Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes
Abstract
1. Introduction
2. Type 2 Diabetes and Obesity-Induced Inflammation
2.1. Inflammation and Impaired Insulin Signaling
2.2. Adipose Tissue Inflammation and Immune Cells
3. Myeloid-Derived Suppressor Cells (MDSCs) and Inflammation
3.1. MDSC Origin and Phenotypes
3.2. The Role of MDSCs in Immune Regulation
4. MDSCs and Adipose Tissue Inflammation in T2D
4.1. Recruitment of MDSCs in Adipose Tissue in T2D
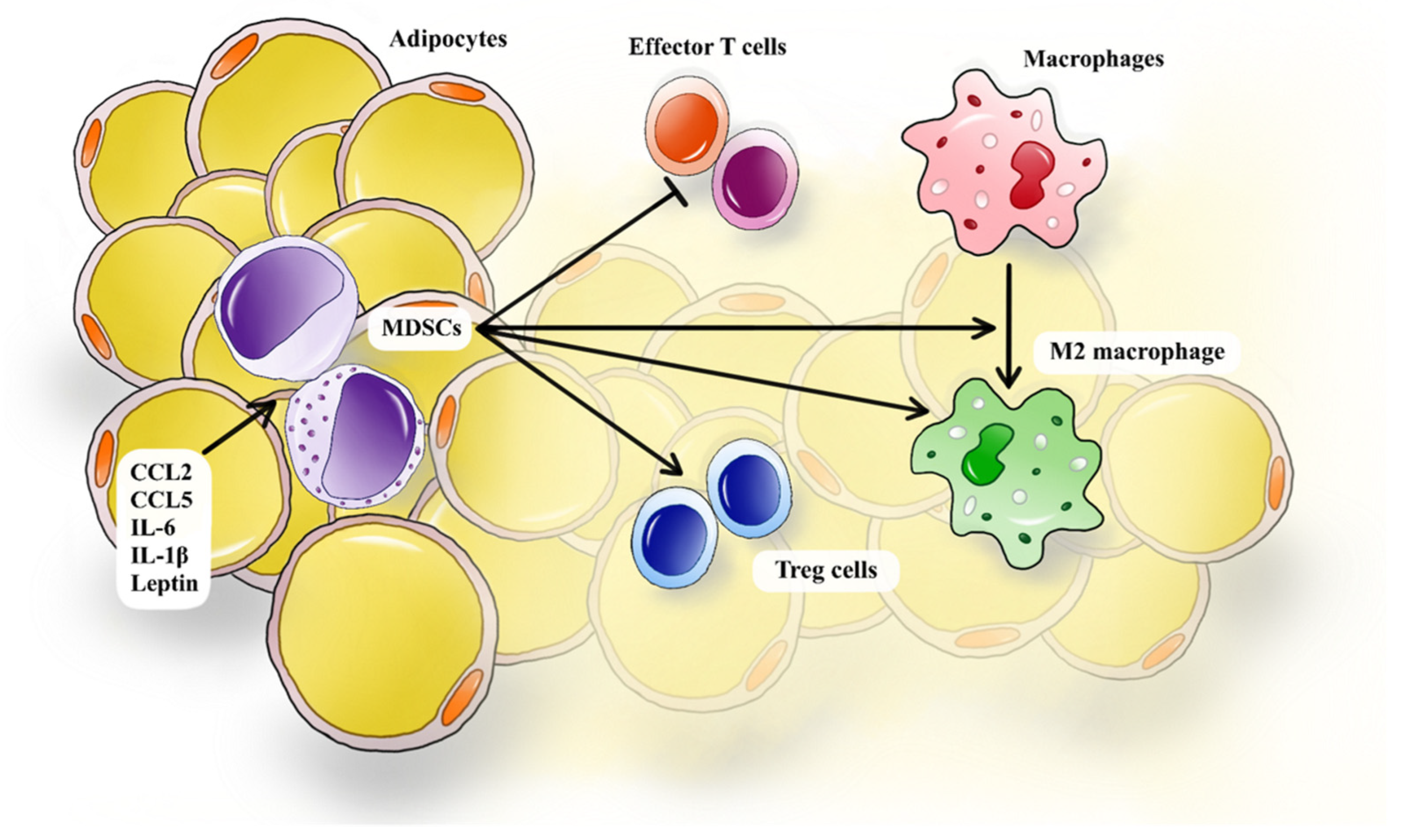
4.2. The Interplay Between MDSCs and Immune Cells in T2D
4.2.1. MDSCs and Macrophages
4.2.2. MDSCs and Effector T Cells
4.2.3. MDSCs and Tregs
5. MDSCs and Diabetic Complications
6. MDSCs and Anti-Diabetic Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Cho, Y.; Park, H.-S.; Huh, B.W.; Seo, S.H.; Seo, D.H.; Ahn, S.H.; Hong, S.; Suh, Y.J.; Kim, S.H. Prevalence and risk of diabetic complications in young-onset versus late-onset type 2 diabetes mellitus. Diabetes Metab. 2022, 48, 101389. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.G.; Sousa, L.P.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019, 13, 1165–1172. [Google Scholar] [CrossRef]
- Li, J.; Niu, Q.; Wu, A.; Zhang, Y.; Hong, L.; Wang, H. Causal relationship between circulating immune cells and the risk of type 2 diabetes: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1210415. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- O’hearn, M.; Lara-Castor, L.; Cudhea, F.; Miller, V.; Reedy, J.; Shi, P.; Zhang, J.; Wong, J.B.; Economos, C.D.; Micha, R.; et al. Incident type 2 diabetes attributable to suboptimal diet in 184 countries. Nat. Med. 2023, 29, 982–995. [Google Scholar] [CrossRef]
- Suárez-Torres, I.; García-García, F.; Morales-Romero, J.; Melgarejo-Gutiérrez, M.; Demeneghi-Marini, V.P.; Luna-Ceballos, R.I.; Hernández-Trejo, C.; Carmona-Cortés, D.A. Poor quality of sleep in Mexican patients with type 2 diabetes and its association with lack of glycemic control. Prim. Care Diabetes 2023, 17, 155–160. [Google Scholar] [CrossRef]
- Maftei, G.-A.; Martu, M.-A.; Martu, M.-C.; Popescu, D.; Surlin, P.; Tatarciuc, D.; Popa, C.; Foia, L.-G. Correlations between Salivary Immuno-Biochemical Markers and HbA1c in Type 2 Diabetes Subjects before and after Dental Extraction. Antioxidants 2021, 10, 1741. [Google Scholar] [CrossRef]
- Pasarin, L.; Martu, M.-A.; Ciurcanu, O.E.; Luca, E.O.; Salceanu, M.; Anton, D.; Martu, C.; Martu, S.; Esanu, I.M. Influence of Diabetes Mellitus and Smoking on Pro- and Anti-Inflammatory Cytokine Profiles in Gingival Crevicular Fluid. Diagnostics 2023, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Sufaru, I.-G.; Martu, M.-A.; Luchian, I.; Stoleriu, S.; Diaconu-Popa, D.; Martu, C.; Teslaru, S.; Pasarin, L.; Solomon, S.M. The Effects of 810 nm Diode Laser and Indocyanine Green on Periodontal Parameters and HbA1c in Patients with Periodontitis and Type II Diabetes Mellitus: A Randomized Controlled Study. Diagnostics 2022, 12, 1614. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Xia, S.; Sha, H.; Yang, L.; Ji, Y.; Ostrand-Rosenberg, S.; Qi, L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem. 2011, 286, 23591–23599. [Google Scholar] [CrossRef]
- Miracle, C.E.; McCallister, C.L.; Egleton, R.D.; Salisbury, T.B. Mechanisms by which obesity regulates inflammation and anti-tumor immunity in cancer. Biochem. Biophys. Res. Commun. 2024, 733, 150437. [Google Scholar] [CrossRef]
