Assessing the Agreement Between Diffusion Tension Imaging (DTI) and T2-Weighted MRI Sequence for Biometry of the Fetal Corpus Callosum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MR Imaging Technique
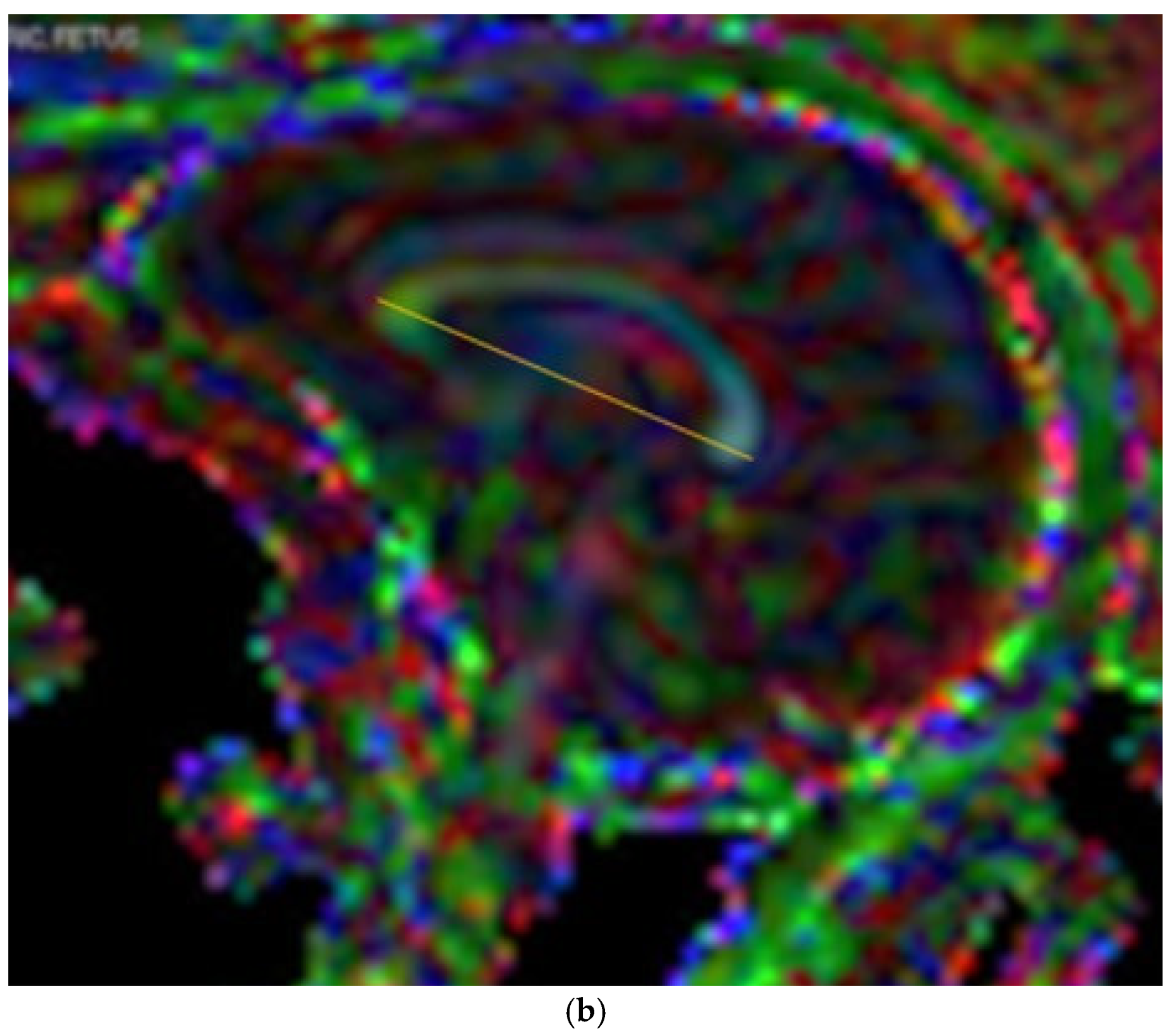
2.3. Measurements
2.4. Stastistical Analysis
3. Results
3.1. Inter-Observer and Intra-Observer Agreement
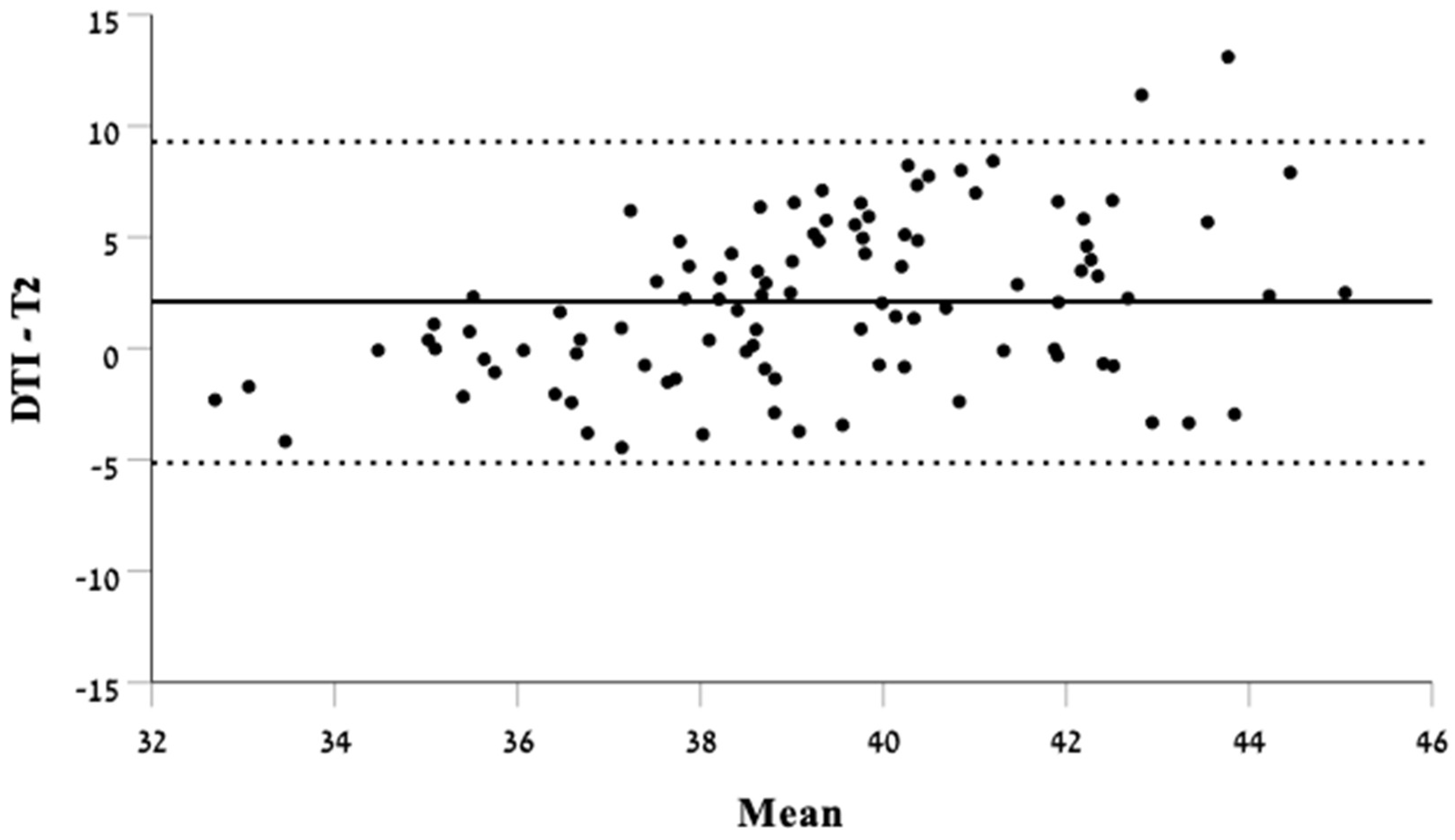
3.2. Biometry Measurements in T2-Weighted Imaging Compared with DTI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Innocenti, G.M.; Schmidt, K.; Milleret, C.; Fabri, M.; Knyazeva, M.G.; Battaglia-Mayer, A.; Aboitiz, F.; Ptito, M.; Caleo, M.; Marzi, C.A.; et al. The Functional Characterization of Callosal Connections. Prog. Neurobiol. 2022, 208, 102186. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Taherian, R.; Tahmasebpour, A.R.; Sanei Taheri, M.; Kazemi, M.A.; Pak, N.; Shirazi, M.; Radmanesh, A.; Oztekin, O.; Arab-Ahmadi, M. Fetal Corpus Callosum Abnormalities: Ultrasound and Magnetic Resonance Imaging Role. J. Clin. Ultrasound 2022, 50, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Malinger, G.; Zakut, H. The Corpus Callosum: Normal Fetal Development as Shown by Transvaginal Sonography. Am. J. Roentgenol. 1993, 161, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Marsh, S.; Swistun, M.D.; Al-Gazali, M.L.; Zaki, M.M.; Abdel-Salam, G.; Al-Tawari, A.; Bastaki, L.; Kayserili, H.; Rajab, A.; et al. Distinguishing 3 Classes of Corpus Callosal Abnormalities in Consanguineous Families. Neurology 2011, 76, 373–382. [Google Scholar] [CrossRef]
- Tsur, A.; Weisz, B.; Rosenblat, O.; Shai, D.; Derazne, E.; Stevenson, D.K.; Achiron, R.; Katorza, E. The Journal of Maternal-Fetal & Neonatal Medicine Personalized Charts for the Fetal Corpus Callosum Length. J. Matern. Neonatal Med. 2018, 32, 3931–3938. [Google Scholar] [CrossRef]
- Achiron, R.; Achiron, A. Development of the Human Fetal Corpus Callosum: A High-Resolution, Cross-Sectional Sonographic Study. Ultrasound Obstet. Gynecol. 2001, 18, 343–347. [Google Scholar] [CrossRef]
