Peptide Receptor Radionuclide Therapy Using 90Y- and 177Lu-DOTATATE Modulating Atherosclerotic Plaque Inflammation: Longitudinal Monitoring by 68Ga-DOTATATE Positron Emissions Tomography/Computer Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. 68Ga-DOTATATE Imaging Technique
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Correlation of Calcified Plaque Score, Overall Vessel Uptake (OVU), and Age
3.3. Correlation of Each Vessel Segment to OVU
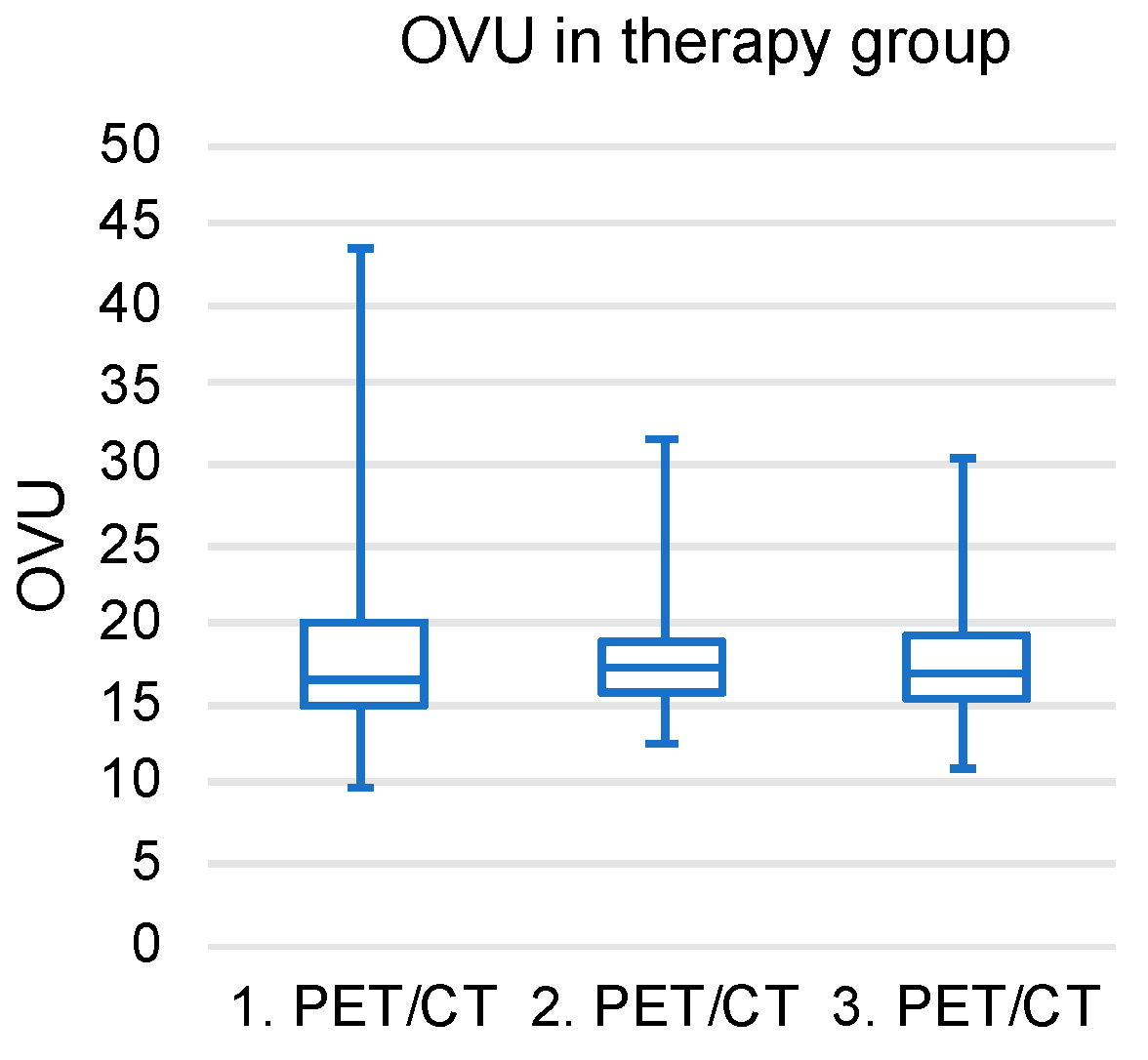
3.4. Longitudinal OVU Evaluation in the Therapy Group
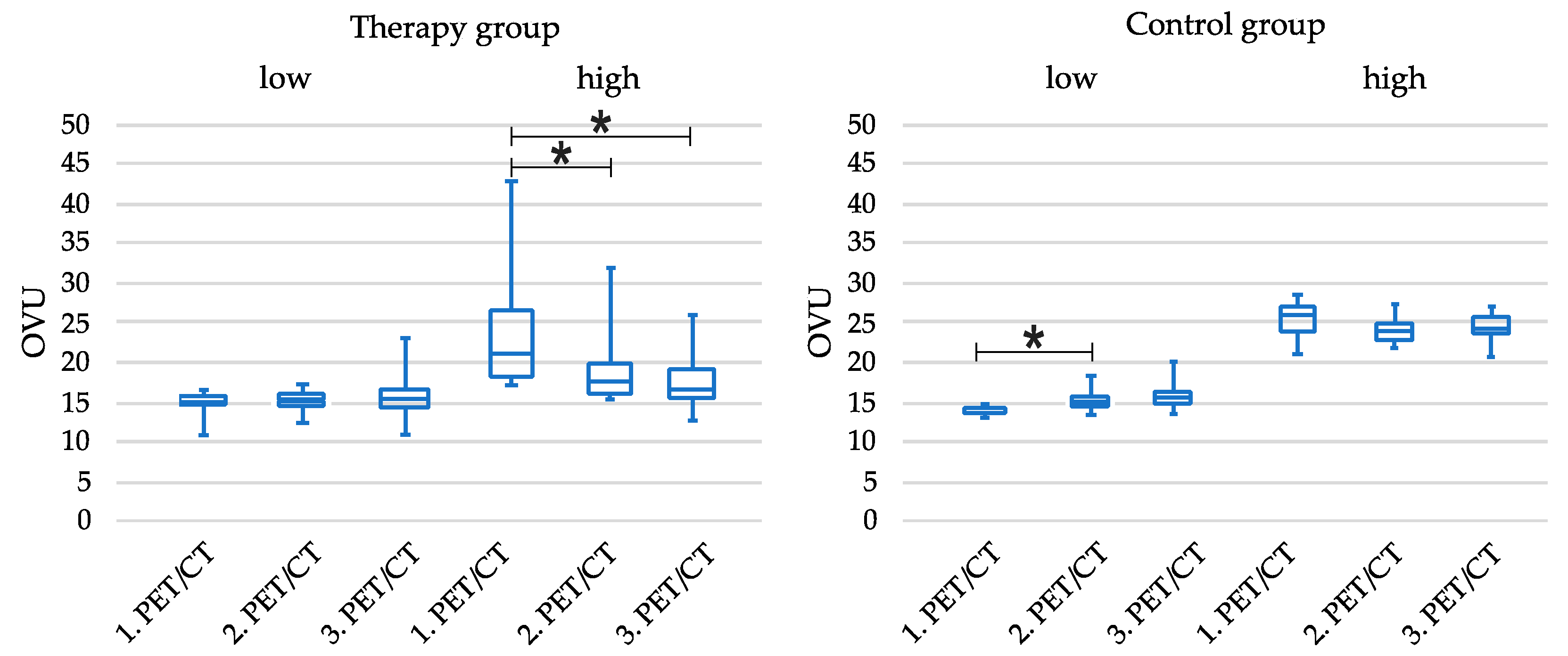
3.5. Tercile-Based Analysis of Patient Cohorts
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.F.; Hyafil, F.; Fayad, Z.A. Inflammation Imaging in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Tabas, I.; Fredman, G.; Fisher, E.A. Inflammation and Its Resolution as Determinants of Acute Coronary Syndromes. Circ. Res. 2014, 114, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Tomey, M.I.; Narula, J.; Kovacic, J.C. Advances in the Understanding of Plaque Composition and Treatment Options: Year in Review. J. Am. Coll. Cardiol. 2014, 63, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Zerizer, I.; Tan, K.; Khan, S.; Barwick, T.; Marzola, M.C.; Rubello, D.; Al-Nahhas, A. Role of FDG-PET and PET/CT in the Diagnosis and Management of Vasculitis. Eur. J. Radiol. 2010, 73, 504–509. [Google Scholar] [CrossRef]
- Rudd, J.H.F.; Narula, J.; Strauss, H.W.; Virmani, R.; Machac, J.; Klimas, M.; Tahara, N.; Fuster, V.; Warburton, E.A.; Fayad, Z.A.; et al. Imaging Atherosclerotic Plaque Inflammation by Fluorodeoxyglucose With Positron Emission Tomography. J. Am. Coll. Cardiol. 2010, 55, 2527–2535. [Google Scholar] [CrossRef]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In Vivo 18F-Fluorodeoxyglucose Positron Emission Tomography Imaging Provides a Noninvasive Measure of Carotid Plaque Inflammation in Patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef]
- Saam, T.; Rominger, A.; Wolpers, S.; Nikolaou, K.; Rist, C.; Greif, M.; Cumming, P.; Becker, A.; Foerster, S.; Reiser, M.F.; et al. Association of Inflammation of the Left Anterior Descending Coronary Artery with Cardiovascular Risk Factors, Plaque Burden and Pericardial Fat Volume: A PET/CT Study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1203–1212. [Google Scholar] [CrossRef]
