Non-Invasive Ventilation Failure in Pediatric ICU: A Machine Learning Driven Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Outcome and Predictors
2.4. Ethic Committee Approval
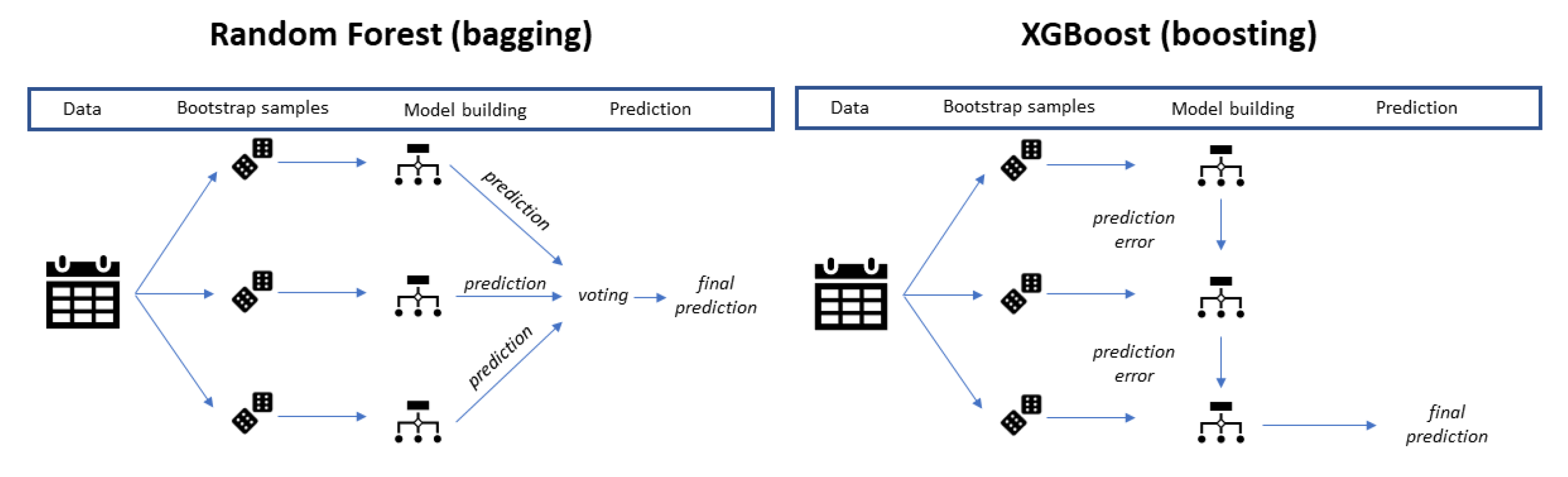
2.5. Machine Learning Techniques
2.6. Model Optimization and Predicted Probability Threshold
2.7. Statistical Analysis
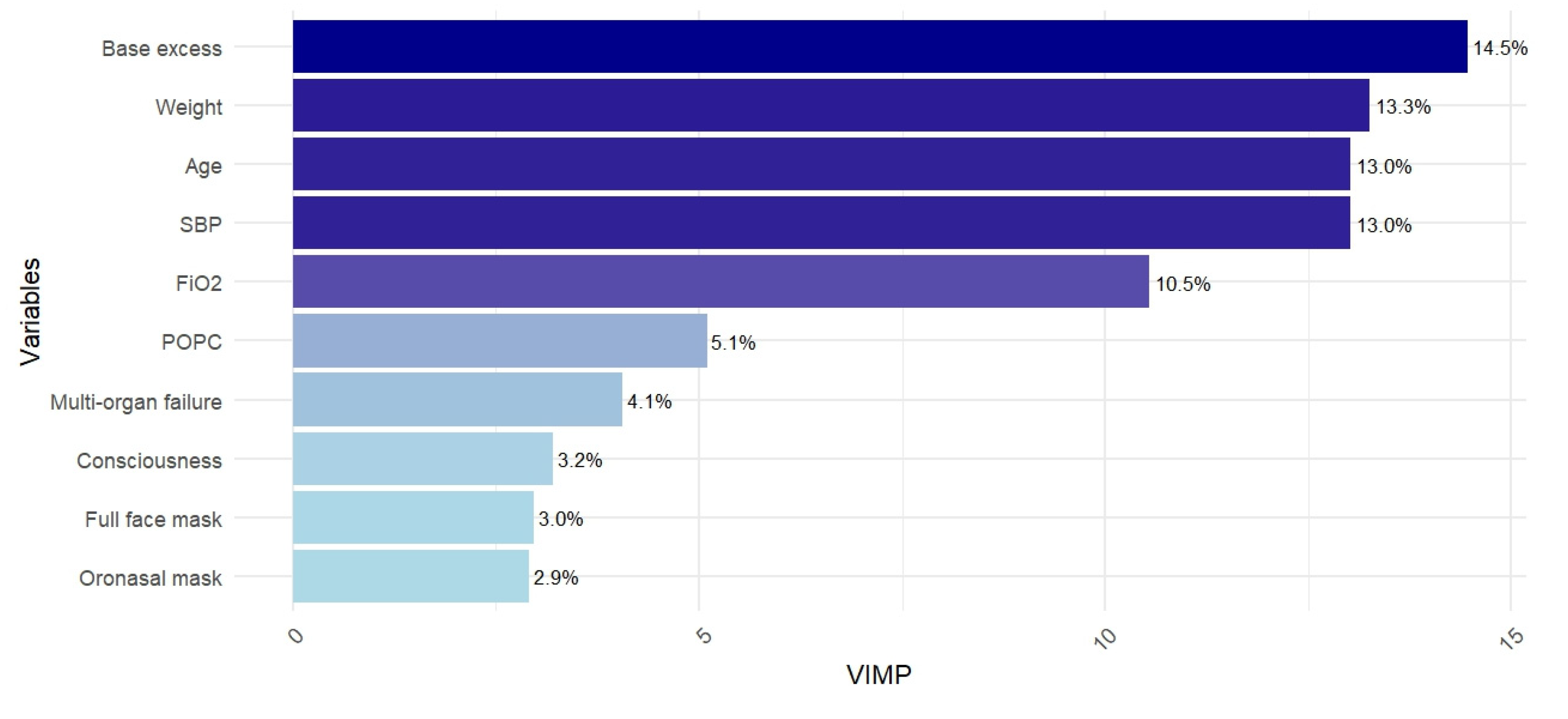
3. Results
4. Discussion
4.1. Main Results
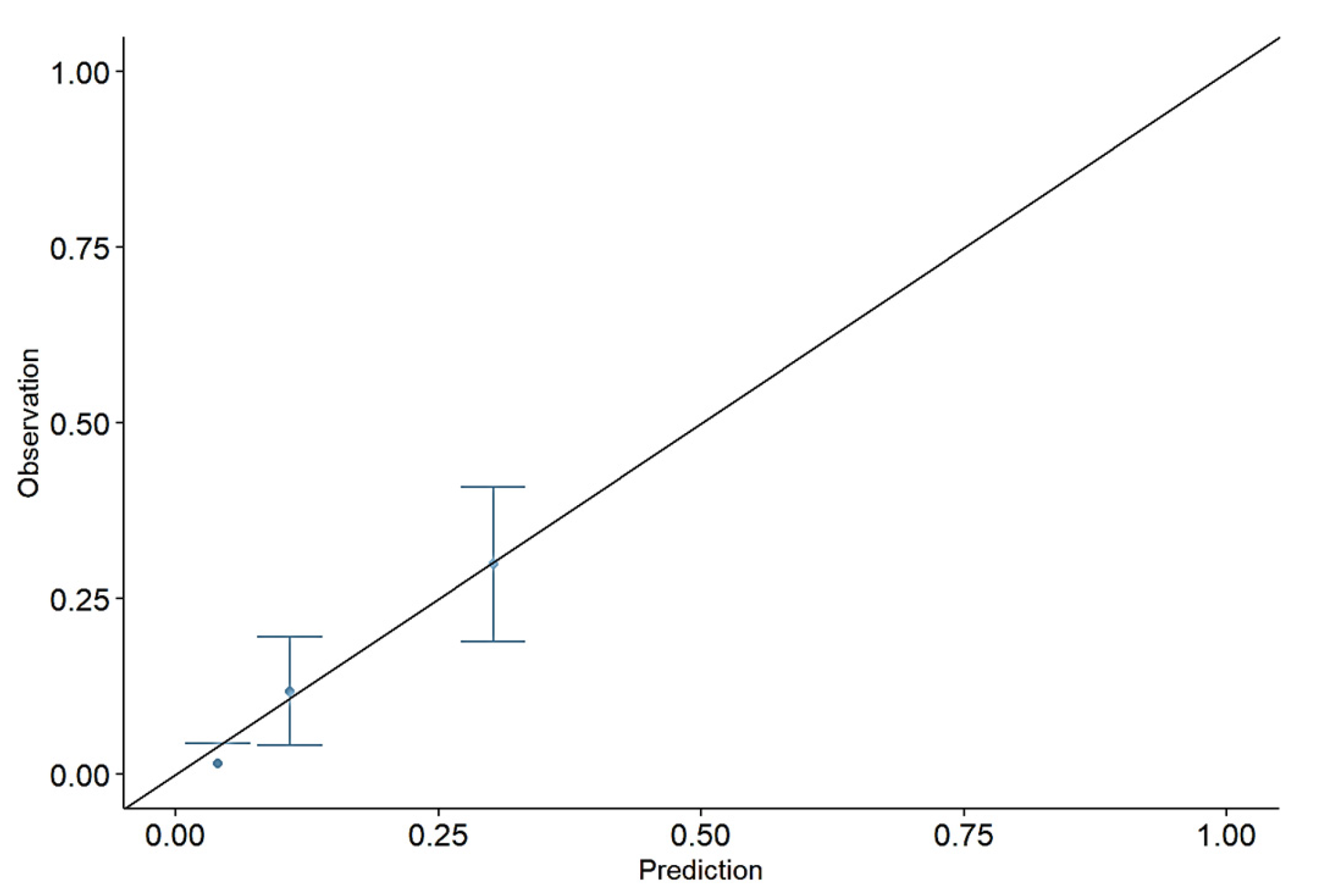
4.2. The Model Calibration
4.3. Study Limitation
4.4. Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mortamet, G.; Emeriaud, G.; Jouvet, P.; Fauroux, B.; Essouri, S. Intérêt de la ventilation non invasive en réanimation pédiatrique: Doit-on espérer un autre niveau de preuve ? Arch. De Pédiatrie 2017, 24, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ganu, S.S.; Gautam, A.; Wilkins, B.; Egan, J. Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med. 2012, 38, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Al-Mukhaini, K.S.; Al-Rahbi, N.M. Noninvasive Ventilation and High-Flow Nasal Cannulae Therapy for Children with Acute Respiratory Failure: An overview. Sultan Qaboos Univ. Med. J. 2018, 18, 278. [Google Scholar] [CrossRef] [PubMed]
- Al Sutari, M.M.; Abdalrahim, M.S.; Hamdan-Mansour, A.M.; Ayasrah, S.M. Pain among mechanically ventilated patients in critical care units. J. Res. Med. Sci. 2014, 19, 726–732. [Google Scholar]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies With All-Cause Mortality in Adults With Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-analysis. JAMA 2020, 324, 57. [Google Scholar] [CrossRef]
- Van Wyk, L.; Applegate, J.T.; Salie, S. Ventilator-associated pneumonia in PICU—How are we doing? S. Afr. J. Crit. Care 2022, 38, 71–74. [Google Scholar] [CrossRef]
- Navarra, S.M.; Congedo, M.T.; Pennisi, M.A. Indications for Non-Invasive Ventilation in Respiratory Failure. Rev. Recent Clin. Trials 2021, 15, 251–257. [Google Scholar] [CrossRef]
- Baker, A.K.; Beardsley, A.L.; Leland, B.D.; Moser, E.A.; Lutfi, R.L.; Cristea, A.I.; Rowan, C.M. Predictors of Failure of Noninvasive Ventilation in Critically Ill Children. J. Pediatr. Intensive Care 2023, 12, 196–202. [Google Scholar] [CrossRef]
- Ongun, E.A.; Dursun, O.; Anıl, A.B.; Altuğ, Ü.; Temel Köksoy, Ö.; Akyıldız, B.N.; Özsoylu, S.; Kendirli, T.; Özcan, S.; Yıldızdaş, R.D.; et al. A multicentered study on efficiency of noninvasive ventilation procedures (SAFE-NIV). Turk. J. Med. Sci. 2021, 51, 1159–1171. Available online: https://journals.tubitak.gov.tr/medical/vol51/iss3/32 (accessed on 9 June 2024).