- Mahallati, H.; Sotiriadis, A.; Celestin, C.; Millischer, A.E.; Sonigo, P.; Grevent, D.; O’Gorman, N.; Bahi-Buisson, N.; Attié-Bitach, T.; Ville, Y.; et al. Heterogeneity in Defining Fetal Corpus Callosal Pathology: Systematic Review. Ultrasound Obstet. Gynecol. 2021, 58, 11–18. [Google Scholar] [CrossRef]
- De Keersmaecker, B.; Jansen, K.; Aertsen, M.; Naulaers, G.; De Catte, L. Outcome of Partial Agenesis of Corpus Callosum. Am. J. Obstet. Gynecol. 2023, 230, 456.e1–456.e9. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q. Magnetic Resonance Imaging (MRI) Diagnosis of Fetal Corpus Callosum Abnormalities and Follow-up Analysis. J. Child Neurol. 2021, 36, 1017–1026. [Google Scholar] [CrossRef]
- Greenbaum, L.; Maya, I.; Sagi-Dain, L.; Sukenik-Halevy, R.; Berkenstadt, M.; Yonath, H.; Rienstein, S.; Shalata, A.; Katorza, E.; Singer, A. Chromosomal Microarray Analysis in Pregnancies With Corpus Callosum or Posterior Fossa Anomalies. Neurol. Genet. 2021, 7, e585. [Google Scholar] [CrossRef]
- Paules, C.; Miranda, J.; Policiano, C.; Crovetto, F.; Youssef, L.; Hahner, N.; Nakaki, A.; Crispi, F.; Gratacós, E.; Eixarch, E. Fetal Neurosonography Detects Differences in Cortical Development and Corpus Callosum in Late-Onset Small Fetuses. Ultrasound Obstet. Gynecol. 2021, 58, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Garel, C. Magnetic Resonance Imaging Examination of the Fetal Brain. Ultrasound Obstet. Gynecol. 2007, 30, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Weissbach, T.; Massarwa, A.; Hadi, E.; Lev, S.; Haimov, A.; Katorza, E.; Brenner-Weissmann, A.; Krampl-Bettelheim, E.; Kasprian, G.; Sharon, R.; et al. Early Fetal Corpus Callosum: Demonstrating Normal Growth and Detecting Pathologies in Early Pregnancy. Am. J. Neuroradiol. 2023, 44, 199–204. [Google Scholar] [CrossRef] [PubMed]
- José, H.; Milani, F.; Quindere De Sá Barreto, E.; Araujo Júnior, E.; Borges Peixoto, A.; Marcondes, L.; Nardozza, M.; Fernandes Moron, A.; Araujo, E.; Rua Belchior De Azevedo, J. Ultrasonographic Evaluation of the Fetal Central Nervous System: Review of Guidelines. Radiol. Bras. 2019, 52, 176–181. [Google Scholar] [CrossRef]
- Tilea, B.; Alberti, C.; Adamsbaum, C.; Armoogum, P.; Oury, J.F.; Cabrol, D.; Sebag, G.; Kalifa, G.; Garel, C. Cerebral Biometry in Fetal Magnetic Resonance Imaging: New Reference Data. Ultrasound Obstet. Gynecol. 2009, 33, 173–181. [Google Scholar] [CrossRef]