- Rominger, A.; Saam, T.; Wolpers, S.; Cyran, C.C.; Schmidt, M.; Foerster, S.; Nikolaou, K.; Reiser, M.F.; Bartenstein, P.; Hacker, M. 18F-FDG PET/CT Identifies Patients at Risk for Future Vascular Events in an Otherwise Asymptomatic Cohort with Neoplastic Disease. J. Nucl. Med. 2009, 50, 1611–1620. [Google Scholar] [CrossRef]
- Tahara, N.; Kai, H.; Ishibashi, M.; Nakaura, H.; Kaida, H.; Baba, K.; Hayabuchi, N.; Imaizumi, T. Simvastatin Attenuates Plaque Inflammation. Evaluation by Fluorodeoxyglucose Positron Emission Tomography. J. Am. Coll. Cardiol. 2006, 48, 1825–1831. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Dweck, M.R.; Evans, N.R.; Takx, R.A.P.; Brown, A.J.; Tawakol, A.; Fayad, Z.A.; Rudd, J.H.F. Imaging Atherosclerosis. Circ. Res. 2016, 118, 750–769. [Google Scholar] [CrossRef] [PubMed]
- Alie, N.; Eldib, M.; Fayad, Z.A.; Mani, V. Inflammation, Atherosclerosis, and Coronary Artery Disease: PET/CT for the Evaluation of Atherosclerosis and Inflammation. Clin. Med. Insights Cardiol. 2014, 8, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.J.A.; Lindner, O.; Sciagra, R.; Agostini, D.; Übleis, C.; Gimelli, A.; Hacker, M. Position Paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET Imaging of Atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The Immune System in Cardiac Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef]
- Dalm, V.A.S.H.; Martin Van Hagen, P.; van Koetsveld, P.M.; Achilefu, S.; Houtsmuller, A.B.; Pols, D.H.J.; van der Lely, A.-J.; Lamberts, S.W.J.; Hofland, L.J. Expression of Somatostatin, Cortistatin, and Somatostatin Receptors in Human Monocytes, Macrophages, and Dendritic Cells. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 344–353. [Google Scholar] [CrossRef]
- Armani, C.; Catalani, E.; Balbarini, A.; Bagnoli, P.; Cervia, D. Expression, Pharmacology, and Functional Role of Somatostatin Receptor Subtypes 1 and 2 in Human Macrophages. J. Leukoc. Biol. 2007, 81, 845–855. [Google Scholar] [CrossRef]