- Asif, H.; McNeer, J.L.; Ghanayem, N.S.; Cursio, J.F.; Kane, J.M. First-Line Respiratory Support for Children With Hematologic Malignancy and Acute Respiratory Failure. Crit. Care Explor. 2024, 6, e1076. [Google Scholar] [CrossRef]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Koh, Y.; Lim, C.-M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.-S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Napolitano, N.; Pons-Òdena, M.; Iyer, N.P.; Korang, S.K.; Essouri, S.; on behalf of the Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Noninvasive Respiratory Support for Pediatric Acute Respiratory Distress Syndrome: From the Second Pediatric Acute Lung Injury Consensus Conference. Pediatr. Crit. Care Med. 2023, 24, S135–S147. [Google Scholar] [CrossRef]
- Varpaei, H.A.; Bayraktar, N.; Mohammadi, M. Predictors of Non-invasive Ventilation Failure and Associated Factors Among the COVID-19 Patients Admitted to Intensive Care Unit. Anesth. Pain Med. 2023, 13, e140847. Available online: https://brieflands.com/articles/aapm-140847 (accessed on 11 December 2024). [CrossRef] [PubMed]
- Aparecida Alves Grande, R.; Albuquerque Fernandes, G.; Pascoal Andrade, D.; Yumi Matsunaga, N.; De Oliveira, T.; Cruz Bresciani Almeida, C.; Cohen, M.A. Noninvasive ventilation in a pediatric ICU: Factors associated with failure. J. Bras. Pneumol. 2020, 46, e20180053. [Google Scholar] [CrossRef]
- Rowan, C.M.; Fitzgerald, J.C.; Agulnik, A.; Zinter, M.S.; Sharron, M.P.; Slaven, J.E.; Kreml, E.M.; Bajwa, R.P.; Mahadeo, K.M.; Moffet, J.; et al. Risk Factors for Noninvasive Ventilation Failure in Children Post-Hematopoietic Cell Transplant. Front. Oncol. 2021, 11, 653607. [Google Scholar] [CrossRef]
- Aydın, O.; Aydın, E.A.; Birbilen, A.Z.; Tekşam, Ö. Predictive factors of high-flow nasal cannula oxygen therapy failure in children with respiratory distress treated in a Pediatric Emergency Department. Turk. J. Pediatr. 2021, 63, 1012–1019. [Google Scholar] [CrossRef]
- Thomrongpairoj, P.; Tongyoo, S.; Tragulmongkol, W.; Permpikul, C. Factors predicting failure of noninvasive ventilation assist for preventing reintubation among medical critically ill patients. J. Crit. Care 2017, 38, 177–181. [Google Scholar] [CrossRef]
- Corrêa, T.D.; Sanches, P.R.; De Morais, L.C.; Scarin, F.C.; Silva, E.; Barbas, C.S.V. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: A prospective, observational, cohort study. BMC Pulm. Med. 2015, 15, 144. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ferri, C.; Martin-Loeches, I.; Díaz, E.; Masclans, J.R.; Gordo, F.; Sole-Violán, J.; Bodí, M.; Avilés-Jurado, F.X.; Trefler, S.; et al. Risk Factors for Noninvasive Ventilation Failure in Critically Ill Subjects With Confirmed Influenza Infection. Respir. Care. 2017, 62, 1307–1315. [Google Scholar] [CrossRef]
- Liengswangwong, W.; Yuksen, C.; Thepkong, T.; Nakasint, P.; Jenpanitpong, C. Early detection of non-invasive ventilation failure among acute respiratory failure patients in the emergency department. BMC Emerg. Med. 2020, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.S.; Priya, A.; Pekow, P.S.; Steingrub, J.S.; Hill, N.S.; Lagu, T.; Raghunathan, K.; Bhat, A.G.; Lindenauer, P.K. A scoring system derived from electronic health records to identify patients at high risk for noninvasive ventilation failure. BMC Pulm. Med. 2021, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Pan, S.; Yan, M.; Shen, Y.; Liu, X.; Cai, G.; Ning, G. Dynamic prediction of late noninvasive ventilation failure in intensive care unit using a time adaptive machine model. Comput. Methods Programs Biomed. 2021, 208, 106290. [Google Scholar] [CrossRef] [PubMed]
