MRI-Based Circumferential Strain in Boys with Early Duchenne Muscular Dystrophy Cardiomyopathy
Abstract
1. Introduction
2. Methods
2.1. Study Enrollment
2.2. CMR Imaging
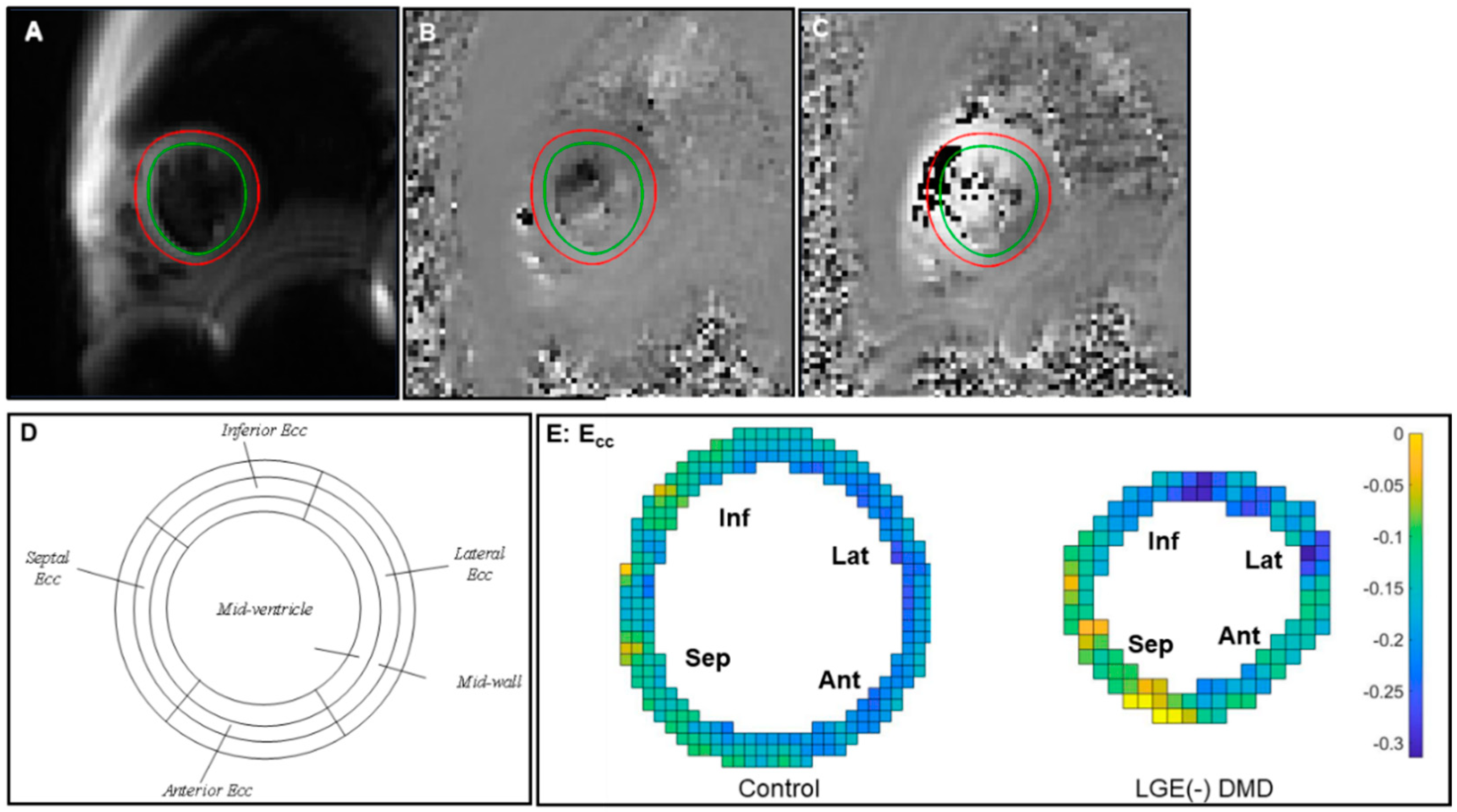
2.3. Post-Processing and Analysis
2.4. Statistics
3. Results
3.1. Demographics
3.2. LV and RV Volume and Function
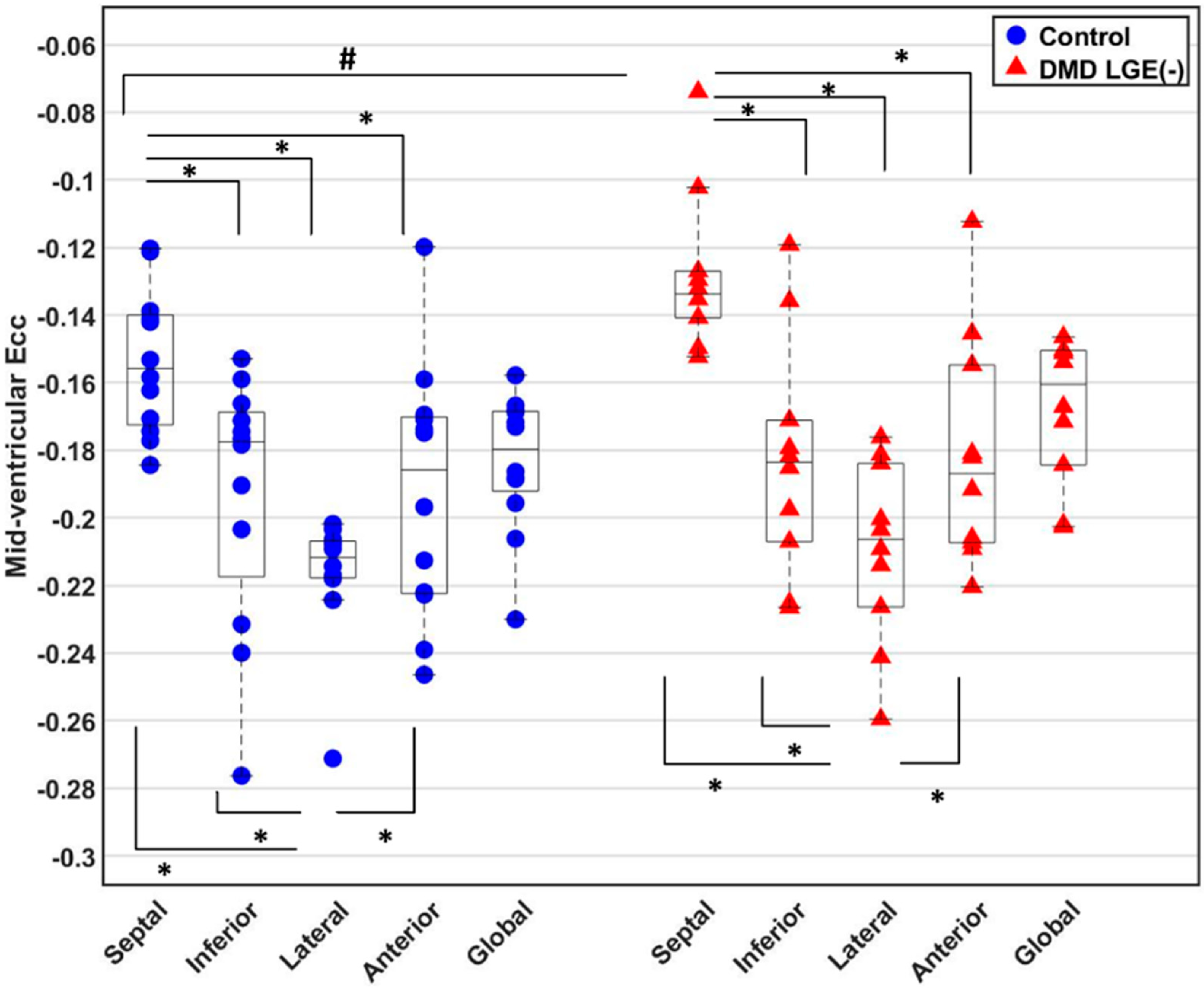
3.3. Global and Regional Ecc
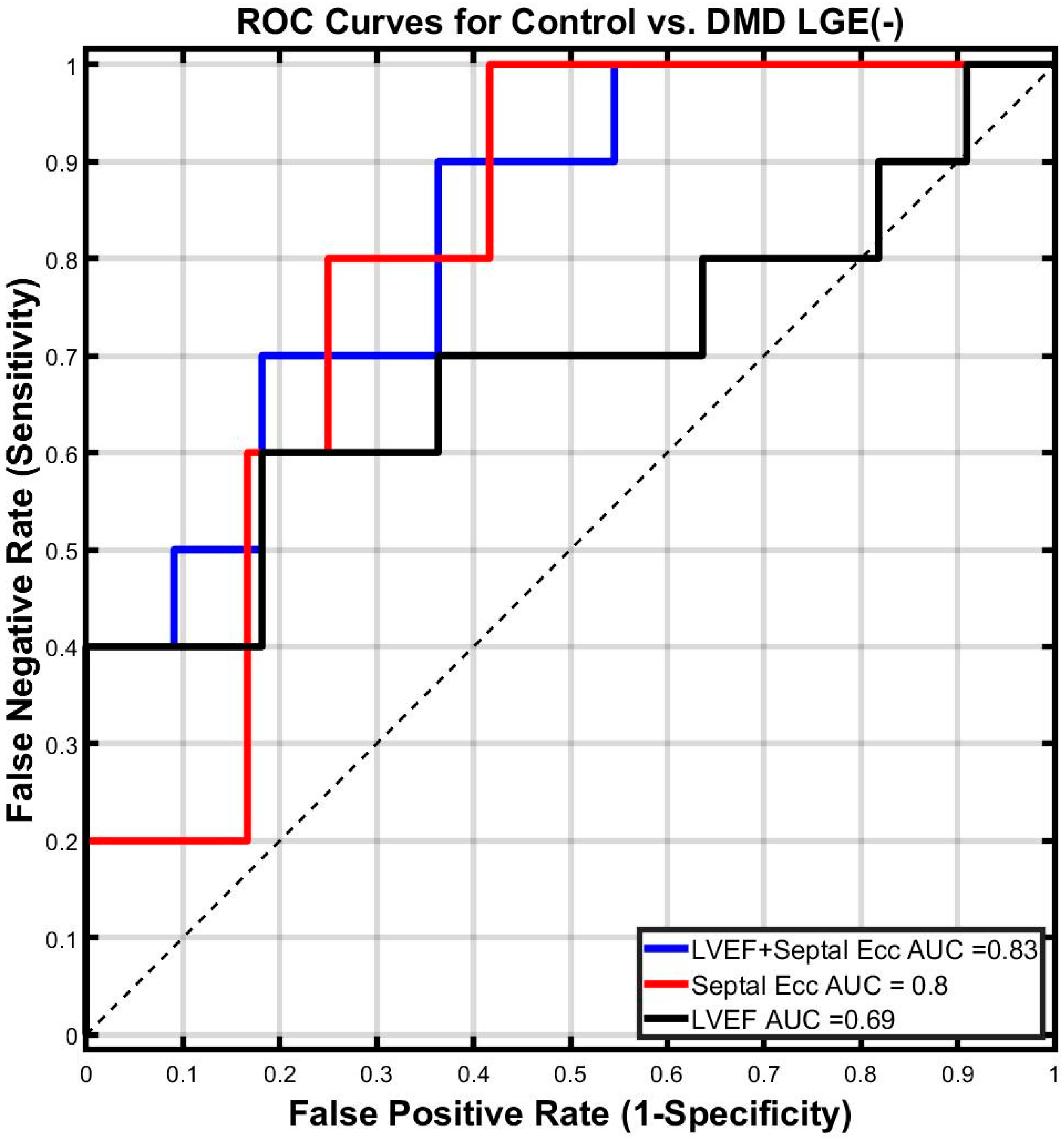
3.4. Binomial Logistic Regression
3.5. Best Fit Regression Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNally, E.M.; Kaltman, J.R.; Benson, D.W.; Canter, C.E.; Cripe, L.H.; Duan, D.; Finder, J.D.; Groh, W.J.; Hoffman, E.P.; Judge, D.P. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation 2015, 131, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. Lancet Neurol. 2010, 9, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Cheeran, D.; Khan, S.; Khera, R.; Bhatt, A.; Garg, S.; Grodin, J.L.; Morlend, R.; Araj, F.G.; Amin, A.A.; Thibodeau, J.T.; et al. Predictors of Death in Adults With Duchenne Muscular Dystrophy–Associated Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e006340. [Google Scholar] [CrossRef] [PubMed]