- Glenn, O.A.; Barkovich, J. Magnetic Resonance Imaging of the Fetal Brain and Spine: An Increasingly Important Tool in Prenatal Diagnosis: Part 2. Am. J. Neuroradiol. 2006, 27, 1807–1814. [Google Scholar]
- Griffiths, P.D.; Bradburn, M.; Campbell, M.J.; Cooper, C.L.; Embleton, N.; Graham, R.; Hart, A.R.; Jarvis, D.; Kilby, M.D.; Lie, M.; et al. MRI in the Diagnosis of Fetal Developmental Brain Abnormalities: The MERIDIAN Diagnostic Accuracy Study. Health Technol. Assess. 2019, 23, 1–144. [Google Scholar] [CrossRef]
- Rüland, A.M.; Berg, C.; Gembruch, U.; Geipel, A. Prenatal Diagnosis of Anomalies of the Corpus Callosum over a 13-Year Period. Ultraschall Med. 2016, 37, 598–603. [Google Scholar] [CrossRef]
- Blondiaux, E.; Garel, C. Fetal Cerebral Imaging-Ultrasound vs. MRI: An Update. Acta Radiol. 2013, 54, 1046–1054. [Google Scholar] [CrossRef]
- Lamon, S.; De Dumast, P.; Sanchez, T.; Dunet, V.; Pomar, L.; Vial, Y.; Koob, M.; Bach Cuadra, M. Assessment of Fetal Corpus Callosum Biometry by 3D Super-Resolution Reconstructed T2-Weighted Magnetic Resonance Imaging. Front. Neurol. 2024, 15, 1358741. [Google Scholar] [CrossRef]
- Bookstein, S.; Nachmias, N.; Katorza, E. Agreement between Fetal Brain Ultrasonography and Magnetic Resonance Imaging in the Measurements of the Corpus Callosum and Transverse Cerebellar Diameter. Diagnostics 2024, 14, 366. [Google Scholar] [CrossRef] [PubMed]
- Manevich-Mazor, M.; Weissmann-Brenner, A.; Bar Yosef, O.; Hoffmann, C.; Mazor, R.; Mosheva, M.; Achiron, R.; Katorza, E. Added Value of Fetal MRI in the Evaluation of Fetal Anomalies of the Corpus Callosum: A Retrospective Analysis of 78 Cases. Ultraschall Med.-Eur. J. Ultrasound 2018, 39, 513–525. [Google Scholar] [CrossRef]
- Al-Mukhtar, A.; Kasprian, G.; Schmook, M.T.; Brugger, P.C.; Prayer, D. Diagnostic Pitfalls in Fetal Brain MRI. Semin Perinatol 2009, 33, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Pasternak, O. Diffusion Tensor Imaging (DTI)-Based White Matter Mapping in Brain Research: A Review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Hüppi, P.S. Cortical Development in the Fetus and the Newborn Advanced MR Techniques. Top. Magn. Reson. Imaging 2011, 22, 33–38. [Google Scholar] [CrossRef]