- Pappy, G.; Aczon, M.; Wetzel, R.; Ledbetter, D. Predicting High Flow Nasal Cannula Failure in an Intensive Care Unit Using a Recurrent Neural Network With Transfer Learning and Input Data Perseveration: Retrospective Analysis. JMIR Med. Inform. 2022, 10, e31760. [Google Scholar] [CrossRef]
- Bose, S.N.; Defante, A.; Greenstein, J.L.; Haddad, G.G.; Ryu, J.; Winslow, R.L. A data-driven model for early prediction of need for invasive mechanical ventilation in pediatric intensive care unit patients. Ajagbe SA, curatore. PLoS ONE 2023, 18, e0289763. [Google Scholar] [CrossRef]
- Lins, A.R.B.D.S.; Duarte, M.D.C.M.B.; Andrade, L.B.D. Noninvasive ventilation as the first choice of ventilatory support in children. Rev. Bras. Ter. Intensiv. 2019, 31, 333–339. Available online: https://criticalcarescience.org/article/noninvasive-ventilation-as-the-first-choice-of-ventilatory-support-in-children/ (accessed on 9 June 2024). [CrossRef]
- Chen, W.; Yang, K.; Yu, Z.; Shi, Y.; Chen, C.L.P. A survey on imbalanced learning: Latest research, applications and future directions. Artif. Intell. Rev. 2024, 57, 137. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Gregori, D.; Petrinco, M.; Bo, S.; Rosato, R.; Pagano, E.; Berchialla, P.; Merletti, F. Using Data Mining Techniques in Monitoring Diabetes Care. The Simpler the Better? J. Med. Syst. 2011, 35, 277–281. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: San Francisco, CA, USA, 2016; pp. 785–794. Available online: https://dl.acm.org/doi/10.1145/2939672.2939785 (accessed on 9 June 2024).
- Zhou, Z.-H. Ensemble Methods: Foundations and Algorithms; Taylor & Francis: Boca Raton, FL, USA, 2012. [Google Scholar]
- Bishop, C.M. Neural Networks for Pattern Recognition; Clarendon Pr: Oxford, UK, 1996. [Google Scholar]
- Polley, E. SuperLearner: Super Learner Prediction. 2024. Available online: https://CRAN.R-project.org/package=SuperLearner (accessed on 9 June 2024).
- Wei, P.; Lu, Z.; Song, J. Variable importance analysis: A comprehensive review. Reliab. Eng. Syst. Saf. 2015, 142, 399–432. [Google Scholar] [CrossRef]
- Kuhn, M.; Johnson, K. Applied predictive modeling. In Corrected at 5th Printing; Springer: New York, NY, USA, 2016. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Nembrini, S.; König, I.R.; Wright, M.N. The revival of the Gini importance? Bioinformatics 2018, 34, 3711–3718. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.D. vimp: Perform Inference on Algorithm-Agnostic Variable Importance. 2023. Available online: https://CRAN.R-project.org/package=vimp (accessed on 9 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Breiman, L.; Liaw, A. randomForest: Breiman and Cutler’s Random Forests for Classification and Regression. 2024. Available online: https://CRAN.R-project.org/package=randomForest (accessed on 9 June 2024).
- Chen, T. xgboost: Extreme Gradient Boosting. 2024. Available online: https://CRAN.R-project.org/package=xgboost (accessed on 9 June 2024).
- Ripley, B. nnet: Feed-Forward Neural Networks and Multinomial Log-Linear Models. 2023. Available online: https://CRAN.R-project.org/package=nnet (accessed on 9 June 2024).
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Soft 2008, 28, 1–26. Available online: http://www.jstatsoft.org/v28/i05/ (accessed on 9 June 2024). [CrossRef]
- Rhodes, S. Pmcalibration. 2023. Available online: https://CRAN.R-project.org/package=pmcalibration (accessed on 9 June 2024).
- Silva Filho, T.M.; Kull, M. Betacal: Beta Calibration. 2017. Available online: https://CRAN.R-project.org/package=DMwR2 (accessed on 9 June 2024).
