streamlining the process and identifying critical details that might otherwise be missed during conventional clinical visits [63]. |
| AI-Enhanced Stethoscopes: Digital stethoscopes equipped with heart murmur interpretation algorithms improve the detection of structural heart murmurs in athletes [64]. | |
| Electrocardiography | ECG Interpretation: AI models provide enhanced precision in interpreting ECG results, offering improved detection of CV conditions linked to SCD and the ability to predict clinical outcomes [65]. |
| Exercise Testing: Advanced AI algorithms enhance the diagnostic accuracy of exercise stress tests and continuous ECG monitoring, particularly in identifying coronary artery disease and heart rhythm disorders [66]. | |
| Imaging | Echocardiography: AI-based tools analyse echocardiographic patterns, aiding clinicians in differentiating physiological athletic heart adaptations from pathological conditions [67]. |
| Advanced Imaging: AI-driven analysis in cardiac magnetic resonance imaging, coronary computed tomography, and nuclear cardiology assists in diagnosing conditions within the “grey zone” of athlete’s heart [7]. | |
| Genetic Testing | Genomic Data Analysis: AI models applied to large genomic datasets help identify genetic markers and disease subtypes, enabling more accurate risk stratification and supporting personalized treatment strategies [68]. |
| Wearable Technology | Continuous Monitoring: AI integration with wearable devices extends their capabilities, enabling continuous cardiac monitoring and enhanced risk assessment in athletes [60]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dores, H.; Dinis, P.; Viegas, J.M.; Freitas, A. Preparticipation Cardiovascular Screening of Athletes: Current Controversies and Challenges for the Future. Diagnostics 2024, 14, 2445. https://doi.org/10.3390/diagnostics14212445
Dores H, Dinis P, Viegas JM, Freitas A. Preparticipation Cardiovascular Screening of Athletes: Current Controversies and Challenges for the Future. Diagnostics. 2024; 14(21):2445. https://doi.org/10.3390/diagnostics14212445
Chicago/Turabian StyleDores, Hélder, Paulo Dinis, José Miguel Viegas, and António Freitas. 2024. "Preparticipation Cardiovascular Screening of Athletes: Current Controversies and Challenges for the Future" Diagnostics 14, no. 21: 2445. https://doi.org/10.3390/diagnostics14212445
APA StyleDores, H., Dinis, P., Viegas, J. M., & Freitas, A. (2024). Preparticipation Cardiovascular Screening of Athletes: Current Controversies and Challenges for the Future. Diagnostics, 14(21), 2445. https://doi.org/10.3390/diagnostics14212445