- Hor, K.N.; Taylor, M.D.; Al-Khalidi, H.R.; Cripe, L.H.; Raman, S.V.; Jefferies, J.L.; O’Donnell, R.; Benson, D.W.; Mazur, W. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: Effect of age and left ventricular systolic function. J. Cardiovasc. Magn. Reson. 2013, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Villa, C.R.; Hor, K.N.; Jefferies, J.L.; Gao, Z.; Towbin, J.A.; Wong, B.L.; Mazur, W.; Fleck, R.J.; Sticka, J.J. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in Duchenne muscular dystrophy. J. Am. Heart Assoc. 2015, 4, e001338. [Google Scholar] [CrossRef]
- Silva, M.C.; Meira, Z.M.A.; Giannetti, J.G.; da Silva, M.M.; Campos, A.F.O.; de Melo Barbosa, M.; Starling Filho, G.M.; de Aguiar Ferreira, R.; Zatz, M.; Rochitte, C.E. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J. Am. Coll. Cardiol. 2007, 49, 1874–1879. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Papavasiliou, A.; Giannakopoulou, K.; Markousis-Mavrogenis, G.; Pons, M.R.; Karanasios, E.; Nikas, I.; Papadopoulos, G.; Kolovou, G.; Chrousos, G.P. Oedema-fibrosis in Duchenne Muscular Dystrophy: Role of cardiovascular magnetic resonance imaging. Eur. J. Clin. Investig. 2017, 47, e12843. [Google Scholar] [CrossRef]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.V.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Influence of Ejection Fraction on Cardiovascular Outcomes in a Broad Spectrum of Heart Failure Patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef]