- Li, X.; Bauer, W.; Kreissl, M.C.; Weirather, J.; Bauer, E.; Israel, I.; Richter, D.; Riehl, G.; Buck, A.; Samnick, S. Specific Somatostatin Receptor II Expression in Arterial Plaque: 68Ga-DOTATATE Autoradiographic, Immunohistochemical and Flow Cytometric Studies in ApoE-Deficient Mice. Atherosclerosis 2013, 230, 33–39. [Google Scholar] [CrossRef]
- Rinne, P.; Hellberg, S.; Kiugel, M.; Virta, J.; Li, X.-G.; Käkelä, M.; Helariutta, K.; Luoto, P.; Liljenbäck, H.; Hakovirta, H.; et al. Comparison of Somatostatin Receptor 2-Targeting PET Tracers in the Detection of Mouse Atherosclerotic Plaques. Mol. Imaging Biol. 2016, 18, 99–108. [Google Scholar] [CrossRef]
- Malmberg, C.; Ripa, R.S.; Johnbeck, C.B.; Knigge, U.; Langer, S.W.; Mortensen, J.; Oturai, P.; Loft, A.; Hag, A.M.; Kjer, A. 64Cu-DOTATATE for Noninvasive Assessment of Atherosclerosis in Large Arteries and Its Correlation with Risk Factors: Head-to-Head Comparison with 68Ga-DOTATOC in 60 Patients. J. Nucl. Med. 2015, 56, 1895–1900. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Sandholt, B.V.; Keller, S.H.; Hansen, A.E.; Clemmensen, A.E.; Sillesen, H.; Højgaard, L.; Ripa, R.S.; Kjær, A. 64Cu-DOTATATE PET/MRI for Detection of Activated Macrophages in Carotid Atherosclerotic Plaques: Studies in Patients Undergoing Endarterectomy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1696–1703. [Google Scholar] [CrossRef]
- Wan, M.Y.S.; Endozo, R.; Michopoulou, S.; Shortman, R.; Rodriguez-Justo, M.; Menezes, L.; Yusuf, S.; Richards, T.; Wild, D.; Waser, B.; et al. PET/CT Imaging of Unstable Carotid Plaque with 68Ga-Labeled Somatostatin Receptor Ligand. J. Nucl. Med. 2017, 58, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.Z.; Kochiadakis, G.; Lazopoulos, G.; Marias, K.; Klapsinos, N.; Hannah-Shmouni, F.; Igoumenaki, G.G.; Nikolouzakis, T.K.; Kteniadakis, S.; Spandidos, D.A.; et al. Targeting Vulnerable Atherosclerotic Plaque via PET-Tracers Aiming at Cell-Surface Overexpression of Somatostatin Receptors. Biomed. Rep. 2020, 13, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, A.; Alavi, A.; Thamake, S.; Amerinia, R.; Ranganathan, D.; Tworowska, I.; Delpassand, E.S. Assessment of Vulnerable Atherosclerotic and Fibrotic Plaques in Coronary Arteries Using (68)Ga-DOTATATE PET/CT. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 65–71. [Google Scholar] [PubMed]
