Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit—Incidence and Strategies for Prevention
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- The study included patients who were admitted to the neonatal intensive care unit (NICU) and were less than 28 days old at the time of hospitalization;
- (2)
- A case–control or cohort study;
- (3)
- Ventilator-associated pneumonia (VAP) was suspected 48 h after the initiation of mechanical ventilation;
- (4)
- Published in the English language.
- (1)
- It was an animal study;
- (2)
- Included participants already diagnosed with VAP;
- (3)
- It was a duplicated study;
- (4)
- The study was case-report;
- (5)
- The time of mechanical ventilation for the included participants was < 48 h or not specified in the study.
3. VAP Definition
3.1. Clinical Findings
3.2. Radiological Findings
3.3. Microbiological Findings
4. VAP Incidence
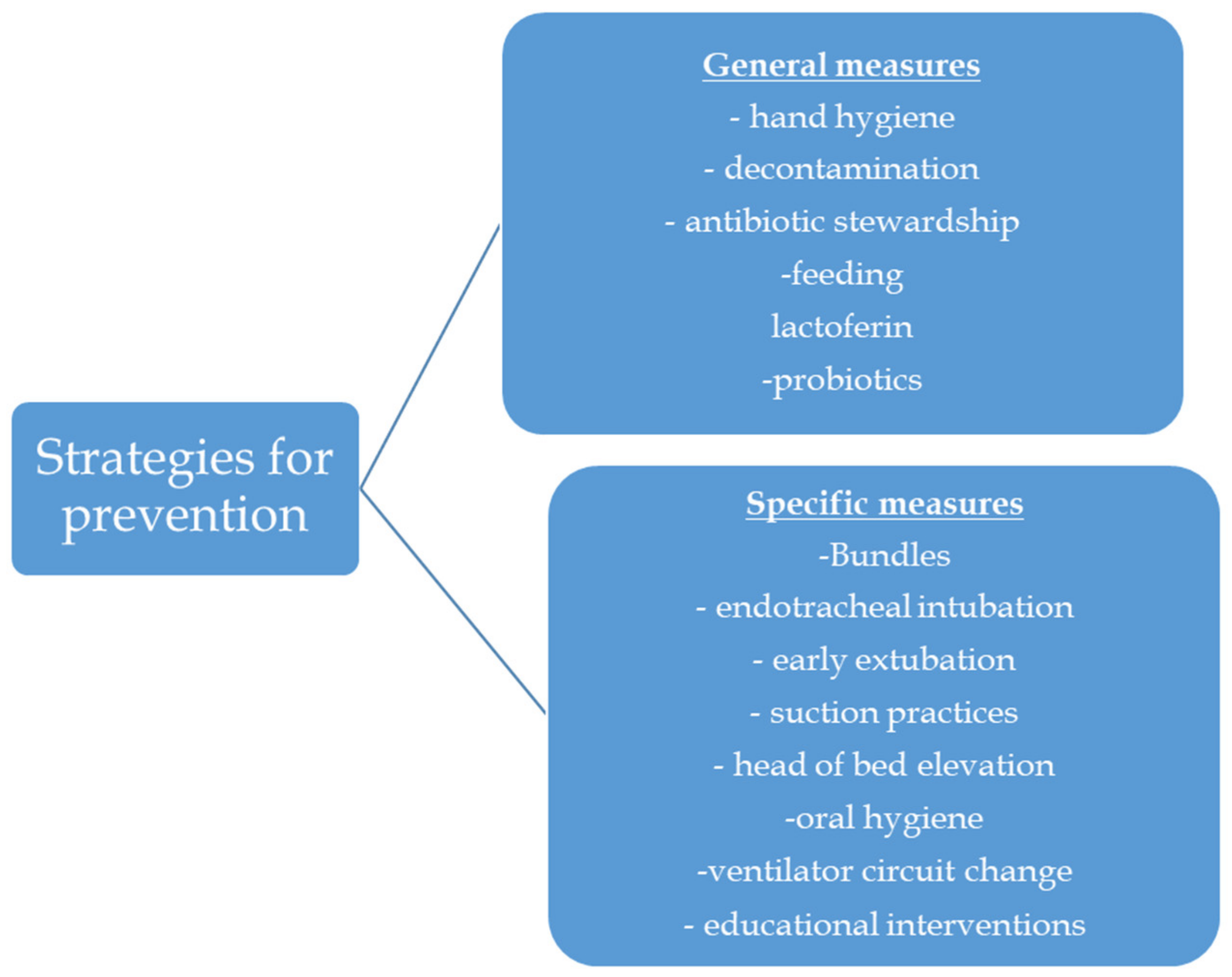
5. Prevention Strategies
5.1. General Measures for Prevention of VAP
5.1.1. Hand Hygiene
5.1.2. Decontamination
5.1.3. Antibiotic Stewardship
5.1.4. Feeding
5.2. General Preventive Strategies Which Need Further Evaluation
5.2.1. Probiotics
5.2.2. Lactoferin
5.3. Specific Measures for Prevention of VAP
5.3.1. Bundles
5.3.2. Endotracheal Intubation, Suction Practices and Early Extubation
5.3.3. Head Positioning
5.3.4. Ventilator Circuit Changes
5.3.5. Educational Interventions
5.3.6. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foglia, E.; Meier, M.D.; Edward, A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin. Micro. Boil. Rev. 2007, 20, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, F.; De La Rosa, G.; Gómez, E.; Múnera, P.; Ramírez, J.; Castrillón, S. Incidence and risk factors for ventilator-associated pneumonia in a developing country: Where is the difference? Respir. Med. 2007, 101, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.S. Strategies to prevent ventilator-associated pneumonia in neonates. Clin. Perinatol. 2010, 3, 629–643. [Google Scholar] [CrossRef] [PubMed]
- National Healthcare Safety Network, Pneumonia (Ventilator-Associated [VAP] and Non-Ventilator-Associated Pneumonia [PNEU]) Event. January 2023. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf (accessed on 1 November 2023).