- Chen, R.; Sun, C.; Liu, T.; Liao, Y.; Wang, J.; Sun, Y.; Zhang, Y.; Wang, G.; Wu, D. Deciphering the Developmental Order and Microstructural Patterns of Early White Matter Pathways in a Diffusion MRI Based Fetal Brain Atlas. Neuroimage 2022, 264, 119700. [Google Scholar] [CrossRef]
- Jakab, A.; Pogledic, I.; Schwartz, E.; Gruber, G.; Mitter, C.; Brugger, P.C.; Langs, G.; Schöpf, V.; Kasprian, G.; Prayer, D. Fetal Cerebral Magnetic Resonance Imaging Beyond Morphology. Semin. Ultrasound CT MRI 2015, 36, 465–475. [Google Scholar] [CrossRef]
- Millischer, A.E.; Grevent, D.; Sonigo, P.; Bahi-Buisson, N.; Desguerre, I.; Mahallati, H.; Bault, J.P.; Quibel, T.; Couderc, S.; Moutard, M.L.; et al. Feasibility and Added Value of Fetal DTI Tractography in the Evaluation of an Isolated Short Corpus Callosum: Preliminary Results. Am. J. Neuroradiol. 2022, 43, 132–138. [Google Scholar] [CrossRef]
- Song, J.W.; Gruber, G.M.; Patsch, J.M.; Seidl, R.; Prayer, D.; Kasprian, G. How Accurate Are Prenatal Tractography Results? A Postnatal in Vivo Follow-up Study Using Diffusion Tensor Imaging. Pediatr. Radiol. 2018, 48, 486–498. [Google Scholar] [CrossRef]
- Jakab, A.; Tuura, R.; Kellenberger, C.; Scheer, I. In Utero Diffusion Tensor Imaging of the Fetal Brain: A Reproducibility Study. Neuroimage Clin. 2017, 15, 601–612. [Google Scholar] [CrossRef]
- Santirocco, M.; Rodó, C.; Illescas, T.; Vázquez, É.; Garrido, M.; Higueras, T.; Arévalo, S.; Maiz, N.; Carreras, E. Accuracy of Prenatal Ultrasound in the Diagnosis of Corpus Callosum Anomalies. J. Matern. Neonatal Med. 2021, 34, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kasprian, G.; Brugger, P.C.; Weber, M.; Krssák, M.; Krampl, E.; Herold, C.; Prayer, D. In Utero Tractography of Fetal White Matter Development. Neuroimage 2008, 43, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Harreld, J.H.; Bhore, R.; Chason, D.P.; Twickler, D.M. Corpus Callosum Length by Gestational Age as Evaluated by Fetal MR Imaging. Am. J. Neuroradiol. 