Could SLC26A7 Be a Promising Marker for Preoperative Diagnosis of High-Grade Papillary Thyroid Carcinoma?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
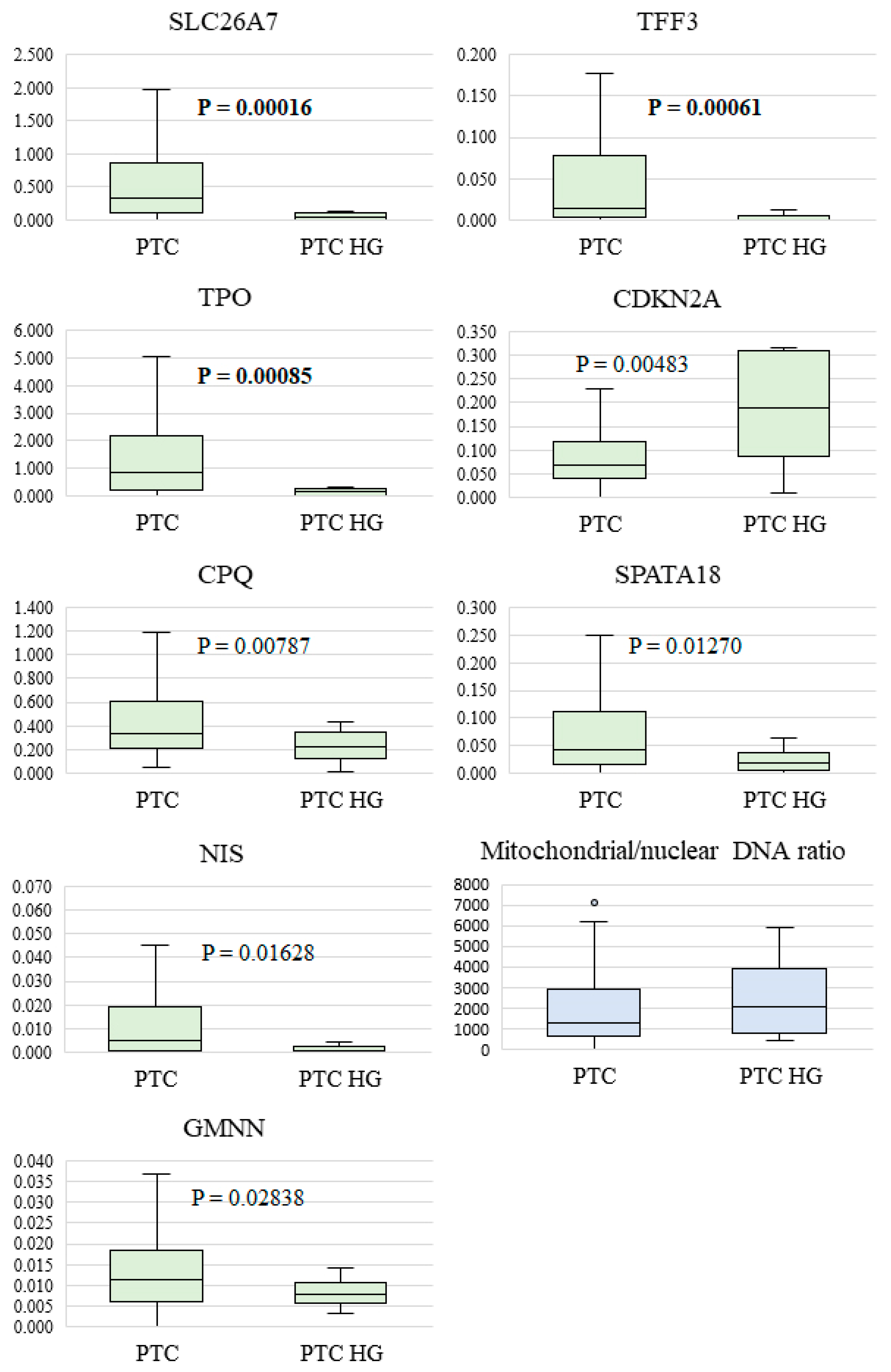
- The TPO gene (p = 0.00085) encodes thyroid peroxidase, which plays an important role in thyroid hormones’ synthesis and maintenance of stable thyroid function. According to other studies, TPO is highly expressed, mainly in normal thyroid tissue or benign lesions, whereas in thyroid cancer, its expression is reduced or absent [37].