- Kam, B.L.R.; Teunissen, J.J.M.; Krenning, E.P.; de Herder, W.W.; Khan, S.; van Vliet, E.I.; Kwekkeboom, D.J. Lutetium-Labelled Peptides for Therapy of Neuroendocrine Tumours. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 103–112. [Google Scholar] [CrossRef]
- Khan, S.; Krenning, E.P.; van Essen, M.; Kam, B.L.; Teunissen, J.J.; Kwekkeboom, D.J. Quality of Life in 265 Patients with Gastroenteropancreatic or Bronchial Neuroendocrine Tumors Treated with [ 177Lu-DOTA 0,Tyr 3]Octreotate. J. Nucl. Med. 2011, 52, 1361–1368. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the Radiolabeled Somatostatin Analog [177Lu- DOTA0,Tyr3]Octreotate: Toxicity, Efficacy, and Survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Rominger, A.; Saam, T.; Vogl, E.; Übleis, C.; la Fougère, C.; Förster, S.; Haug, A.; Cumming, P.; Reiser, M.F.; Nikolaou, K.; et al. In Vivo Imaging of Macrophage Activity in the Coronary Arteries Using 68Ga-DOTATATE PET/CT: Correlation with Coronary Calcium Burden and Risk Factors. J. Nucl. Med. 2010, 51, 193–197. [Google Scholar] [CrossRef]
- Rudd, J.H.F.; Myers, K.S.; Bansilal, S.; Machac, J.; Rafique, A.; Farkouh, M.; Fuster, V.; Fayad, Z.A. 18Fluorodeoxyglucose Positron Emission Tomography Imaging of Atherosclerotic Plaque Inflammation Is Highly Reproducible. Implications for Atherosclerosis Therapy Trials. J. Am. Coll. Cardiol. 2007, 50, 892–896. [Google Scholar] [CrossRef]
- Brown, E.R.; Kronmal, R.A.; Bluemke, D.A.; Guerci, A.D.; Carr, J.J.; Goldin, J.; Detrano, R. Coronary Calcium Coverage Score: Determination, Correlates, and Predictive Accuracy in the Multi-Ethnic Study of Atherosclerosis. Radiology 2008, 247, 669–678. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the Vulnerable Plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Sadeghi, M.M.; Glover, D.K.; Lanza, G.M.; Fayad, Z.A.; Johnson, L.L. Imaging Atherosclerosis and Vulnerable Plaque. J. of Nucl. Med. 2010, 51, 51S–65S. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.F.; Myers, K.S.; Bansilal, S.; Machac, J.; Woodward, M.; Fuster, V.; Farkouh, M.E.; Fayad, Z.A. Relationships among Regional Arterial Inflammation, Calcification, Risk Factors, and Biomarkers: A Prospective Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Imaging Study. Circ. Cardiovasc. Imaging 2009, 2, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.F.; Myers, K.S.; Bansilal, S.; Machac, J.; Pinto, C.A.; Tong, C.; Rafique, A.; Hargeaves, R.; Farkouh, M.; Fuster, V.; et al. Atherosclerosis Inflammation Imaging with 18F-FDG PET: Carotid, Iliac, and Femoral Uptake Reproducibility, Quantification Methods, and Recommendations. J. Nucl. Med. 2008, 49, 871–878. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.v.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Gholami, S.; Salavati, A.; Houshmand, S.; Werner, T.J.; Alavi, A. Assessment of Atherosclerosis in Large Vessel Walls: A Comprehensive Review of FDG-PET/CT Image Acquisition Protocols and Methods for Uptake Quantification. J. Nucl. Cardiol. 2015, 22, 468–479. [Google Scholar] [CrossRef]
- Scherer, D.J.; Psaltis, P.J. Future Imaging of Atherosclerosis: Molecular Imaging of Coronary Atherosclerosis with 18F Positron Emission Tomography. Cardiovasc. Diagn. Ther. 2016, 6, 354–367. [Google Scholar] [CrossRef]
- Derlin, T.; Richter, U.; Bannas, P.; Begemann, P.; Buchert, R.; Mester, J.; Klutmann, S. Feasibility of 18F-Sodium Fluoride PET/CT for Imaging of Atherosclerotic Plaque. J. Nucl. Med. 2010, 51, 862–865. [Google Scholar] [CrossRef]
- Derlin, T.; Wisotzki, C.; Richter, U.; Apostolova, I.; Bannas, P.; Weber, C.; Mester, J.; Klutmann, S. In Vivo Imaging of Mineral Deposition in Carotid Plaque Using 18F-Sodium Fluoride PET/CT: Correlation with Atherogenic Risk Factors. J. Nucl. Med. 2011, 52, 362–368. [Google Scholar] [CrossRef]
- Janssen, T.; Bannas, P.; Herrmann, J.; Veldhoen, S.; Busch, J.D.; Treszl, A.; Münster, S.; Mester, J.; Derlin, T. Association of Linear 18F-Sodium Fluoride Accumulation in Femoral Arteries as a Measure of Diffuse Calcification with Cardiovascular Risk Factors: A PET/CT Study. J. Nucl. Cardiol. 