2011, 32, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Corroenne, R.; Grevent, D.; Kasprian, G.; Stirnemann, J.; Ville, Y.; Mahallati, H.; Salomon, L.J. Corpus Callosal Reference Ranges: Systematic Review of Methodology of Biometric Chart Construction and Measurements Obtained. Ultrasound Obstet. Gynecol. 2023, 62, 175–184. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Gafner, M.; Kedar Sade, E.; Barzilay, E.; Katorza, E. Sexual Dimorphism of the Fetal Brain Biometry: An MRI-Based Study. Arch. Gynecol. Obstet. 2023, 308, 1257–1262. [Google Scholar] [CrossRef]
- Gerke, O. Reporting Standards for a Bland–Altman Agreement Analysis: A Review of Methodological Reviews. Diagnostics 2020, 10, 334. [Google Scholar] [CrossRef]
- Van Doorn, M.; Oude Rengerink, K.; Newsum, E.A.; Reneman, L.; Majoie, C.B.; Pajkrt, E. Added Value of Fetal MRI in Fetuses with Suspected Brain Abnormalities on Neurosonography: A Systematic Review and Meta-Analysis. J. Matern. Neonatal Med. 2016, 29, 2949–2961. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, S.; Sharma, R.; Gadodia, A.; Roy, K.K.; Sharma, J.B. The Role of Magnetic Resonance Imaging in Fetal Renal Anomalies. Int. J. Gynecol. Obstet. 2010, 111, 209–212. [Google Scholar] [CrossRef]
- Cassart, M.; Massez, A.; Metens, T.; Rypens, F.; Lambot, M.; Hall, M.; Avni, F.E. Complementary Role of MRI After Sonography in Assessing Bilateral Urinary Tract Anomalies in the Fetus. Am. J. Roentgenol. 2004, 182, 689–695. [Google Scholar] [CrossRef]
- Jiang, S.; Xue, H.; Counsell, S.; Anjari, M.; Allsop, J.; Rutherford, M.; Rueckert, D.; Hajnal, J.V. Diffusion Tensor Imaging (DTI) of the Brain in Moving Subjects: Application to in-Utero Fetal and Ex-Utero Studies. Magn. Reson. Med. 2009, 62, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Brugger, P.C.; Stuhr, F.; Lindner, C.; Prayer, D. Methods of Fetal MR: Beyond T2-Weighted Imaging. Eur. J. Radiol. 2006, 57, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Uus, A.U.; Collado, A.E.; Roberts, T.A.; Hajnal, J.V.; Rutherford, M.A.; Deprez, M. Retrospective Motion Correction in Foetal MRI for Clinical Applications: Existing Methods, Applications and Integration into Clinical Practice. Br. J. Radiol. 2023, 96, 20220071. [Google Scholar] [CrossRef] [PubMed]