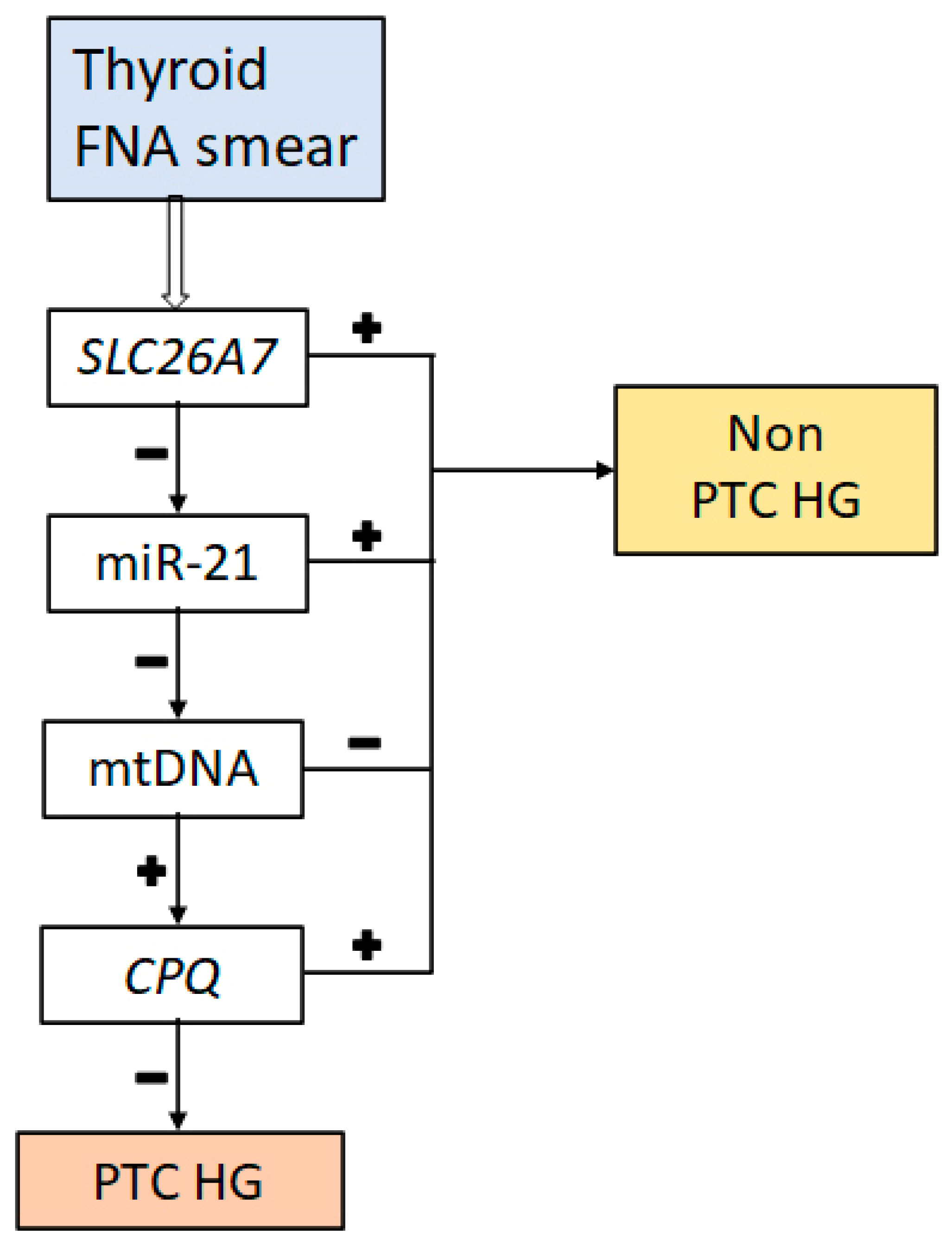
- The SLC26A7 gene (p = 0.000166) codes for a transmembrane Cl−/HCO3− ion transporter that controls the acid–base balance in renal tubules and the gastric epithelium and appears to perform a similar function in thyroid follicular cells. This is because an optimal ionic milieu and pH are known to be critical for enzymatic activity (of TPO, for example) during thyroid hormone biosynthesis [38]. There is evidence that decreased SLC26A7 expression accompanies the development of anaplastic carcinoma and significantly correlates with a poor prognosis among the patients [26], whereas in PTC, SLC26A7 downregulation has been associated with an elevated risk of metastases outside the thyroid [39].