- Păunică, I.; Giurgiu, M.; Dumitriu, A.S.; Păunică, S.; Pantea Stoian, A.M.; Martu, M.-A.; Serafinceanu, C. The Bidirectional Relationship between Periodontal Disease and Diabetes Mellitus—A Review. Diagnostics 2023, 13, 681. [Google Scholar] [CrossRef]
- Martu, M.A.; Maftei, G.A.; Luchian, I.; Popa, C.; Filioreanu, A.M.; Tatarciuc, D.; Nichitean, G.; Hurjui, L.L.; Foia, L.G. Wound healing of periodontal and oral tissues: Part II—Patho-phisiological conditions and metabolic diseases. Rom. J. Oral Rehabil. 2020, 12, 30–40. [Google Scholar]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of adipose tissue inflammation to the development of type 2 diabetes HHS Public Access. Physiol. Behav. 2019, 9, 1–58. [Google Scholar]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.M.; Guarino, M.P.; Barroso, S.; Gil, M.M. Phytopharmacological Strategies in the Management of Type 2 Diabetes Mellitus. Foods 2020, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Heendeniya, S.N.; Keerthirathna, L.R.; Manawadu, C.K.; Dissanayake, I.H.; Ali, R.; Mashhour, A.; Alzahrani, H.; Godakumbura, P.; Boudjelal, M.; Peiris, D.C.; et al. Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites. Biomolecules 2020, 10, 165. [Google Scholar] [CrossRef]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef]
- Shih, Y.L.; Huang, T.C.; Shih, C.C.; Chen, J.Y. Relationship between Leptin and Insulin Resistance among Community—Dwelling Middle-Aged and Elderly Populations in Taiwan. J. Clin. Med. 2022, 11, 5357. [Google Scholar] [CrossRef]
- Dagogo-Jack, S. Leptin and Insulin Sensitivity: Endogenous Signals of Metabolic Homeostasis. J. Clin. Endocrinol. Metab. 2024, 109, e1402–e1403. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef]
- Yeo, G.S.; Chao, D.H.M.; Siegert, A.-M.; Koerperich, Z.M.; Ericson, M.D.; Simonds, S.E.; Larson, C.M.; Luquet, S.; Clarke, I.; Sharma, S.; et al. The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol. Metab. 2021, 48, 101206. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]
- Cojocaru, K.-A.; Luchian, I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.; Zhao, Y.; Wang, J.; Liu, B.; Yu, S.; Fu, J.; Liu, Y.; Su, J. Macrophage Polarization Mediated by Mitochondrial Dysfunction Induces Adipose Tissue Inflammation in Obesity. Int. J. Mol. Sci. 2022, 23, 9252. [Google Scholar] [CrossRef]
- Senkus, K.E.; Crowe-White, K.M.; Bolland, A.C.; Locher, J.L.; Ard, J.D. Changes in adiponectin:leptin ratio among older adults with obesity following a 12-month exercise and diet intervention. Nutr. Diabetes 2022, 12, 30. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, obesity, and cancer: Clash of the bigwigs in health and disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, X.; You, L.; Li, F.; Lian, H.; Wang, J.; Mao, N.; Ren, M.; Li, Y.; Wang, C.; et al. Association between adiponectin and newly diagnosed type 2 diabetes in population with the clustering of obesity, dyslipidaemia and hypertension: A cross-sectional study. BMJ Open 2023, 13, e060377. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Chiu, C.-J.; Hsing, C.-H.; Hsu, Y.-H. Interferon Family Cytokines in Obesity and Insulin Sensitivity. Cells 2022, 11, 4041. [Google Scholar] [CrossRef]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Crunkhorn, S. Metabolic disorders: Breaking the links between inflammation and diabetes. Nat. Rev. Drug. Discov. 2013, 12, 261. [Google Scholar] [CrossRef]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of Obesity-Induced Inflammation and Insulin Resistance: Insights into the Emerging Role of Nutritional Strategies. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Singh, B.P.; Sandhu, N.; Singh, N.; Kaur, R.; Rokana, N.; Singh, K.S.; Chaudhary, V.; Panwar, H. Probiotic mediated NF-κB regulation for prospective management of type 2 diabetes. Mol. Biol. Rep. 2020, 47, 2301–2313. [Google Scholar] [CrossRef]
