Significance of Detecting Serum Antibodies to Outer Surface Protein A of Lyme Disease Borreliae in PCR-Confirmed Blood Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
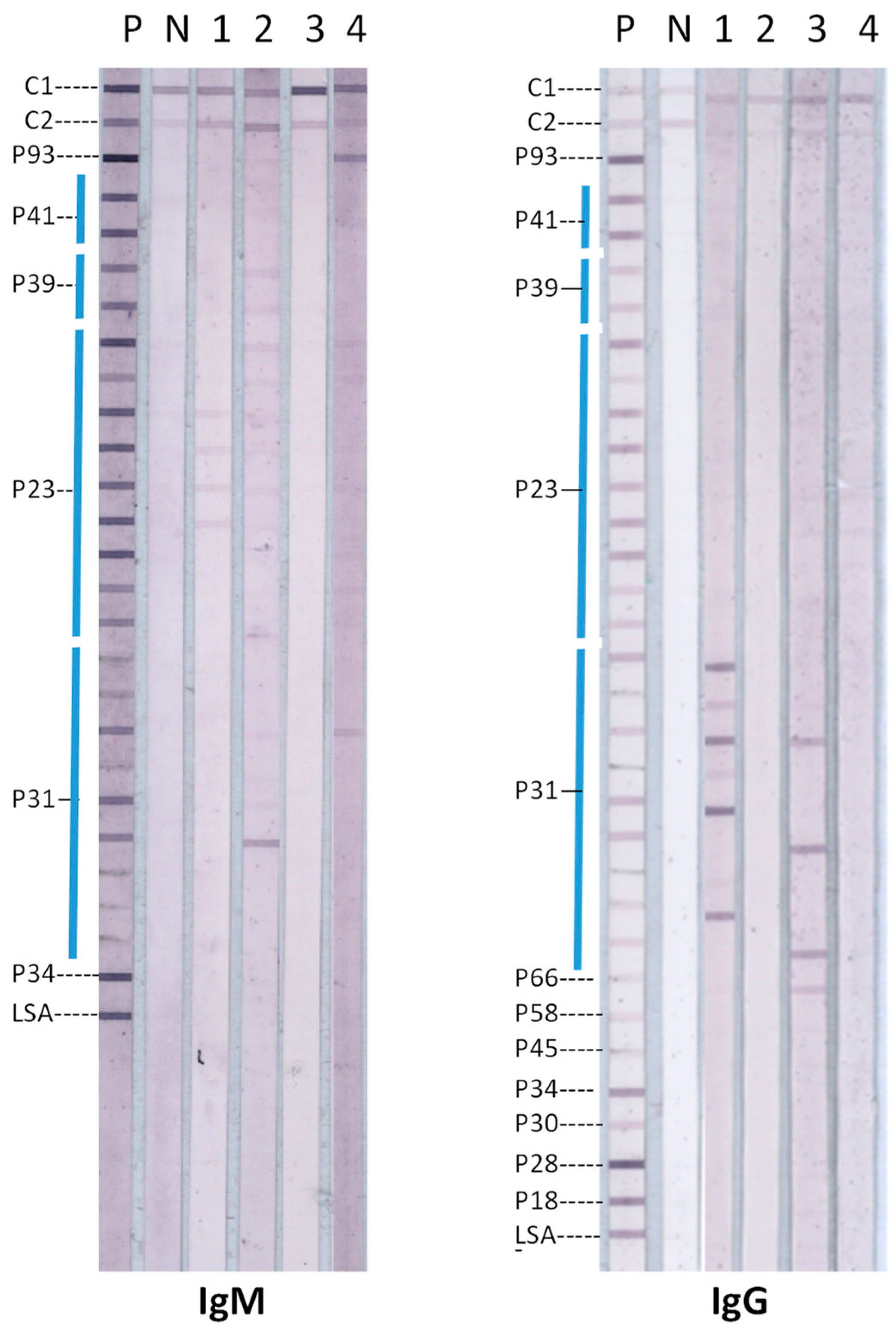
2.2. Lyme Disease Immunoblots
2.3. Ethical Considerations
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Lyme Disease. Available online: https://www.cdc.gov/lyme/index.html (accessed on 18 September 2024).