- The TFF3 gene (p = 0.000614) encodes a small molecular peptide that belongs to a family of small secretory molecules involved in the protection and repair of the gastrointestinal mucosa. TFF proteins participate in the maintenance and restoration of epithelial structural integrity by activating key signaling pathways for epithelial–cell migration, proliferation, and invasiveness [40]. TFF3 expression is diminished in PTC, and patients with a lower gene expression have poorer disease-free survival but a higher immune-cell infiltration [41].
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basolo, F.; Macerola, E.; Poma, A.M.; Torregrossa, L. The 5th edition of WHO classification of tumors of endocrine organs: Changes in the diagnosis of follicular-derived thyroid carcinoma. Endocrine 2023, 80, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Harahap, A.S.; Roren, R.S.; Imtiyaz, S. A Comprehensive Review and Insights into the New Entity of Differentiated High-Grade Thyroid Carcinoma. Curr. Oncol. 2024, 31, 3311–3328. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; David, J.; Dogan, S.; Landa, I.; Katabi, N.; Saliba, M.; Khimraj, A.; Sherman, E.J.; Tuttle, R.M.; Tallini, G.; et al. Primary high-grade non-anaplastic thyroid carcinoma: A retrospective study of 364 cases. Histopathology 2022, 80, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Ghossein, R.A.; Schoder, H.; Gomez, D.; Larson, S.M.; Tuttle, R.M. Histopathologic characterization of radioactive iodine-refractory fuorodeoxyglucosepositron emission tomography-positive thyroid carcinoma. Cancer 2008, 113, 48–56. [Google Scholar] [CrossRef]
- Wong, K.S.; Dong, F.; Telatar, M.; Lorch, J.H.; Alexander, E.K.; Marqusee, E.; Cho, N.L.; Nehs, M.A.; Doherty, G.M.; Afkhami, M.; et al. Papillary Thyroid Carcinoma with High-Grade Features Versus Poorly Diferentiated Thyroid Carcinoma: An Analysis of Clinicopathologic and Molecular Features and Outcome. Thyroid 2021, 31, 933–940. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R.A. Advances in Thyroid Pathology: High Grade Follicular Cell-derived Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Adv. Anat. Pathol. 2023, 30, 3–10. [Google Scholar] [CrossRef]
- Ghossein, R.; Katabi, N.; Dogan, S.; Shaha, A.R.; Tuttle, R.M.; Fagin, J.A.; Ganly, I.; Xu, B. Papillary thyroid carcinoma tall cell subtype (PTC-TC) and high-grade differentiated thyroid carcinoma tall cell phenotype (HGDTC-TC) have different clinical behaviour: A retrospective study of 1456 patients. Histopathology 2024, 84, 1130–1138. [Google Scholar] [CrossRef]
- Paulsson, J.O.; Backman, S.; Wang, N.; Stenman, A.; Crona, J.; Thutkawkorapin, J.; Ghaderi, M.; Tham, E.; Stalberg, P.; Zedenius, J.; et al. Whole-genome sequencing of synchronous thyroid carcinomas identifies aberrant DNA repair in thyroid cancer dediferentiation. J. Pathol. 2020, 250, 183–194. [Google Scholar] [CrossRef]