- Sepehri, Z.; Kiani, Z.; Afshari, M.; Kohan, F.; Dalvand, A.; Ghavami, S. Inflammasomes and type 2 diabetes: An updated systematic review. Immunol. Lett. 2017, 192, 97–103. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Huang, F.; Liu, Y.; Liang, Y.; Wei, Z.; Ke, H.; Zeng, Z.; Huang, W.; He, Y. NLRP3 Inflammasome as a Molecular Marker in Diabetic Cardiomyopathy. Front. Physiol. 2017, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Jin, C.; Chen, Y.; Flavell, R.A. Inflammasome activation and metabolic disease progression. Cytokine Growth Factor Rev. 2014, 25, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Satheesan, A.; Kumar, J.; Leela, K.V.; Murugesan, R.; Chaithanya, V.; Angelin, M. Review on the role of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome pathway in diabetes: Mechanistic insights and therapeutic implications. Inflammopharmacology 2024, 32, 2753–2779. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hee, S.-W.; Chuang, L.-M. T helper 17 cells: A new actor on the stage of type 2 diabetes and aging? J. Diabetes Investig. 2021, 12, 909–913. [Google Scholar] [CrossRef]
- Greco, M.; Mirabelli, M.; Tocci, V.; Mamula, Y.; Salatino, A.; Brunetti, F.S.; Dragone, F.; Sicilia, L.; Tripolino, O.; Chiefari, E.; et al. Prothymosin-Alpha, a Novel and Sensitive Biomarker of the Inflammatory and Insulin-Resistant Statuses of Obese Individuals: A Pilot Study Involving Humans. Endocrines 2023, 4, 427–436. [Google Scholar] [CrossRef]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, inflammation, and prostate cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef]
- Chan, P.-C.; Lu, C.-H.; Chien, H.-C.; Tian, Y.-F.; Hsieh, P.-S. Adipose Tissue-Derived CCL5 Enhances Local Pro-Inflammatory Monocytic MDSCs Accumulation and Inflammation via CCR5 Receptor in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 14226. [Google Scholar] [CrossRef]
- Bergenfelz, C.; Leandersson, K. The Generation and Identity of Human Myeloid-Derived Suppressor Cells. Front. Oncol. 2020, 10, 109. [Google Scholar] [CrossRef]
- Shi, Z.-Y.; Yang, C.; Lu, L.-Y.; Lin, C.-X.; Liang, S.; Li, G.; Zhou, H.-M.; Zheng, J.-M. Inhibition of hexokinase 2 with 3-BrPA promotes MDSCs differentiation and immunosuppressive function. Cell Immunol. 2023, 385, 104688. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.H.; Emmons, T.R.; Wong, J.T.; Alqassim, E.; Singel, K.L.; Mark, J.; Smith, B.E.; Tario, J.D.; Eng, K.H.; Moysich, K.B.; et al. Quantification of Early-Stage Myeloid-Derived Suppressor Cells in Cancer Requires Excluding Basophils. Cancer Immunol. Res. 2020, 8, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Varela, V.A.; Heinen, L.B.d.S.; Marti, L.C.; Caraciolo, V.B.; Datoguia, T.S.; Amano, M.T.; Pereira, W.O. In vitro differentiation of myeloid suppressor cells (MDSC-like) from an immature myelomonocytic precursor THP-1. J. Immunol. Methods 2023, 515, 113441. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Vanhaver, C.; van der Bruggen, P.; Bruger, A.M. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872. [Google Scholar] [CrossRef]