- Shah, J.S.; Burrascano, J.J.; Ramasamy, R. Recombinant protein immunoblots for differential diagnosis of tick-borne relapsing fever and Lyme disease. J. Vector Borne Dis. 2023, 60, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, D.A.; Oliver, J.H., Jr.; Kolbert, C.P.; Tullson, E.D.; Johnson, B.J.; Campbell, G.L.; Mitchell, P.D.; Reed, K.D.; Telford, S.R., 3rd; Anderson, J.F.; et al. Genetic heterogeneity of Borrelia burgdorferi in the United States. J. Infect. Dis. 1997, 175, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, P.; Fryland, L.; Börjesson, S.; Nordgren, J.; Bergström, S.; Ernerudh, J.; Forsberg, P.; Lindgren, P.E. Prevalence and diversity of Borrelia species in ticks that have bitten humans in Sweden. J. Clin. Microbiol. 2010, 48, 4169–4176, Erratum in J. Clin. Microbiol. 2011, 49, 481. [Google Scholar] [CrossRef] [PubMed]
- Branda, J.A.; Steere, A.C. Laboratory diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 2021, 34, e00018-19. [Google Scholar] [CrossRef]
- Hyde, J.A. Borrelia burgdorferi Keeps moving and carries on: A review of borrelial dissemination and invasion. Front. Immunol. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Tracy, K.E.; Baumgarth, N. Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Front. Immunol. 2017, 8, 116. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Notice to readers: Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morb. Mortal. Wkly. Rep. 1995, 44, 590–591. [Google Scholar]
- Barbour, A.G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J. Clin. Microbiol. 1988, 26, 475–478. [Google Scholar] [CrossRef]
- Li, H.; Dunn, J.J.; Luft, B.J.; Lawson, C.L. Crystal structure of Lyme disease antigen outer surface protein A complexed with a Fab. Proc. Natl. Acad. Sci. USA 1997, 94, 3584–3589. [Google Scholar] [CrossRef]
- Rudolph, M.J.; Davis, S.A.; Haque, H.M.E.; Ejemel, M.; Cavacini, L.A.; Vance, D.J.; Willsey, G.G.; Piazza, C.L.; Weis, D.D.; Wang, Y.; et al. Structure of a transmission blocking antibody in complex with outer surface protein A from the Lyme disease spirochete, Borreliella burgdorferi. Proteins 2023, 91, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.; de Silva, A.M.; Montgomery, R.R.; Fish, D.; Anguita, J.; Anderson, J.F.; Lobet, Y.; Fikrig, E. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 2000, 106, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Pal, U.; Alani, S.M.; Fikrig, E.; Norgard, M.V. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 2004, 199, 641–648. [Google Scholar] [CrossRef]
- Pal, U.; Li, X.; Wang, T.; Montgomery, R.R.; Ramamoorthi, N.; De Silva, A.M.; Bao, F.; Yang, X.; Pypaert, M.; Pradhan, D.; et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 2004, 119, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Piesman, J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 2000, 38, 382–388. [Google Scholar] [CrossRef]
- de Silva, A.M.; Telford, S.R., 3rd; Brunet, L.R.; Barthold, S.W.; Fikrig, E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 1996, 183, 271–275. [Google Scholar] [CrossRef]
- O’Bier, N.S.; Hatke, A.L.; Camire, A.C.; Marconi, R.T. Human and veterinary vaccines for Lyme disease. Curr. Issues Mol. Biol. 2021, 42, 191–222. [Google Scholar] [PubMed]
- Steere, A.C.; Sikand, V.K.; Meurice, F.; Parenti, D.L.; Fikrig, E.; Schoen, R.T.; Nowakowski, J.; Schmid, C.H.; Laukamp, S.; Buscarino, C.; et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N. Engl. J. Med. 1998, 339, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Comstedt, P.; Schueler, W.; Meinke, A.; Lundberg, U. The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes. PLoS ONE 2017, 12, e0184357. [Google Scholar] [CrossRef] [PubMed]