- Wong, K.S.; Lorch, J.H.; Alexander, E.K.; Marqusee, E.; Cho, N.L.; Nehs, M.A.; Doherty, G.M.; Barletta, J.A. Prognostic Signifcance of Extent of Invasion in Poorly Diferentiated Thyroid Carcinoma. Thyroid 2019, 29, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Chernock, R.D.; Rivera, B.; Borrelli, N.; Hill, D.A.; Fahiminiya, S.; Shah, T.; Chong, A.S.; Aqil, B.; Mehrad, M.; Giordano, T.J.; et al. Poorly diferentiated thyroid carcinoma of childhood and adolescence: A distinct entity characterized by DICER1 mutations. Mod. Pathol. 2020, 33, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Sistrunk, J.W.; Shifrin, A.; Frager, M.; Bardales, R.H.; Thomas, J.; Fishman, N.; Goldberg, P.; Guttler, R.; Grant, E. Clinical performance of multiplatform mutation panel and microRNA risk classifier in indeterminate thyroid nodules. J. Am. Soc. Cytopathol. 2020, 9, 232–241. [Google Scholar] [CrossRef]
- Patel, K.N.; Angell, T.E.; Babiarz, J.; Barth, N.M.; Blevins, T.; Duh, Q.Y.; Ghossein, R.A.; Harrell, R.M.; Huang, J.; Kennedy, G.C.; et al. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg. 2018, 153, 817–824. [Google Scholar] [CrossRef]
- Santos, M.; Buzolin, A.L.; Gama, R.R.; Silva, E.; Dufloth, R.M.; Figueiredo, D.; Carvalho, A.L. Molecular Classification of Thyroid Nodules with Indeterminate Cytology: Development and Validation of a Highly Sensitive and Specific New miRNA-Based Classifier Test Using Fine-Needle Aspiration Smear Slides. Thyroid 2018, 28, 1618–1626. [Google Scholar] [CrossRef]
- Titov, S.; Demenkov, P.S.; Lukyanov, S.A.; Sergiyko, S.V.; Katanyan, G.A.; Veryaskina, Y.A.; Ivanov, M.K. Preoperative detection of malignancy in fine-needle aspiration cytology (FNAC) smears with indeterminate cytology (Bethesda III, IV) by a combined molecular classifier. J. Clin. Pathol. 2020, 73, 722–727. [Google Scholar] [CrossRef]
- Lukyanov, S.A.; Sergiyko, S.V.; Titov, S.E.; Beltsevich, D.G.; Veryaskina, Y.A.; Vanushko, V.E.; Urusova, L.S.; Mikheenkov, A.A.; Kozorezova, E.S.; Vorobyov, S.L.; et al. New Opportunities for Preoperative Diagnosis of Medullary Thyroid Carcinoma. Biomedicines 2023, 11, 1473. [Google Scholar] [CrossRef]
- Titov, S.E.; Lukyanov, S.A.; Kozorezova, E.S.; Demenkov, P.S.; Sergiyko, S.V.; Veryaskina, Y.A.; Vorobyev, S.L.; Sleptsov, I.V.; Gostimskii, A.V. Validation of preoperative diagnosis of malignant thyroid tumors using a molecular classifier. Vopr. Onkol. 2022, 68, 741–751. [Google Scholar] [CrossRef]
- Titov, S.E.; Kozorezova, E.S.; Demenkov, P.S.; Veryaskina, Y.A.; Kuznetsova, I.V.; Vorobyev, S.L.; Chernikov, R.A.; Sleptsov, I.V.; Timofeeva, N.I.; Ivanov, M.K. Preoperative Typing of Thyroid and Parathyroid Tumors with a Combined Molecular Classifier. Cancers 2021, 13, 237. [Google Scholar] [CrossRef]