- Raycheva, R.; Rangelova, V.; Kevorkyan, A. Cost Analysis for Patients with Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit. Healthcare 2022, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D. Neurodevelopmental and growth impairment among extremely low-birthweight infants with neonatal infection. JAMA 2004, 17, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- van der Zwet, W.C.; Kaiser, A.M.; van Elburg, R.M.; Berkhof, J.; Fetter, W.P.; Parlevliet, G.A.; Vandenbroucke-Grauls, C.M. Nosocomial infections in a Dutch neonatal intensive care unit: Surveillance study with definitions for infection specifically adapted for neonates. J. Hosp. Infect. 2005, 61, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, R.P.; Edwards, J.R.; Jarvis, W.R.; Culver, D.H.; Tolson, J.S.; Martone, W.J. Nosocomial infections among neonates in high-risk nurseries in the United States. National Nosocomial Infections Surveillance System. Pediatrics 1996, 98, 357–361. [Google Scholar] [CrossRef]
- Aelami, M.H.; Lotfi, M.; Zingg, W. Ventilator-associated pneumonia in neonates, infants and children. Antimicrob. Resist. Infect. Control. 2014, 3, 30. [Google Scholar] [CrossRef]
- Hatachi, T.; Tachibana, K.; Takeuchi, M. Incidences and influences of device-associated healthcare-associated infections in a pediatric intensive care unit in Japan: A retrospective surveillance study. J. Intensive. Care 2015, 26, 44. [Google Scholar] [CrossRef]
- Wojkowska-Mach, J.; Merritt, T.A.; Borszewska-Kornacka, M.; Domańska, J.; Gulczyńska, E.; Nowiczewski, M.; Helwich, E.; Kordek, A.; Pawlik, D.; Adamski, P. Device-associated pneumonia of very low birth weight infants in Polish Neonatal Intensive Care Units. Adv. Med. Sci. 2015, 61, 90–95. [Google Scholar] [CrossRef]
- Ferrari, R. Writing narrative style literature reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Baltimore, R.S. The difficulty of diagnosing ventilator-associated pneumonia. Pediatrics 2003, 112, 1420–1421. [Google Scholar] [CrossRef] [PubMed]
- Krankenhaus Infektions Surveillance System: Protokoll. Surveillance Nosokomialer INFEKTIONEN bei Frühgeborenen Mit einem Geburtsgewicht < 1.500 g (NEO-KISS). 2009. Available online: http://www.nrz-hygiene.de/fileadmin/nrz/download/NEOKISSProtokoll221209.pdf (accessed on 1 November 2023).