- Bline, K.; Andrews, A.; Moore-Clingenpeel, M.; Mertz, S.; Ye, F.; Best, V.; Sayegh, R.; Tomatis-Souverbielle, C.; Quintero, A.M.; Maynard, Z.; et al. Myeloid-Derived Suppressor Cells and Clinical Outcomes in Children with COVID-19. Front. Pediatr. 2022, 10, 893045. [Google Scholar] [CrossRef]
- Grohová, A.; Dáňová, K.; Adkins, I.; Šumník, Z.; Petruželková, L.; Obermannová, B.; Koloušková, S.; Špíšek, R.; Palová-Jelínková, L. Myeloid—Derived suppressor cells in Type 1 diabetes are an expanded population exhibiting diverse T-cell suppressor mechanisms. PLoS ONE 2020, 15, e0242092. [Google Scholar] [CrossRef]
- Tellez, R.S.L.; Reynolds, L.; Piris, M.A. Myeloid-derived suppressor cells (MDSCs): What do we currently know about the effect they have against anti-PD-1/PD-L1 therapies? Ecancermedicalscience 2023, 17, 1556. [Google Scholar]
- Cassetta, L.; Bruderek, K.; Skrzeczynska-Moncznik, J.; Osiecka, O.; Hu, X.; Rundgren, I.M.; Lin, A.; Santegoets, K.; Horzum, U.; Godinho-Santos, A.; et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J. Immunother. Cancer 2020, 8, e001223. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Wang, J.C.; Sun, L. PD-1/PD-L1, MDSC Pathways, and Checkpoint Inhibitor Therapy in Ph(-) Myeloproliferative Neoplasm: A Review. Int. J. Mol. Sci. 2022, 23, 5837. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Sommer, M.; Strobel, S.; Thrum, S.; Blüher, M.; Wagner, U.; Rossol, M. Perturbation of the Monocyte Compartment in Human Obesity. Front. Immunol. 2019, 10, 1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, Q.; Hou, Y.; Dou, H. Emerging Roles of Myeloid-Derived Suppressor Cells in Diabetes. Front. Pharmacol. 2021, 12, 798320. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mukherjee, M.B.; Lin, J. Coordinated Regulation of Myeloid-Derived Suppressor Cells by Cytokines and Chemokines. Cancers 2022, 14, 1236. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Singh, K.; Agrawal, N.K.; Gupta, S.K.; Mohan, G.; Chaturvedi, S.; Singh, K. Increased expression of endosomal members of toll-like receptor family abrogates wound healing in patients with type 2 diabetes mellitus. Int. Wound J. 2016, 13, 927–935. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.C.; Ávila, J.C.G.-D.; Martínez-Fierro, M.L.; Garza-Veloz, I.; Cervantes-Villagrana, A.R.; Valtierra-Alvarado, M.A.; Serrano, C.J.; García-Hernández, M.H.; Enciso-Moreno, J.A.; Castañeda-Delgado, J.E. Myeloid-Derived Suppressor Cells Show Different Frequencies in Diabetics and Subjects with Arterial Hypertension. J. Diabetes Res. 2019, 2019, 1568457. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Liu, A. Chemokines in Prediabetes and Type 2 Diabetes: A Meta-Analysis. Front. Immunol. 2021, 12, 622438. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; Gutiérrez-García, C.; Sánchez-Jiménez, F.; Vilariño-García, T.; Flores-Campos, R.; Pérez-Pérez, A.; Garnacho, C.; Sánchez-León, M.L.; García-Domínguez, D.J.; Hontecillas-Prieto, L.; et al. Impact of obesity associated myeloid derived suppressor cells on cancer risk and progression (Review). Int. J. Oncol. 2024, 65, 79. [Google Scholar] [CrossRef]