- Torous, V.F.; Jitpasutham, T.; Baloch, Z.; Cantley, R.L.; Kerr, D.A.; Liu, X.; Maleki, Z.; Merkin, R.; Nosé, V.; Pantanowitz, L.; et al. Cytologic features of differentiated high-grade thyroid carcinoma: A multi-institutional study of 40 cases. Cancer Cytopathol. 2024, 132, 525–536. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.Y.; Xiong, G.F.; Zhao, Z.R.; Gu, X.; Huang, X.S. Comprehensive Identification of Potential Crucial Genes and miRNA-mRNA Regulatory Networks in Papillary Thyroid Cancer. Biomed Res. Int. 2021, 2021, 6752141. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, B.; Pfeifer, A.; Oczko-Wojciechowska, M.; Krajewska, J.; Czarniecka, A.; Kukulska, A. Gene Expression (mRNA) Markers for Differentiating between Malignant and Benign Follicular Thyroid Tumours. Int. J. Mol. Sci. 2017, 18, 1184. [Google Scholar] [CrossRef] [PubMed]
- Mussazhanova, Z.; Shimamura, M.; Kurashige, T.; Ito, M.; Nakashima, M.; Nagayama, Y. Causative role for defective expression of mitochondria-eating protein in accumulation of mitochondria in thyroid oncocytic cell tumors. Cancer Sci. 2020, 111, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Yang, M.; Mylona, N.; Wennerberg, J.; Paulsson, K. Global RNA Expression and DNA Methylation Patterns in Primary Anaplastic Thyroid Cancer. Cancers 2020, 12, 680. [Google Scholar] [CrossRef]
- Colombo, C.; Minna, E.; Gargiuli, C.; Muzza, M.; Dugo, M.; De Cecco, L.; Pogliaghi, G.; Tosi, D.; Bulfamante, G.; Greco, A.; et al. The molecular and gene/miRNA expression profiles of radioiodine resistant papillary thyroid cancer. J. Exp. Clin. Cancer Res. 2020, 39, 245. [Google Scholar] [CrossRef]
- Kebebew, E.; Peng, M.; Reiff, E.; Duh, Q.Y.; Clark, O.H.; McMillan, A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann. Surg. 2005, 242, 353–361. [Google Scholar] [CrossRef]
- Binabaj, M.M.; Soleimani, A.; Rahmani, F.; Avan, A.; Khazaei, M.; Fiuji, H.; Soleimanpour, S.; Ryzhikov, M.; Ferns, G.A.; Bahrami, A.; et al. Prognostic value of high mobility group protein A2 (HMGA2) over-expression in cancer progression. Gene 2019, 706, 131–139. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Y.; Zhao, D.; Zou, Q.; Yu, F.; Zhang, L.; Xu, L. Comprehensive Analysis Revealed that CDKN2A is a Biomarker for Immune Infiltrates in Multiple Cancers. Front. Cell Dev. Biol. 2021, 9, 808208. [Google Scholar] [CrossRef]
- Titov, S.E.; Ivanov, M.K.; Demenkov, P.S.; Katanyan, G.A.; Kozorezova, E.S.; Malek, A.V.; Veryaskina, Y.A.; Zhimulev, I.F. Combined quantitation of HMGA2 mRNA, microRNAs, and mitochondrial-DNA content enables the identification and typing of thyroid tumors in fine-needle aspiration smears. BMC Cancer 2019, 19, 1010. [Google Scholar] [CrossRef]
- Titov, S.E.; Ivanov, M.K.; Karpinskaya, E.V.; Tsivlikova, E.V.; Shevchenko, S.P.; Veryaskina, Y.A.; Akhmerova, L.G.; Poloz, T.L.; Klimova, O.A.; Gulyaeva, L.F.; et al. miRNA profiling, detection of BRAF V600E mutation and RET-PTC1 translocation in patients from Novosibirsk oblast (Russia) with different types of thyroid tumors. BMC Cancer 2016, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Maggisano, V.; Capriglione, F.; Verrienti, A.; Celano, M.; Gagliardi, A.; Bulotta, S.; Sponziello, M.; Mio, C.; Pecce, V.; Durante, C.; et al. Identification of Exosomal microRNAs and Their Targets in Papillary Thyroid Cancer Cells. Biomedicines 2022, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, S.L. C4.5: Programs for Machine Learning; Quinlan, J.R., Ed.; Morgan Kaufmann Publishers, Inc.: Burlington, MA, USA, 1993; Volume 16, pp. 235–240. [Google Scholar]