2013, 20, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Morbelli, S.; Fiz, F.; Piccardo, A.; Picori, L.; Massollo, M.; Pestarino, E.; Marini, C.; Cabria, M.; Democrito, A.; Cittadini, G.; et al. Divergent Determinants of 18F-NaF Uptake and Visible Calcium Deposition in Large Arteries: Relationship with Framingham Risk Score. Int. J. Cardiovasc. Imaging 2014, 30, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Tóth, Z.; Papp, L.; Wisotzki, C.; Apostolova, I.; Habermann, C.R.; Mester, J.; Klutmann, S. Correlation of Inflammation Assessed By18F-FDG PET, Active Mineral Deposition Assessed By18F-Fluoride PET, and Vascular Calcification in Atherosclerotic Plaque: A Dual-Tracer PET/CT Study. J. Nucl. Med. 2011, 52, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Jenkins, W.S.A.; Vesey, A.T.; Pringle, M.A.H.; Chin, C.W.L.; Malley, T.S.; Cowie, W.J.A.; Tsampasian, V.; Richardson, H.; Fletcher, A.; et al. 18F-Sodium Fluoride Uptake Is a Marker of Active Calcification and Disease Progression in Patients With Aortic Stenosis. Circ. Cardiovasc. Imaging 2014, 7, 371–378. [Google Scholar] [CrossRef]
- Dweck, M.R.; Chow, M.W.L.; Joshi, N.V.; Williams, M.C.; Jones, C.; Fletcher, A.M.; Richardson, H.; White, A.; McKillop, G.; van Beek, E.J.R.; et al. Coronary Arterial 18F-Sodium Fluoride Uptake: A Novel Marker of Plaque Biology. J. Am. Coll. Cardiol. 2012, 59, 1539–1548. [Google Scholar] [CrossRef]
- Joshi, N.v.; Vesey, A.T.; Williams, M.C.; Shah, A.S.V.; Calvert, P.A.; Craighead, F.H.M.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-Fluoride Positron Emission Tomography for Identification of Ruptured and High-Risk Coronary Atherosclerotic Plaques: A Prospective Clinical Trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Schatka, I.; Wollenweber, T.; Haense, C.; Brunz, F.; Gratz, K.F.; Bengel, F.M. Peptide Receptor-Targeted Radionuclide Therapy Alters Inflammation in Atherosclerotic Plaques. J. Am. Coll. Cardiol. 2013, 62, 2344–2345. [Google Scholar] [CrossRef]
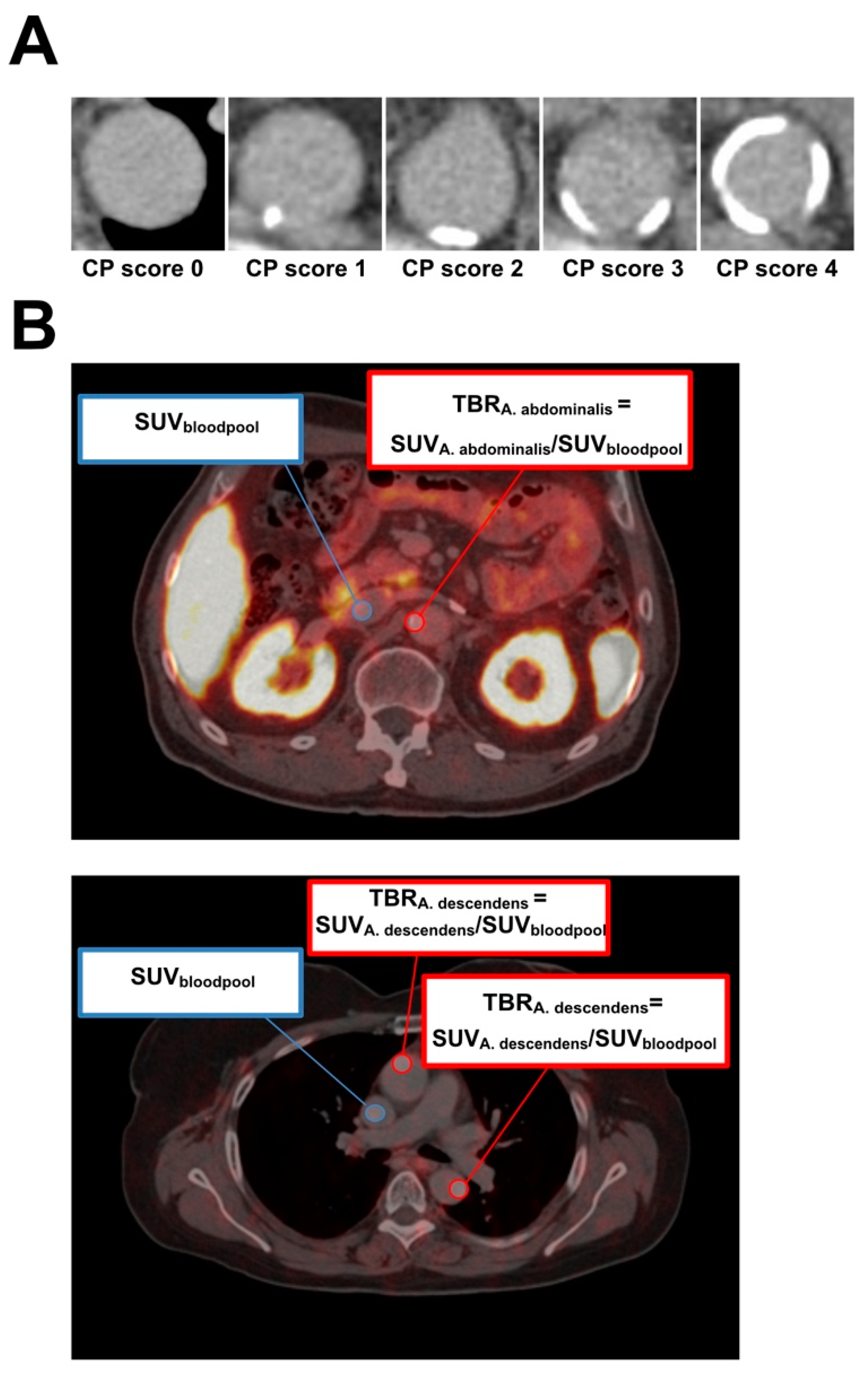

| Study Design | |
|---|---|
| Therapy Group | Control Group |
| 1. 68Ga-DOTATATE PET/CT (Baseline) | 1. 68Ga-DOTATATE PET/CT (Baseline) |
| |
| |
| 2. 68Ga-DOTATATE PET/CT | 2. 68Ga-DOTATATE PET/CT |
| |
| |
| 3. 68Ga-DOTATATE PET/CT | 3. 68Ga-DOTATATE PET/CT |