- Turbitt, W.J.; Collins, S.D.; Meng, H.; Rogers, C.J. Increased Adiposity Enhances the Accumulation of MDSCs in the Tumor Microenvironment and Adipose Tissue of Pancreatic Tumor-Bearing Mice and in Immune Organs of Tumor-Free Hosts. Nutrients 2019, 11, 3012. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, B.; Kang, J.; Li, A.; Sun, J. Obesity Promotes Tumor Immune Evasion in Ovarian Cancer Through Increased Production of Myeloid-Derived Suppressor Cells via IL-6. Cancer Manag. Res. 2021, 13, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Ribeiro, V.S.; Dinarello, C.A.; Ostrand-Rosenberg, S.; Di Santo, J.P.; Apte, R.N.; Vosshenrich, C.A. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 2010, 40, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The role of interleukin-1β in type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef] [PubMed]
- Clements, V.K.; Long, T.; Long, R.; Figley, C.; Smith, D.M.C.; Ostrand-Rosenberg, S. Frontline Science: High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J. Leukoc. Biol. 2018, 103, 395–407. [Google Scholar] [CrossRef]
- Fujisaka, S. The role of adipose tissue M1/M2 macrophages in type 2 diabetes mellitus. Diabetol. Int. 2021, 12, 74–79. [Google Scholar] [CrossRef]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef]
- Orliaguet, L.; Dalmas, E.; Drareni, K.; Venteclef, N.; Alzaid, F. Mechanisms of Macrophage Polarization in Insulin Signaling and Sensitivity. Front. Endocrinol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Jahan, H.; Choudhary, M.I. Gliclazide alters macrophages polarization state in diabetic atherosclerosis in vitro via blocking AGE-RAGE/TLR4-reactive oxygen species-activated NF-kβ nexus. Eur. J. Pharmacol. 2021, 894, 173874. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, Y.; He, B. New Insights into Adipose Tissue Macrophages in Obesity and Insulin Resistance. Cells 2022, 11, 1424. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Sheng, H.; Liang, C.; Liu, H.; Guerrero, J.A.M.; Lu, Z.; Mao, W.; Dai, Z.; Liu, X.; et al. Hyperoside Suppresses Renal Inflammation by Regulating Macrophage Polarization in Mice with Type 2 Diabetes Mellitus. Front. Immunol. 2021, 12, 733808. [Google Scholar] [CrossRef]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Stivers, K.B.; Chilton, P.M.; E Beare, J.; Dale, J.R.; Alard, P.; LeBlanc, A.J.; Hoying, J.B. Adipose-resident myeloid-derived suppressor cells modulate immune cell homeostasis in healthy mice. Immunol. Cell Biol. 2020, 98, 650–666. [Google Scholar] [CrossRef] [PubMed]
- Okwan-Duodu, D.; Umpierrez, G.E.; Brawley, O.W.; Diaz, R. Obesity-driven inflammation and cancer risk: Role of myeloid derived suppressor cells and alternately activated macrophages. Am. J. Cancer Res. 2013, 3, 21–33. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23359288%0A (accessed on 8 October 2024). [PubMed]
- Frkic, R.L.; Richter, K.; Bruning, J.B. The therapeutic potential of inhibiting PPARγ phosphorylation to treat type 2 diabetes. J. Biol. Chem. 2021, 297, 101030. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Kammoun, H.L.; Kraakman, M.J.; Febbraio, M.A. Adipose tissue inflammation in glucose metabolism. Rev. Endocr. Metab. Disord. 2014, 15, 31–44. [Google Scholar] [CrossRef]
- Xia, C.; Rao, X.; Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J. Diabetes Res. 2017, 2017, 6494795. [Google Scholar] [CrossRef]
- Shirakawa, K.; Yan, X.; Shinmura, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yamamoto, T.; Anzai, A.; Isobe, S.; Yoshida, N.; et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Investig. 2016, 126, 4626–4639. [Google Scholar] [CrossRef]