- Apisarnthanarak, A.; Holzmann-Pazgal, G.; Hamvas, A.; Olsen, M.A.; Fraser, V.J. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: Characteristics, risk factors, and outcomes. Pediatrics 2003, 112, 1283.e9. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.; El-Lahony, D.; Soliman, W.F. Ventilator-associated pneumonia in the neonatal intensive care unit. Menoufia. Med. J. 2014, 27, 73–77. [Google Scholar] [CrossRef]
- Venkatachalam, V.; Hendley, J.O.; Willson, D.F. The diagnostic dilemma of ventilator-associated pneumonia in critically ill children. Pediatr. Crit. Care Med. 2011, 12, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Haney, P.J.; Bohlman, M.; Sun, C.C. Radiographic findings in neonatal pneumonia. AJR Am. J. Roentgenol. 1984, 143, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Cernada, M.; Aguar, M.; Brugada, M.; Gutiérrez, A.; López, J.L.; Castell, M.; Vento, M. Ventilator-Associated Pneumonia in Newborn Infants Diagnosed with an Invasive Bronchoalveolar Lavage Technique. Pediatr. Crit. Care Med. 2013, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.C.; Kefala, K.; Simpson, A.J.; Wilkinson, T.S.; Everingham, K.; Kerslake, D.; Raby, S.; Laurenson, I.F.; Swann, D.G.; Walsh, T.S. Evaluation of the effect of diagnostic methodology on the reported incidence of ventilator-associated pneumonia. Thorax 2009, 64, 516–522. [Google Scholar] [CrossRef]
- Cardeñosa Cendrero, J.A.; Solé-Violán, J.; Bordes Benítez, A.; Noguera Catalán, J.; Arroyo Fernández, J.; Saavedra Santana, P.; Rodríguez de Castro, F. Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest 1999, 116, 462–470. [Google Scholar] [CrossRef]
- Feldman, C.; Kassel, M.; Cantrell, J.; Kaka, S.; Morar, R.; Goolam Mahomed, A.; Philips, J.I. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur. Respir. J. 1999, 13, 546–551. [Google Scholar] [CrossRef]
- Cordero, L.; Ayers, L.W.; Miller, R.R.; Seguin, J.H.; Coley, B.D. Surveillance of ventilator-associated pneumonia in very-low-birth-weight infants. Am. J. Infect. Control 2002, 30, 32–39. [Google Scholar] [CrossRef]
- Stevens, J.P.; Kachniarz, B.; Wright, S.B.; Gillis, J.; Talmor, D.; Clardy, P.; Howell, M.D. When policy gets it right: Variability in US hospitals’ diagnosis of ventilator-associated pneumonia. Crit. Care Med. 2014, 42, 497–503. [Google Scholar] [CrossRef]
- Klompas, M. Interobserver variability in ventilator-associated pneumonia surveillance. Am. J. Infect. Control 2010, 38, 237–239. [Google Scholar] [CrossRef]
- Magill, S.S.; Klompas, M.; Balk, R.; Burns, S.M.; Deutschman, C.S.; Diekema, D.; Fridkin, S.; Greene, L.; Guh, A.; Gutterman, D.; et al. Developing a new, national approach to surveillance for ventilator-associated events. Am. J. Crit. Care 2013, 22, 469–473. [Google Scholar] [CrossRef]
- National Healthcare Safety Network, Pediatric Ventilator-Associated Event (PedVAE). 2023. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/pedvae-current-508.pdf (accessed on 1 November 2023).