- Li, X.; Cheng, R. TPO as an indicator of lymph node metastasis and recurrence in papillary thyroid carcinoma. Sci. Rep. 2023, 13, 10848. [Google Scholar] [CrossRef] [PubMed]
- Massart, C.; Hoste, C.; Virion, A.; Ruf, J.; Dumont, J.E.; Van Sande, J. Cell biology of H2O2 generation in the thyroid: Investigation of the control of dual oxidases (DUOX) activity in intact ex vivo thyroid tissue and cell lines. Mol. Cell. Endocrinol. 2011, 343, 32–44. [Google Scholar] [CrossRef]
- Huang, F.; Xiao, J.; Wang, L.; Xie, Y.; Jia, H. Relationships between SLC26A7 expressions and extra-thyroid metastasis of papillary thyroid carcinoma. Chin. Med. J. 2021, 135, 225–227. [Google Scholar] [CrossRef]
- Jahan, R.; Shah, A.; Kisling, S.G.; Macha, M.A.; Thayer, S.; Batra, S.K.; Kaur, S. Odyssey of trefoil factors in cancer: Diagnostic and therapeutic implications. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188362. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Zhang, J.; Liu, Y.; Ji, T.; Mou, J.; Fang, X.; Wang, S.; Chen, J. Low expression of TFF3 in papillary thyroid carcinoma may correlate with poor prognosis but high immune cell infiltration. Future Oncol. 2022, 18, 333–348. [Google Scholar] [CrossRef]
- Kushwaha, P.P.; Rapalli, K.C.; Kumar, S. Geminin a multi task protein involved in cancer pathophysiology and developmental process: A review. Biochimie 2016, 131, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Liggett, W.H.; Jr Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Nikiforov, N.G.; Zhuravlev, A.D.; Orekhov, N.A.; Mikhaleva, L.M.; Orekhov, A.N. The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment. Int. J. Mol. Sci. 2021, 23, 460. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.; Coelho, M.J.; Eloy, C.; Melo, M.; da Rocha, A.G.; Pestana, A.; Batista, R.; Ferreira, L.B.; Rios, E.; Selmi-Ruby, S.; et al. NIS expression in thyroid tumors, relation with prognosis clinicopathological and molecular features. Endocr. Connect. 2018, 7, 78–90. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Kloppel, G.; Rosai, J. World Health Organization Classification of Tumours of Endocrine Organs, 4th ed.; IARC Press: Lyon, France, 2017; Volume 10. [Google Scholar]
- Kazaure, H.S.; Roman, S.A.; Sosa, J.A. Aggressive variants of papillary thyroid cancer: Incidence, characteristics and predictors of survival among 43,738 patients. Ann. Surg. Oncol. 2012, 19, 1874–1880. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Biddinger, P.W.; Thompson, L.D. Diagnostic Pathology and Molecular Genetics of the Thyroid; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- GTEx Portal (dbGaP Accession phs000424.v8.p2). Available online: https://www.gtexportal.org/home/gene/TFF3 (accessed on 22 November 2024).