- Mahlangu, T.; Dludla, P.V.; Nyambuya, T.M.; Mxinwa, V.; Mazibuko-Mbeje, S.E.; Cirilli, I.; Marcheggiani, F.; Tiano, L.; Louw, J.; Nkambule, B.B. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine 2020, 126, 154892. [Google Scholar] [CrossRef]
- Wang, T.; Wen, Y.; Fan, X. Myeloid-derived suppressor cells suppress CD4+ T cell activity and prevent the development of type 2 diabetes. Acta Biochim. Biophys. Sin. 2018, 50, 362–369. [Google Scholar] [CrossRef]
- Van Herck, M.A.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; De Winter, B.Y.; Francque, S.M.; Vonghia, L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front. Immunol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Negura, I.; Pavel-Tanasa, M.; Danciu, M. Regulatory T cells in gastric cancer: Key controllers from pathogenesis to therapy. Cancer Treat. Rev. 2023, 120, 102629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Song, H.; Zhang, L.; Meng, H. Characterization of IL-17-producing Treg cells in type 2 diabetes patients. Immunol. Res. 2019, 67, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Eller, K.; Kirsch, A.; Wolf, A.M.; Sopper, S.; Tagwerker, A.; Stanzl, U.; Wolf, D.; Patsch, W.; Rosenkranz, A.R.; Eller, P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes 2011, 60, 2954–2962. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
- Bao, Y.; Mo, J.; Ruan, L.; Li, G. Increased monocytic CD14+HLADRlow/- myeloid-derived suppressor cells in obesity. Mol. Med. Rep. 2015, 11, 2322–2328. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Myeloid derived-suppressor cells: Their role in cancer and obesity. Curr. Opin. Immunol. 2018, 51, 68–75. [Google Scholar] [CrossRef]
- Sun, P.; Jin, Q.; Nie, S.; Jia, S.; Li, Y.; Li, X.; Guo, F. Unlike PD-L1, PD-1 Is Downregulated on Partial Immune Cells in Type 2 Diabetes. J. Diabetes Res. 2019, 2019, 5035261. [Google Scholar] [CrossRef]
- Islam, J.; Lee, H.J.; Yang, S.H.; Kim, D.K.; Joo, K.W.; Kim, Y.S.; Seo, S.-U.; Seong, S.-Y.; Lee, D.-S.; Youn, J.-I.; et al. Decreased programmed cell death ligand 2-positive monocytic myeloid-derived suppressor cells and programmed cell death protein 1-positive T-regulatory cells in patients with type 2 diabetes: Implications for immunopathogenesis. Endocr. Connect. 2023, 12, e230218. [Google Scholar]
- Hsieh, C.-C.; Chang, C.-C.; Hsu, Y.-C.; Lin, C.-L. Immune Modulation by Myeloid-Derived Suppressor Cells in Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 13263. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Lee, H.J.; Yang, S.H.; Kim, D.K.; Joo, K.W.; Kim, Y.S.; Seo, S.-U.; Seong, S.-Y.; Lee, D.-S.; Youn, J.-I.; et al. Expansion of Myeloid-Derived Suppressor Cells Correlates with Renal Progression in Type 2 Diabetic Nephropathy. Immune Netw. 2020, 20, e18. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Lin, C.-L.; He, J.-T.; Chiang, M.; Wang, Y.; Tsai, Y.-C.; Hung, C.-H.; Chang, P.-J. Administration of cytokine-induced myeloid-derived suppressor cells ameliorates renal fibrosis in diabetic mice. Stem Cell Res. Ther. 2018, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Lin, C.-L.; Chiang, M.; He, J.-T.; Hung, C.-H.; Hsieh, C.-C. Cytokine-Induced Myeloid-Derived Suppressor Cells Demonstrate Their Immunoregulatory Functions to Prolong the Survival of Diabetic Mice. Cells 2023, 12, 1507. [Google Scholar] [CrossRef]