- Cotter, J.; Schmiege, S.; Moss, A.; Ambroggio, L. How to Interact With Interactions: What Clinicians Should Know About Statistical Interactions. Hosp. Pediatr. 2023, 13, e319–e323. [Google Scholar] [CrossRef]
- Manera, U.; Torrieri, M.C.; Bellocchia, M.; Ribolla, F.; Canosa, A.; Vasta, R.; Mora, G.; Mattei, A.; Moglia, C.; Calvo, A.; et al. The Use of Blood Carbonate (HCO3−) and Base-Excess (SBE) in Predicting Non-Invasive Ventilation (NIV) Adaptation (P1-1.Virtual). Neurology 2022, 98, 2938. [Google Scholar] [CrossRef]
- Pons-Odena, M.; Palanca, D.; Modesto, V.; Esteban, E.; González-Lamuño, D.; Carreras, R.; Palomeque, A. SpO2/FiO2 as a predictor of non-invasive ventilation failure in children with hypoxemic respiratory insufficiency. J. Pediatr. Intensive Care 2015, 02, 111–119. [Google Scholar] [CrossRef]
- Langer, T.; Brusatori, S.; Gattinoni, L. Understanding base excess (BE): Merits and pitfalls. Intensive Care Med. 2022, 48, 1080–1083. [Google Scholar] [CrossRef]
- Otero, R.; Trujillo-Santos, J.; Cayuela, A.; Rodriguez, C.; Barron, M.; Martin, J.J.; Monrea, M. Haemodynamically unstable pulmonary embolism in the RIETE Registry: Systolic blood pressure or shock index? Eur. Respir. J. 2007, 30, 1111–1116. [Google Scholar] [CrossRef]
- Harrell, F. Classification vs. Prediction. Statistical Thinking. 2017. Available online: https://www.fharrell.com/post/classification/index.html (accessed on 9 June 2024).
- Housseine, N.; Rijken, M.J.; Weller, K.; Nassor, N.H.; Gbenga, K.; Dodd, C.; Debray, T.; Meguid, T.; Franx, A.; Grobbee, D.E.; et al. Development of a clinical prediction model for perinatal deaths in low resource settings. eClinicalMedicine 2022, 44, 101288. [Google Scholar] [CrossRef]
| Characteristics | NIV Failure, N = 241 1 | NIV Success, N = 1620 1 | p-Value 2 | q-Value 3 |
|---|---|---|---|---|
| Age (months) | 32.94 (2.50, 39.90) | 25.77 (2.40, 26.90) | 0.071 | 0.10 |
| Sex (Female) | 123 (51%) | 897 (55%) | 0.2 | 0.3 |
| Weight | 12.26 (4.70, 13.00) | 10.93 (5.00, 12.00) | 0.6 | 0.7 |
| Ethnicity | 0.038 | 0.059 | ||
| ⠀⠀Caucasian | 194 (80%) | 1204 (74%) | ||
| ⠀⠀Other | 47 (20%) | 416 (26%) | ||
| Chronic disease | 105 (44%) | 468 (29%) | <0.001 | <0.001 |
| Systolic Blood Pressure (SBP) | 102.56 (90.00, 119.00) | 100.78 (90.00, 117.00) | 0.3 | 0.3 |
| ⠀⠀(Missing) | 4 | 22 | ||
| FiO2 (fraction of inspired oxygen) | 0.54 (0.40, 0.60) | 0.58 (0.30, 0.50) | <0.001 | <0.001 |
| ⠀⠀(Missing) | 120 | 928 | ||
| Base excess | 2.28 (−2.00, 2.30) | 0.31 (−0.85, 1.60) | >0.9 | >0.9 |
| ⠀⠀(Missing) | 4 | 44 | ||
| Priority of admission | 0.001 | 0.002 | ||
| ⠀⠀High | 147 (79%) | 883 (69%) | ||
| ⠀⠀Medium | 32 (17%) | 365 (29%) | ||
| ⠀⠀Low | 8 (4.3%) | 29 (2.3%) | ||
| ⠀⠀(Missing) | 54 | 343 | ||
| State of consciousness | <0.001 | <0.001 | ||
| ⠀⠀Conscious | 177 (73%) | 1447 (89%) | ||
| ⠀⠀Pharmacological sedation | 13 (5.4%) | 54 (3.3%) | ||
| ⠀⠀Other | 51 (21%) | 118 (7.3%) | ||
| ⠀⠀(Missing) | 0 | 1 | ||
| Paediatric overall performance category (POPC—min = 1; max = 6) | 2.02 (1.00, 3.00) | 1.72 (1.00, 2.00) | 0.001 | 0.002 |