- Cangul, H.; Liao, X.H.; Schoenmakers, E.; Kero, J.; Barone, S.; Srichomkwun, P.; Iwayama, H.; Serra, E.G.; Saglam, H.; Eren, E.; et al. Homozygous loss-of-function mutations in SLC26A7 cause goitrous congenital hypothyroidism. JCI Insight 2018, 3, e99631. [Google Scholar] [CrossRef]
- Ishii, J.; Suzuki, A.; Kimura, T.; Tateyama, M.; Tanaka, T.; Yazawa, T.; Arimasu, Y.; Chen, I.S.; Aoyama, K.; Kubo, Y.; et al. Congenital goitrous hypothyroidism is caused by dysfunction of the iodide transporter SLC26A7. Commun. Biol. 2019, 2, 270. [Google Scholar] [CrossRef]

| Characteristic | Value, n (%) | |
|---|---|---|
| PTC (n = 97) | PTC HG (n = 13) | |
| Age, median (Q1-Q3) | 47.5 (37–60.25) | 64 (48.75–74.25) |
| Sex ratio (M/F) | 21/76 | 3/10 |
| Metastasis to central cervical lymph nodes N1a | 26 (26.8%) | 2 (15.4%) |
| Metastasis to lateral cervical lymph nodes N1b | 19 (19.6%) | 6 (46.2%) |
| Multifocality | 58 (59.8%) | 3 (23%) |
| Extrathyroidal extension (macroscopic invasion) | 18 (18.6%) | 7 (53.8%) |
| Distant metastasis | 0 | 0 |
| Marker | p-Value |
|---|---|
| miR-146b | 0.452583 |
| miR-199b | 0.086416 |
| miR-221 | 0.744162 |
| miR-223 | 0.204396 |
| miR-31 | 0.067463 |
| miR-375 | 0.767790 |
| miR-551b | 0.629983 |
| miR-21 | 0.359206 |
| mtDNA | 0.206242 |
| FN1 | 0.142648 |
| GMNN | 0.028378 |
| CDKN2A | 0.004832 |
| TPO | 0.000850 |
| SLC26A7 | 0.000166 |
| HMGA2 | 0.558307 |
| CPQ | 0.007873 |
| SPATA18 | 0.012699 |
| APOE | 0.104936 |
| DIO1 | 0.929108 |
| NIS | 0.016281 |
| TFF3 | 0.000614 |
| TMPRSS4 | 0.547838 |
| TSHR | 0.458833 |
| Diagnostic Characteristics | Decision Tree | SLC26A7 |
|---|---|---|
| Sensitivity | 53.8% (25.1–80.8%) | 53.8% (25.1–80.8%) |
| Specificity | 100.0% (96.3–100.0%) | 87.6% (79.4–93.4%) |
| Accuracy | 94.5% (88.5–97.8%) | 83.6% (75.5–90.0%) |
| Positive predictive value | 100.0% (59.0–100.0%) | 36.8% (36.8–54.8%) |
| Negative predictive value | 94.2% (90.0–96.7%) | 93.4% (88.7–96.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titov, S.E.; Kozorezova, E.S.; Lukyanov, S.A.; Sergiyko, S.V.; Demenkov, P.S.; Veryaskina, Y.A.; Vorobyev, S.L.; Sleptsov, I.V.; Chernikov, R.A.; Timofeeva, N.I.; et al. Could SLC26A7 Be a Promising Marker for Preoperative Diagnosis of High-Grade Papillary Thyroid Carcinoma? Diagnostics 2024, 14, 2652. https://doi.org/10.3390/diagnostics14232652
Titov SE, Kozorezova ES, Lukyanov SA, Sergiyko SV, Demenkov PS, Veryaskina YA, Vorobyev SL, Sleptsov IV, Chernikov RA, Timofeeva NI, et al. Could SLC26A7 Be a Promising Marker for Preoperative Diagnosis of High-Grade Papillary Thyroid Carcinoma? Diagnostics. 2024; 14(23):2652. https://doi.org/10.3390/diagnostics14232652
Chicago/Turabian StyleTitov, Sergei E., Evgeniya S. Kozorezova, Sergei A. Lukyanov, Sergei V. Sergiyko, Pavel S. Demenkov, Yulia A. Veryaskina, Sergey L. Vorobyev, Ilya V. Sleptsov, Roman A. Chernikov, Natalia I. Timofeeva, and et al. 2024. "Could SLC26A7 Be a Promising Marker for Preoperative Diagnosis of High-Grade Papillary Thyroid Carcinoma?" Diagnostics 14, no. 23: 2652. https://doi.org/10.3390/diagnostics14232652
APA StyleTitov, S. E., Kozorezova, E. S., Lukyanov, S. A., Sergiyko, S. V., Demenkov, P. S., Veryaskina, Y. A., Vorobyev, S. L., Sleptsov, I. V., Chernikov, R. A., Timofeeva, N. I., Barashkova, S. V., Lushnikova, E. L., Uspenskaya, A. A., Zolotoukho, A. V., Romanova, O. V., & Zhimulev, I. F. (2024). Could SLC26A7 Be a Promising Marker for Preoperative Diagnosis of High-Grade Papillary Thyroid Carcinoma? Diagnostics, 14(23), 2652. https://doi.org/10.3390/diagnostics14232652