- Qin, L.; Li, Q.; Wang, L.; Huang, Y. Mass cytometry reveals the corneal immune cell changes at single cell level in diabetic mice. Front. Endocrinol. 2023, 14, 1253188. [Google Scholar] [CrossRef]
- Li, C.; Cai, Q. Two ferroptosis-specific expressed genes NOX4 and PARP14 are considered as potential biomarkers for the diagnosis and treatment of diabetic retinopathy and atherosclerosis. Diabetol. Metab. Syndr. 2024, 16, 61. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, L.; Yu, J.; Wang, N.; Bu, S.; Wang, H.; Zhang, J.; Luo, X.; Liu, Y.; Nie, C. Biomedicine & Pharmacotherapy MDSCs promote pathological angiogenesis in ocular neovascular disease. Biomed. Pharmacother. 2024, 178, 117222. [Google Scholar] [CrossRef]
- Fernando, W.; MacLean, E.; Monro, S.; Coombs, M.R.P.; Marcato, P.; Rupasinghe, H.P.V.; Hoskin, D.W. Phloridzin Docosahexaenoate, an Omega-3 Fatty Acid Ester of a Flavonoid Precursor, Inhibits Angiogenesis by Suppressing Endothelial Cell Proliferation, Migration, and Differentiation. Biomolecules 2024, 14, 769. [Google Scholar] [CrossRef]
- Kauppinen, A.; Kaarniranta, K.; Salminen, A. Potential Role of Myeloid-Derived Suppressor Cells (MDSCs) in Age-Related Macular Degeneration (AMD). Front. Immunol. 2020, 11, 384. [Google Scholar] [CrossRef]
- Wei, C.; Mi, Y.; Sun, L.; Luo, J.; Zhang, J.; Gao, Y.; Yu, X.; Ge, H.; Liu, P. Cannabidiol alleviates suture-induced corneal pathological angiogenesis and inflammation by inducing myeloid-derived suppressor cells. Int. Immunopharmacol. 2024, 137, 112429. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, X.; Yan, X.; Lin, Y.; Tan, Q.; Hou, Y. mTOR inhibitor INK128 promotes wound healing by regulating MDSCs. Stem Cell Res. Ther. 2021, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Nabi, A.H.M.N.; Ebihara, A.; Shekhar, H.U. Impacts of SARS-CoV-2 on diabetes mellitus: A pre and post pandemic evaluation. World J. Virol. 2023, 12, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Rey-Reñones, C.; Martinez-Torres, S.; Martín-Luján, F.M.; Pericas, C.; Redondo, A.; Vilaplana-Carnerero, C.; Dominguez, A.; Grau, M. Type 2 Diabetes Mellitus and COVID-19: A Narrative Review. Biomedicines 2022, 10, 2089. [Google Scholar] [CrossRef] [PubMed]
- Martu, M.A.; Maftei, G.A.; Sufaru, I.G.; Jelihovschi, I.; Luchian, I.; Hurjui, L.; Martu, I.; Pasarin, L. COVID-19 and Periodontal Disease-Ethiopathogenic and Clinical Implications. Rom. J. Oral Rehabil. 2020, 12, 116–124. [Google Scholar]
- Miller, M.G.; Terebuh, P.; Kaelber, D.C.; Xu, R.; Davis, P.B. SARS-CoV-2 Infection and New-Onset Type 2 Diabetes Among Pediatric Patients, 2020 to 2022. JAMA Netw. Open 2024, 7, e2439444. [Google Scholar] [CrossRef]
- Akachar, J.; Bouricha, E.M.; Hakmi, M.; Belyamani, L.; El Jaoudi, R.; Ibrahimi, A. Identify-ing epitopes for cluster of differentiation and design of new peptides inhibitors against human SARS-CoV-2 spike RBD by an in-silico approach. Heliyon 2020, 6, e05739. [Google Scholar] [CrossRef]
- Drzymała, A. The Functions of SARS-CoV-2 Receptors in Diabetes-Related Severe COVID-19. Int. J. Mol. Sci. 2024, 25, 9635. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Ali, M.M.; Mirza, I.; Naquiallah, D.; Hassan, C.; Masrur, M.; Bianco, F.M.; Mahmoud, A.M. CD147 Levels in Blood and Adipose Tissues Correlate with Vascular Dysfunction in Obese Diabetic Adults. J. Cardiovasc. Dev. Dis. 2021, 9, 7. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Xiao, T.; Li, J.; Guo, A.; Lei, L.; Jin, C.; Long, Q.; Su, J.; Yin, M.; et al. CD147 mediates epidermal malig-nant transformation through the RSK2/AP-1 pathway. J. Exp. Clin. Cancer Res. 2022, 41, 246. [Google Scholar] [CrossRef]