| ⠀⠀(Missing) | 23 | 159 | ||
| Multiorgan failure | 38 (16%) | 43 (2.7%) | <0.001 | <0.001 |
| Bronchiolitis | 89 (37%) | 801 (49%) | <0.001 | <0.001 |
| Asthma | 14 (5.8%) | 141 (8.7%) | 0.13 | 0.2 |
| Characteristic | NIV Failure, N = 241 1 | NIV Success, N = 1620 1 | p-Value 2 | q-Value 3 |
|---|---|---|---|---|
| Nasal mask | 36 (15%) | 287 (18%) | 0.3 | 0.3 |
| Oronasal mask | 47 (20%) | 101 (6.2%) | <0.001 | <0.001 |
| Nasal cannulas | 60 (25%) | 579 (36%) | <0.001 | 0.002 |
| Helmet | 90 (37%) | 693 (43%) | 0.11 | 0.15 |
| Full-face (eyes included) | 42 (17%) | 75 (4.6%) | <0.001 | <0.001 |
| Time Before Failure | N (%) | Cumulative N (%) |
|---|---|---|
| within 24 h | 85 (35%) | 85 (35%) |
| within 48 h | 89 (37%) | 174 (72%) |
| within 3 days | 29 (12%) | 203 (84%) |
| within 4 days | 9 (3.7%) | 212 (87.7%) |
| within 5 days | 7 (2.9%) | 219 (90.6%) |
| within 6 days | 3 (1.2%) | 222 (91.8%) |
| within 7 days | 4 (1.7%) | 226 (93.5%) |
| >7 days | 15 (6.5%) | 241 (100%) |
| Model | Sensitivity | Specificity | AUROC | PPV/NPV |
|---|---|---|---|---|
| GLM | 0.76 (0.56, 0.90) | 0.83 (0.76, 0.88) | 0.81 (0.72, 0.91) | 0.42/0.95 |
| RF | 0.83 (0.64, 0.94) | 0.72 (0.65, 0.78) | 0.82 (0.74, 0.90) | 0.33/0.96 |
| XGBoost | 0.72 (0.53, 0.87) | 0.70 (0.63, 0.77) | 0.72 (0.61, 0.82) | 0.29/0.94 |
| NNET | 0.62 (0.42, 0.79) | 0.52 (0.44, 0.59) | 0.50 (0.50, 0.50) | 0.18/0.89 |
| SuperLearner | 0.76 (0.56, 0.90) | 0.82 (0.76, 0.88) | 0.82 (0.73,0.92) | 0.42/0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaruttini, M.V.; Lorenzoni, G.; Daverio, M.; Marchetto, L.; Izzo, F.; Chidini, G.; Picconi, E.; Nettuno, C.; Zanonato, E.; Sagredini, R.; et al. Non-Invasive Ventilation Failure in Pediatric ICU: A Machine Learning Driven Prediction. Diagnostics 2024, 14, 2857. https://doi.org/10.3390/diagnostics14242857
Chiaruttini MV, Lorenzoni G, Daverio M, Marchetto L, Izzo F, Chidini G, Picconi E, Nettuno C, Zanonato E, Sagredini R, et al. Non-Invasive Ventilation Failure in Pediatric ICU: A Machine Learning Driven Prediction. Diagnostics. 2024; 14(24):2857. https://doi.org/10.3390/diagnostics14242857
Chicago/Turabian StyleChiaruttini, Maria Vittoria, Giulia Lorenzoni, Marco Daverio, Luca Marchetto, Francesca Izzo, Giovanna Chidini, Enzo Picconi, Claudio Nettuno, Elisa Zanonato, Raffaella Sagredini, and et al. 2024. "Non-Invasive Ventilation Failure in Pediatric ICU: A Machine Learning Driven Prediction" Diagnostics 14, no. 24: 2857. https://doi.org/10.3390/diagnostics14242857
APA StyleChiaruttini, M. V., Lorenzoni, G., Daverio, M., Marchetto, L., Izzo, F., Chidini, G., Picconi, E., Nettuno, C., Zanonato, E., Sagredini, R., Rossetti, E., Mondardini, M. C., Cecchetti, C., Vitale, P., Alaimo, N., Colosimo, D., Sacco, F., Genoni, G., Perrotta, D., ... Gregori, D. (2024). Non-Invasive Ventilation Failure in Pediatric ICU: A Machine Learning Driven Prediction. Diagnostics, 14(24), 2857. https://doi.org/10.3390/diagnostics14242857