| Descriptive Statistics for Therapy and Control Group | |||
|---|---|---|---|
| Therapy Group (n = 37) | Control Group (n = 20) | p-Value | |
| Age [y] | 59 ± 10 | 63 ± 12 | 0.17 |
| Male | 23 (62.2%) | 9 (45%) | 0.268 |
| Diabetes mellitus | 6 (16.2%) | 4 (20%) | 0.728 |
| Hypertension | 26 (70.3%) | 12 (60%) | 0.558 |
| BMI [kg/m2] | 25.9 ± 4.8 | 27.5 ± 4.9 | 0.198 |
| OVU | 18.5 ± 6.7 | 19.6 ± 5.7 | 0.707 |
| CP score | 6 ± 6 | 10 ± 9 | 0.156 |
| Dose 1. PET/CT [MBq] | 206 ± 27 | 208 ± 31 | 0.841 |
| Dose 2. PET/CT [MBq] | 218 ± 20 | 221 ± 29 | 0.735 |
| Dose 3. PET/CT [MBq] | 211 ± 23 | 229 ± 40 | 0.036 |
| Cycle count 177Lutetium | 136 | ||
| Dose 177Lutetium [MBq] | 7254 ± 640 | ||
| Cycle count 90Yttrium | 12 | ||
| Dose 90Yttrium [MBq] | 3647 ± 189 | ||
| Time between 1st and 2nd PET/CT [d] | 214 ± 68 | 174 ± 76 | 0.019 |
| Time between 2nd and 3rd PET/CT [d] | 407 ± 356 | 238 ± 100 | 0.408 |
| Time between 1st and 3rd PET/CT [d] | 621 ± 401 | 413 ± 114 | 0.126 |
| Correlation Analysis of Each TBR to OVU | |
|---|---|
| Vessel segment | |
| TBR ascending aorta | R = 0.891; p < 0.001 |
| TBR aortic arch | R = 0.825; p < 0.001 |
| TBR descending aorta | R = 0.887; p < 0.001 |
| TBR abdominal aorta | R = 0.877; p < 0.001 |
| TBR right iliac arteries | R = 0.853; p < 0.001 |
| TBR left iliac arteries | R = 0.804; p < 0.001 |
| TBR right carotid artery | R = 0.784; p < 0.001 |
| TBR left carotid artery | R = 0.848; p < 0.001 |
| Descriptive Statistics for Subgroups | |||||
| Therapy Group (TG) | Control Group (CG) | p Value | |||
| Tercile | Low | High | Low | High | |
| Count | 12 | 12 | 10 | 10 | |
| Age [y] | 57 ± 12 | 62 ± 10 | 63 ± 12 | 64 ± 12 | 0.577 |
| Men | 6 (50%) | 9 (75%) | 3 (30%) | 6 (60%) | 0.197 |
| Diabetes mellitus | 1 (8.3%) | 2 (16.7%) | 3 (30%) | 1 (10%) | 0.519 |
| Hypertension | 5 (41.7%) | 11 (91.7%) | 6 (60%) | 6 (60%) | 0.082 |
| BMI [kg/m2] | 24.2 ± 4.7 | 25.9 ± 3.8 | 26.7 ± 5.9 | 28.4 ± 3.8 | 0.221 |
| OVU | 14.8 ± 1.4 | 23.7 ± 7.7 | 14.4 ± 0.6 | 24.9 ± 2.6 | <0.001 |
| CP score | 4.6 ± 5.2 | 7.2 ± 7.2 | 7.2 ± 7.5 | 12.1 ± 9.7 | 0.146 |
| Dose 1. PET/CT [MBq] | 205 ± 30 | 200 ± 31 | 205 ± 36 | 210 ± 26 | 0.897 |