- Mehmood, M.; Hor, K.N.; Al-Khalidi, H.R.; Benson, D.W.; Jefferies, J.L.; Taylor, M.D.; Egnaczyk, G.F.; Raman, S.V.; Basu, S.K.; Cripe, L.H.; et al. Comparison of right and left ventricular function and size in Duchenne muscular dystrophy. Eur. J. Radiol. 2015, 84, 1938–1942. [Google Scholar] [CrossRef]
- Florian, A.; Ludwig, A.; Engelen, M.; Waltenberger, J.; Rösch, S.; Sechtem, U.; Yilmaz, A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J. Cardiovasc. Magn. Reson. 2014, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Magrath, P.; Maforo, N.; Renella, P.; Nelson, S.F.; Halnon, N.; Ennis, D.B. Cardiac MRI biomarkers for Duchenne muscular dystrophy. Biomark. Med. 2018, 12, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Hor, K.N.; Wansapura, J.; Markham, L.W.; Mazur, W.; Cripe, L.H.; Fleck, R.; Benson, D.W.; Gottliebson, W.M. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: A cardiac magnetic resonance tagging study. J. Am. Coll. Cardiol. 2009, 53, 1204–1210. [Google Scholar] [CrossRef]
- Ashford, M.; Liu, W.; Lin, S.; Abraszewski, P.; Caruthers, S.; Connolly, A.; Yu, X.; Wickline, S.A. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation 2005, 112, 2462–2467. [Google Scholar] [CrossRef]
- Ryan, T.D.; Taylor, M.D.; Mazur, W.; Cripe, L.H.; Pratt, J.; King, E.C.; Lao, K.; Grenier, M.A.; Jefferies, J.L.; Benson, D.W.; et al. Abnormal Circumferential Strain is Present in Young Duchenne Muscular Dystrophy Patients. Pediatr. Cardiol. 2013, 34, 1159–1165. [Google Scholar] [CrossRef]