- Nepal, M.R.; Shah, S.; Kang, K.T. Dual roles of myeloid-derived suppressor cells in various diseases: A review. Arch. Pharmacal Res. 2024, 47, 597–616. [Google Scholar] [CrossRef]
- Płonka-Czerw, J.; Żyrek, L.; Latocha, M. Changes in the Sensitivity of MCF-7 and MCF-7/DX Breast Cancer Cells to Cytostatic in the Presence of Metformin. Molecules 2024, 29, 3531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Xiao, H. Metformin Actions on the Liver: Protection Mechanisms Emerging in Hepatocytes and Immune Cells against NASH-Related HCC. Int. J. Mol. Sci. 2021, 22, 5016. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, A.; Jovanovic, I.; Stojanovic, B.; Stojanovic, M.D.; Stojanovic, B.S.; Jurisevic, M.; Markovic, B.S.; Jovanovic, M.; Jovanovic, M.; Jovanovic, M.; et al. Harnessing Metformin’s Immunomodulatory Effects on Immune Cells to Combat Breast Cancer. Int. J. Mol. Sci. 2024, 25, 5869. [Google Scholar] [CrossRef] [PubMed]
- Hatae, R.; Kyewalabye, K.; Yamamichi, A.; Chen, T.; Phyu, S.; Chuntova, P.; Nejo, T.; Levine, L.S.; Spitzer, M.H.; Okada, H. Enhancing CAR-T cell metabolism to overcome hypoxic conditions in the brain tumor microenvironment. J. Clin. Investig. 2024, 9, 1434495. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, M.; Zhang, Y.; Jia, X.; Xiao, W.; Lu, G.; Chen, W.; Gong, W. Myeloid-derived suppressor cells in the tumor microenvironment reduce uncoupling protein 1 expression to boost immunosuppressive activity. Biochem. Biophys. Res. Commun. 2024, 732, 150408. [Google Scholar] [CrossRef]
- Abdelmoneim, M.; Aboalela, M.A.; Naoe, Y.; Matsumura, S.; Eissa, I.R.; Bustos-Villalobos, I.; Sibal, P.A.; Takido, Y.; Kodera, Y.; Kasuya, H. The Impact of Metformin on Tumor-Infiltrated Immune Cells: Preclinical and Clinical Studies. Int. J. Mol. Sci. 2023, 24, 13353. [Google Scholar] [CrossRef]
| Subset | Phenotype | Cell Type | Reference |
|---|---|---|---|
| G-MDSCs | CD11b+CD14−CD15+ or CD11b+CD14−CD66b+ | human | [61,65] |
| CD11b+Ly6G+Ly6Clo | mice | [61,65] | |
| M-MDSCs | CD11b+CD14+HLA-DR−/lowCD15− | human | [61,65] |
| CD11b+Ly6G−Ly6Chi | mice | [61,65] | |
| E-MDSCs | Lin− HLADR−CD33+ | Human | [66] |
| - | mice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghemiș, L.; Goriuc, A.; Minea, B.; Botnariu, G.E.; Mârțu, M.-A.; Ențuc, M.; Cioloca, D.; Foia, L.G. Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes. Diagnostics 2024, 14, 2453. https://doi.org/10.3390/diagnostics14212453
Ghemiș L, Goriuc A, Minea B, Botnariu GE, Mârțu M-A, Ențuc M, Cioloca D, Foia LG. Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes. Diagnostics. 2024; 14(21):2453. https://doi.org/10.3390/diagnostics14212453
Chicago/Turabian StyleGhemiș, Larisa, Ancuța Goriuc, Bogdan Minea, Gina Eosefina Botnariu, Maria-Alexandra Mârțu, Melissa Ențuc, Daniel Cioloca, and Liliana Georgeta Foia. 2024. "Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes" Diagnostics 14, no. 21: 2453. https://doi.org/10.3390/diagnostics14212453
APA StyleGhemiș, L., Goriuc, A., Minea, B., Botnariu, G. E., Mârțu, M.-A., Ențuc, M., Cioloca, D., & Foia, L. G. (2024). Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes. Diagnostics, 14(21), 2453. https://doi.org/10.3390/diagnostics14212453