- Camire, A.C.; Hatke, A.L.; King, V.L.; Millership, J.; Ritter, D.M.; Sobell, N.; Weber, A.; Marconi, R.T. Comparative analysis of antibody responses to outer surface protein (Osp)A and OspC in dogs vaccinated with Lyme disease vaccines. Vet. J. 2021, 273, 105676. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cruz, I.D.; Ramos, C.C.; Taleon, P.; Ramasamy, R.; Shah, J. Pilot Study of immunoblots with recombinant Borrelia burgdorferi antigens for laboratory diagnosis of Lyme disease. Healthcare 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; D’Cruz, I.; Ward, S.; Harris, N.S.; Ramasamy, R. Development of a sensitive PCR-dot blot assay to supplement serological tests for diagnosing Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Woodman, M.E.; Cooley, A.E.; Stevenson, B. Production of outer surface protein A by Borrelia burgdorferi during transmission from infected mammals to feeding ticks is insufficient to trigger OspA seroconversion. FEMS Immunol. Med. Microbiol. 2008, 54, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Freer, H.; Rollins, A.; Garcia-Tapia, D.; Erb, H.N.; Earnhart, C.; Marconi, R.; Meeus, P. Antibodies to Borrelia burgdorferi OspA, OspC, OspF, and C6 antigens as markers for early and late infection in dogs. Clin. Vaccine Immunol. 2012, 19, 527–535. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Philipp, M.T. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect. Immun. 1998, 66, 5119–5124. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Hasenkampf, N.R.; Jacobs, M.B.; Philipp, M.T. Dynamic longitudinal antibody responses during Borrelia burgdorferi infection and antibiotic treatment of rhesus macaques. Clin. Vaccine Immunol. 2012, 19, 1218–1226. [Google Scholar] [CrossRef]
- O’Bier, N.S.; Camire, A.C.; Patel, D.T.; Billingsley, J.S.; Hodges, K.R.; Marconi, R.T. Development of novel multi-protein chimeric immunogens that protect against infection with the Lyme disease agent Borreliella burgdorferi. mBio 2024, 17, e0215924. [Google Scholar] [CrossRef]
- Kamp, H.D.; Swanson, K.A.; Wei, R.R.; Dhal, P.K.; Dharanipragada, R.; Kern, A.; Sharma, B.; Sima, R.; Hajdusek, O.; Hu, L.T.; et al. Design of a broadly reactive Lyme disease vaccine. NPJ Vaccines 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Pavia, C.S.; Saggio, G.; Plummer, M.M. The major epidemiologic, microbiologic, immunologic, and clinical aspects of Lyme disease that form the basis for a newly developed vaccine that may become available soon for human use. Front. Immunol. 2024, 14, 1326623. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, S.K.; Schneider, M.; Dubischar, K.; Wagner, L.; Kadlecek, V.; Obersriebnig, M.; Hochreiter, R.; Klingler, A.; Larcher-Senn, J.; Derhaschnig, U.; et al. Immunogenicity and safety of an 18-month booster dose of the VLA15 Lyme borreliosis vaccine candidate after primary immunization in healthy adults in the USA: Results of the booster phase of a randomised, controlled, phase 2 trial. Lancet Infect. Dis. 2024, 24, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
| IgG Antibodies Alone | IgM Antibodies Alone | Both IgG and IgM Antibodies | |
|---|---|---|---|
| No. of positive sera | 9 | 5 | 0 |
| Percent positive of the 91 sera tested | 9.9% | 5.5% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.S.; Ramasamy, R. Significance of Detecting Serum Antibodies to Outer Surface Protein A of Lyme Disease Borreliae in PCR-Confirmed Blood Infections. Diagnostics 2024, 14, 2704. https://doi.org/10.3390/diagnostics14232704
Shah JS, Ramasamy R. Significance of Detecting Serum Antibodies to Outer Surface Protein A of Lyme Disease Borreliae in PCR-Confirmed Blood Infections. Diagnostics. 2024; 14(23):2704. https://doi.org/10.3390/diagnostics14232704
Chicago/Turabian StyleShah, Jyotsna S., and Ranjan Ramasamy. 2024. "Significance of Detecting Serum Antibodies to Outer Surface Protein A of Lyme Disease Borreliae in PCR-Confirmed Blood Infections" Diagnostics 14, no. 23: 2704. https://doi.org/10.3390/diagnostics14232704
APA StyleShah, J. S., & Ramasamy, R. (2024). Significance of Detecting Serum Antibodies to Outer Surface Protein A of Lyme Disease Borreliae in PCR-Confirmed Blood Infections. Diagnostics, 14(23), 2704. https://doi.org/10.3390/diagnostics14232704