- Hor, K.N.; Kissoon, N.; Mazur, W.; Gupta, R.; Ittenbach, R.F.; Al-Khalidi, H.R.; Cripe, L.H.; Raman, S.V.; Puchalski, M.D.; Gottliebson, W.M.; et al. Regional Circumferential Strain is a Biomarker for Disease Severity in Duchenne Muscular Dystrophy Heart Disease: A Cross-Sectional Study. Pediatr. Cardiol. 2015, 36, 111–119. [Google Scholar] [CrossRef]
- Lang, S.M.; Shugh, S.; Mazur, W.; Sticka, J.J.; Rattan, M.S.; Jefferies, J.L.; Taylor, M.D. Myocardial Fibrosis and Left Ventricular Dysfunction in Duchenne Muscular Dystrophy Carriers Using Cardiac Magnetic Resonance Imaging. Pediatr. Cardiol. 2015, 36, 1495–1501. [Google Scholar] [CrossRef]
- Lin, K.; Meng, L.; Collins, J.D.; Chowdhary, V.; Markl, M.; Carr, J.C. Reproducibility of cine displacement encoding with stimulated echoes (DENSE) in human subjects. Magn. Reson. Imaging 2017, 35, 148–153. [Google Scholar] [CrossRef]
- Wehner, G.J.; Suever, J.D.; Haggerty, C.M.; Jing, L.; Powell, D.K.; Hamlet, S.M.; Grabau, J.D.; Mojsejenko, W.D.; Zhong, X.; Epstein, F.H.; et al. Validation of in vivo, 2D displacements from spiral cine DENSE at 3T. J. Cardiovasc. Magn. Reson. 2015, 17, 5. [Google Scholar] [CrossRef]
- Zhong, X.; Spottiswoode, B.S.; Meyer, C.H.; Kramer, C.M.; Epstein, F.H. Imaging three-dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI. Magn. Reson. Med. 2010, 64, 1089–1097. [Google Scholar] [CrossRef]