| Dose 2. PET/CT [MBq] | 219 ± 19 | 225 ± 20 | 217 ± 31 | 225 ± 28 | 0.821 |
| Dose 3. PET/CT [MBq] | 207 ± 20 | 214 ± 17 | 232 ± 30 | 227 ± 50 | 0.226 |
| Time from 1. to 3. PET/CT [d] | 438 ± 94 | 370 ± 70 | 412 ± 86 | 413 ± 142 | 0.425 |
| OVU Course | |||
|---|---|---|---|
| 1. PET/CT | 2. PET/CT | 3. PET/CT | |
| TG_low | 14.76 ± 1.44 | 15.4 ± 1.66 | 15.98 ± 3.11 |
| TG_high | 23.69 ± 7.72 | 18.88 ± 4.64 | 18.7 ± 4 |
| CG_low | 14.42 ± 0.56 | 15.45 ± 1.47 | 16.22 ± 2.03 |
| CG_high | 24.89 ± 2.56 | 24.12 ± 1.93 | 24.09 ± 1.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubinstein, G.; Ilhan, H.; Bartenstein, P.; Lehner, S.; Hacker, M.; Todica, A.; Zacherl, M.J.; Fischer, M. Peptide Receptor Radionuclide Therapy Using 90Y- and 177Lu-DOTATATE Modulating Atherosclerotic Plaque Inflammation: Longitudinal Monitoring by 68Ga-DOTATATE Positron Emissions Tomography/Computer Tomography. Diagnostics 2024, 14, 2486. https://doi.org/10.3390/diagnostics14222486
Rubinstein G, Ilhan H, Bartenstein P, Lehner S, Hacker M, Todica A, Zacherl MJ, Fischer M. Peptide Receptor Radionuclide Therapy Using 90Y- and 177Lu-DOTATATE Modulating Atherosclerotic Plaque Inflammation: Longitudinal Monitoring by 68Ga-DOTATATE Positron Emissions Tomography/Computer Tomography. Diagnostics. 2024; 14(22):2486. https://doi.org/10.3390/diagnostics14222486
Chicago/Turabian StyleRubinstein, German, Harun Ilhan, Peter Bartenstein, Sebastian Lehner, Marcus Hacker, Andrei Todica, Mathias Johannes Zacherl, and Maximilian Fischer. 2024. "Peptide Receptor Radionuclide Therapy Using 90Y- and 177Lu-DOTATATE Modulating Atherosclerotic Plaque Inflammation: Longitudinal Monitoring by 68Ga-DOTATATE Positron Emissions Tomography/Computer Tomography" Diagnostics 14, no. 22: 2486. https://doi.org/10.3390/diagnostics14222486
APA StyleRubinstein, G., Ilhan, H., Bartenstein, P., Lehner, S., Hacker, M., Todica, A., Zacherl, M. J., & Fischer, M. (2024). Peptide Receptor Radionuclide Therapy Using 90Y- and 177Lu-DOTATATE Modulating Atherosclerotic Plaque Inflammation: Longitudinal Monitoring by 68Ga-DOTATATE Positron Emissions Tomography/Computer Tomography. Diagnostics, 14(22), 2486. https://doi.org/10.3390/diagnostics14222486