| Indication | Count | Percentage (%) |
|---|---|---|
| CMV Seroconversion | 32 | 32% |
| Small Head Circumference | 11 | 11% |
| Ventricular Asymmetry | 7 | 7% |
| Genetic Findings | 6 | 6% |
| Heart Defect | 6 | 6% |
| Parvo Virus Infection with or without Fetal Anemia | 5 | 5% |
| IUGR | 4 | 4% |
| Bilateral Club Foot | 2 | 2% |
| Polyhydramnios | 2 | 2% |
| Short CC | 2 | 2% |
| Toxoplasma Exposure | 2 | 2% |
| Other | 21 | 21% |
| ICC | 95% CI | Significance | |
|---|---|---|---|
| Inter-observer agreement (2 observers) | |||
| CC biometry with DTI (mm) | 0.963 | 0.925–0.982 | <0.001 |
| CC biometry with T2 (mm) | 0.876 | 0.763–0.937 | <0.001 |
| Intra-observer agreement | |||
| CC biometry with DTI (mm) | 0.967 | 0.933–0.984 | <0.001 |
| CC biometry with T2 (mm) | 0.942 | 0.884–0.971 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohn, L.N.; Bookstein, S.; Laytman Klein, T.; Mordenfeld Kozlovsky, N.; Ziv-Baran, T.; Mayer, A.; Katorza, E. Assessing the Agreement Between Diffusion Tension Imaging (DTI) and T2-Weighted MRI Sequence for Biometry of the Fetal Corpus Callosum. Diagnostics 2024, 14, 2700. https://doi.org/10.3390/diagnostics14232700
Cohn LN, Bookstein S, Laytman Klein T, Mordenfeld Kozlovsky N, Ziv-Baran T, Mayer A, Katorza E. Assessing the Agreement Between Diffusion Tension Imaging (DTI) and T2-Weighted MRI Sequence for Biometry of the Fetal Corpus Callosum. Diagnostics. 2024; 14(23):2700. https://doi.org/10.3390/diagnostics14232700
Chicago/Turabian StyleCohn, Liel N., Shai Bookstein, Tamar Laytman Klein, Nadia Mordenfeld Kozlovsky, Tomer Ziv-Baran, Arnaldo Mayer, and Eldad Katorza. 2024. "Assessing the Agreement Between Diffusion Tension Imaging (DTI) and T2-Weighted MRI Sequence for Biometry of the Fetal Corpus Callosum" Diagnostics 14, no. 23: 2700. https://doi.org/10.3390/diagnostics14232700
APA StyleCohn, L. N., Bookstein, S., Laytman Klein, T., Mordenfeld Kozlovsky, N., Ziv-Baran, T., Mayer, A., & Katorza, E. (2024). Assessing the Agreement Between Diffusion Tension Imaging (DTI) and T2-Weighted MRI Sequence for Biometry of the Fetal Corpus Callosum. Diagnostics, 14(23), 2700. https://doi.org/10.3390/diagnostics14232700