- Kellman, P.; Chefd′hotel, C.; Lorenz, C.H.; Mancini, C.; Arai, A.E.; McVeigh, E.R. High spatial and temporal resolution cardiac cine MRI from retrospective reconstruction of data acquired in real time using motion correction and resorting. Magn. Reson. Med. 2009, 62, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Kellman, P.; LaRocca, G.; Arai, A.E.; Hansen, M.S. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. J. Cardiovasc. Magn. Reson. 2013, 15, 102. [Google Scholar] [CrossRef]
- Reyhan, M.L.; Wang, Z.; Kim, H.J.; Halnon, N.J.; Finn, J.P.; Ennis, D.B. Effect of free-breathing on left ventricular rotational mechanics in healthy subjects and patients with duchenne muscular dystrophy. Magn. Reson. Med. 2017, 77, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Kellman, P.; Larson, A.C.; Hsu, L.-Y.; Chung, Y.-C.; Simonetti, O.P.; McVeigh, E.R.; Arai, A.E. Motion-corrected free-breathing delayed enhancement imaging of myocardial infarction. Magn. Reson. Med. 2005, 53, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction in Perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, A.; Scott, A.; vanMaanen, D.; Suever, J. DENSEanalysis. 2016. Available online: https://github.com/denseanalysis/denseanalysis (accessed on 1 June 2016).
- Spottiswoode, B.S.; Zhong, X.; Lorenz, C.H.; Mayosi, B.M.; Meintjes, E.M.; Epstein, F.H. Motion-guided segmentation for cine DENSE MRI. Med. Image Anal. 2009, 13, 105–115. [Google Scholar] [CrossRef]
- Spottiswoode, B.S.; Zhong, X.; Hess, A.T.; Kramer, C.M.; Meintjes, E.M.; Mayosi, B.M.; Epstein, F.H. Tracking myocardial motion from cine DENSE images using spatiotemporal phase unwrapping and temporal fitting. IEEE Trans. Med. Imaging 2007, 26, 15–30. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Maceira, A.; Valsangiacomo-Buechel, E.R.; Vogel-Claussen, J.; Turkbey, E.B.; Williams, R.; Plein, S.; Tee, M.; Eng, J.; Bluemke, D.A. Normal values for cardiovascular magnetic resonance in adults and children. J. Cardiovasc. Magn. Reson. 2015, 17, 29. [Google Scholar] [CrossRef]
- Naeije, R.; Badagliacca, R. The overloaded right heart and ventricular interdependence. Cardiovasc. Res. 2017, 113, 1474–1485. [Google Scholar] [CrossRef]
- Kohl, P. Cardiac cellular heterogeneity and remodelling. Cardiovasc. Res. 2004, 64, 195–197. [Google Scholar] [CrossRef]
- Kvasnicka, J.; Vokrouhlický, L. Heterogeneity of the myocardium. Function of the left and right ventricle under normal and pathological conditions. Physiol. Res. 1991, 40, 31–37. [Google Scholar]
- Solovyova, O.; Katsnelson, L.B.; Kohl, P.; Panfilov, A.V.; Tsaturyan, A.K.; Tsyvian, P.B. Mechano-electric heterogeneity of the myocardium as a paradigm of its function. Prog. Biophys. Mol. Biol. 2016, 120, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Widmaier, E.; Raff, H.; Strang, K. Cardiovascular patterns in health and disease. In Vander’s Human Physiology: The Mechanism of Body Function, 10th ed.; Mc Graw Hill.: Boston, MA, USA, 2006. [Google Scholar]
- Saleh, S.; Liakopoulos, O.J.; Buckberg, G.D. The septal motor of biventricular function. Eur. J. Cardiothorac. Surg. 2006, 29 (Suppl. S1), S126–S138. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, D.; Hedström, E.; Jögi, J.; Pahlm, U.; Steding-Ehrenborg, K.; Engblom, H.; Arheden, H.; Carlsson, M. Longitudinal shortening remains the principal component of left ventricular pumping in patients with chronic myocardial infarction even when the absolute atrioventricular plane displacement is decreased. BMC Cardiovasc. Disord. 2017, 17, 208. [Google Scholar] [CrossRef]
- Meyers, T.A.; Townsend, D. Early right ventricular fibrosis and reduction in biventricular cardiac reserve in the dystrophin-deficient mdx heart. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H303–H315. [Google Scholar] [CrossRef]
- Larcher, T.; Lafoux, A.; Tesson, L.; Remy, S.; Thepenier, V.; François, V.; Le Guiner, C.; Goubin, H.; Dutilleul, M.; Guigand, L.; et al. Characterization of Dystrophin Deficient Rats: A New Model for Duchenne Muscular Dystrophy. PLoS ONE 2014, 9, e110371. [Google Scholar] [CrossRef]
- Moher, D.; Schulz, K.F.; Altman, D.; CONSORT Group. The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials. JAMA 2001, 285, 1987–1991. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression modeling strategies. BIOS 2017, 330, 14. [Google Scholar]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- McCullagh, P. Generalized Linear Models; Routledge: London, UK, 2018. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
| Controls N = 12 | DMD N = 10 | |
|---|---|---|
| Age (years) | 13 (4.0) range (9–21) | 12.5 (6.0) range (9–21) |
| Male (%) | 100% | 100% |
| Height * (cm) | 165 (22) | 133 (18) |
| Weight (kg) | 51 (18) | 46 (28) |
| BMI (kg/m2) | 18.7 (6.7) | 25.7 (12.6) |
| BSA * (m2) | 1.53 (0.37) | 1.27 (0.49) |
| Heart Rate (bpm) | 78 (30) | 93 (23) |
| Ambulatory (%) | 12 (100%) | 3 (30%) |
| Ventilatory Support (%) | 0% | 0% |
| Control N = 12 | DMD LGE (-) N = 10 | p-Value | Control N = 12 | DMD LGE (-) N = 10 | p-Value | ||
|---|---|---|---|---|---|---|---|
| LVEF (%) | 58 (4) | 55 (10) | 0.149 | RVEF (%) | 54 (8) | 54 (9) | 0.921 |
| LVEDVi (mL/m2) | 84 (17) | 87 (25) | 0.972 | RVEDVi (mL/m2) | 83 (22) | 81 (36) | 0.249 |
| LVESVi (mL/m2) | 36 (5) | 40 (15) | 0.699 | RVESVi (mL/m2) | 39 (11) | 34 (20) | 0.223 |
| LVMi (g/m2) | 38 (14) | 32 (12) | 0.062 | RVMi (g/m2) | 31 (7) | 25 (10) | 0.199 |
| LVEDV (mL) | 141 (64) | 93 (33) | 0.057 | RVEDV (mL) | 142 (53) | 87 (32) | 0.004 * |
| LVESV (mL) | 59 (25) | 42 (17) | 0.149 | RVESV (mL) | 61 (18) | 37 (18) | 0.004 * |
| LVM (g) | 57 (45) | 39 (12) | 0.008 | RVM (g) | 49 (17) | 31 (13) | 0.006 * |
| All Available Predictors in the Study | Age, HR, Height, Weight, BSA, BMI, LVM, LVMi, LVESV, LVEDV, LVEF, LVESVi, LVEDVi, RVM, RVMi, RVESV, RVEDV, RVEF, RVESVi, RVEDVi | |
| Step 1: Exclude derivable predictors | ||
| Remaining Predictors | Age, HR, Height, Weight, BSA−1, BMI, LVM, LVEDV, RVM, LVEF, RVEDV, RVEF | |
| Step 2: Calculate predictor-by-group effect for each predictor | ||
| Regression Model | Pooled Septal Ecc ~ constant + group + X + group × X | |
| Predictors (X) | Interaction Term (group × X) | |
| Coefficients | p-value | |
| LVEF | 0.006 | 0.034 * |
| LVEDV | −0.00075 | 0.019 * |
| RVEDV | −0.00068 | 0.044 * |
| Remaining Predictors | LVEF, LVEDV, RVEDV | |
| Step 3: Calculate inter-predictor correlations | ||
| Predictors | R2 | |
| LVEF & LVEDV | 0.014 | |
| LVEF & RVEDV | 0.021 | |
| LVEDV & RVEDV | 0.500 | |
| Remaining Predictors | LVEF, LVEDV, RVEDV | |
| Step 4: Perform stepwise backward regression using the Akaike information criterion | ||
| Best Fitting Regression Model | Pooled Septal Ecc ~ Group + LVEF + LVEDV + RVEDV + LVEDV×RVEDV + LVEF × Group | |
| Terms | Coefficients | p-value |
| (constant) | 0.7329 | 0.012 * |
| Group | −0.3647 | 0.013 * |
| LVEF | −0.0130 | 0.008 * |
| LVEDV | −0.0015 | 0.011 * |
| RVEDV | −0.0011 | 0.010 * |
| LVEDV × RVEDV | 0.00001 | 0.009 * |
| LVEF × Group | 0.0067 | 0.010 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-Q.; Maforo, N.G.; Magrath, P.; Prosper, A.; Renella, P.; Halnon, N.; Wu, H.H.; Ennis, D.B. MRI-Based Circumferential Strain in Boys with Early Duchenne Muscular Dystrophy Cardiomyopathy. Diagnostics 2024, 14, 2673. https://doi.org/10.3390/diagnostics14232673
Liu Z-Q, Maforo NG, Magrath P, Prosper A, Renella P, Halnon N, Wu HH, Ennis DB. MRI-Based Circumferential Strain in Boys with Early Duchenne Muscular Dystrophy Cardiomyopathy. Diagnostics. 2024; 14(23):2673. https://doi.org/10.3390/diagnostics14232673
Chicago/Turabian StyleLiu, Zhan-Qiu, Nyasha G. Maforo, Patrick Magrath, Ashley Prosper, Pierangelo Renella, Nancy Halnon, Holden H. Wu, and Daniel B. Ennis. 2024. "MRI-Based Circumferential Strain in Boys with Early Duchenne Muscular Dystrophy Cardiomyopathy" Diagnostics 14, no. 23: 2673. https://doi.org/10.3390/diagnostics14232673
APA StyleLiu, Z.-Q., Maforo, N. G., Magrath, P., Prosper, A., Renella, P., Halnon, N., Wu, H. H., & Ennis, D. B. (2024). MRI-Based Circumferential Strain in Boys with Early Duchenne Muscular Dystrophy Cardiomyopathy. Diagnostics, 14(23), 2673. https://doi.org/10.3390/diagnostics14232673