- Ergenekon, E.; Çataltepe, S. Ventilator-associated pneumonia in the NICU: Time to boost diagnostics? Pediatr. Res. 2020, 87, 1143–1144. [Google Scholar] [CrossRef]
- Cernada, M.; Brugada, M.; Golombek, S.; Vento, M. Ventilator-associated pneumonia in neonatal patients: An update. Neonatology 2014, 105, 98–107. [Google Scholar] [CrossRef]
- Gohr, A.R.F.; El Tayeb, A.A.; Shalaby, A.M. An Observational Study on Ventilator-Associated Pneumonia as a Cause for Nosocomial Infection in Mechanically Ventilated Neonates. Ann. Neonatol. J. 2021, 3, 144–164. [Google Scholar] [CrossRef]
- Afjeh, S.A.; Sabzehei, M.K.; Karimi, A.; Shiva, F.; Shamshiri, A.R. Surveillance of ventilator-associated pneumonia in a neonatal intensive care unit: Characteristics, risk factors, and outcome. Arch. Iran. Med. 2012, 15, 567–571. [Google Scholar]
- ELMeneza, S.A.; Gaber, A.; Refaey, A.A. Ventilator-associated pneumonia in neonatal intensive care unit: Characteristics, risk factors and outcome. Azhar. J. Pediatr. 2010, 3, 1–14. [Google Scholar]
- Dudeck, M.A.; Weiner, L.M.; Allen-Bridson, K.; Malpiedi, P.J.; Peterson, K.D.; Pollock, D.A.; Sievert, D.M.; Edwards, J.R. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am. J. Infect. Control 2013, 41, 1148–1166. [Google Scholar] [CrossRef]
- Patrick, S.W.; Kawai, A.T.; Kleinman, K.; Jin, R.; Vaz, L.; Gay, C.; Kassler, W.; Goldmann, D.; Lee, G.M. Health care-associated infections among critically ill children in the US, 2007–2012. Pediatrics 2014, 134, 705–712. [Google Scholar] [CrossRef]
- Urzedo, J.E.; Levenhagen, M.M.M.D.; Pedroso, R.S.; Abdallah, V.O.S.; Sabino, S.S.; Brito, D.V.D. Nosocomial infections in a neonatal intensive care unit during 16 years: 1997–2012. Rev. Soc. Bras. Med. Trop. 2014, 47, 321–326. [Google Scholar] [CrossRef]
- Romanelli, R.M.; Anchieta, L.M.; Mourão, M.V.; Campos, F.A.; Loyola, F.C.; Jesus, L.A.; Armond, G.A.; Clemente, W.T. Infecções relacionadas à assistência a saúde baseada em critérios internacionais, realizada em unidade neonatal de cuidados progressivos de referência de Belo Horizonte, MG [Notification of healthcare associated infections based on international criteria performed in a reference neonatal progressive care unity in Belo Horizonte, MG]. Rev. Bras. Epidemiol. 2013, 16, 77–86. (In Portuguese) [Google Scholar]
- Mir, Z.H.; Ali, I.; Qureshi, O.A.; Wani, G.R. Risk factors, pathogen profile and outcome of ventilator associated pneumonia in a Neonatal intensive care unit. Int. J. Contemp. Pediatr. 2015, 2, 17–20. [Google Scholar] [CrossRef]
- Vijayakanthi, N.; Kitchanan, S.; Arasan, D. Ventilator associated pneumonia (VAP) in neonatal intensive care unit--an emerging problem. Indian J. Pediatr. 2015, 82, 96. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; Mohamed, H.A.; Abdelfattah, M.; ElTatawy, S.S. Device associated infections among neonates in neonatal intensive care units: A single unit survey study in Cairo, Egypt. Int. J. Contemp. Pediatr. 2020, 7, 739. [Google Scholar] [CrossRef]
- Katoch, P.; Singh, S.; Kumar, D. Incidence of ventilator-associated pneumonia and their socio-demographic profile among children admitted in neonatal and pediatric intensive care units. Int. J. Recent Sci. Res. 2021, 9, 43117–43120. [Google Scholar]
- Kawanishi, F.; Yoshinaga, M.; Morita, M.; Shibata, Y.; Yamada, T.; Ooi, Y.; Ukimura, A. Risk factors for ventilator-associated pneumonia in neonatal intensive care unit patients. J. Infect. Chemother. 2014, 20, 627–630. [Google Scholar] [CrossRef]
- Lee, P.L.; Lee, W.T.; Chen, H.L. Ventilator-Associated Pneumonia in Low Birth Weight Neonates at a Neonatal Intensive Care Unit: A Retrospective Observational Study. Pediatr. Neonatol. 2017, 58, 16–21. [Google Scholar] [CrossRef]
- Navoa-Ng, J.A.; Berba, R.; Galapia, Y.A.; Rosenthal, V.D.; Villanueva, V.D.; Tolentino, M.C.; Genuino, G.A.; Consunji, R.J.; Mantaring, J.B., 3rd. Device-associated infections rates in adult, pediatric, and neonatal intensive care units of hospitals inthe Philippines: International Nosocomial Infection Control Consortium(INICC) findings. Am. J. Infect. Control 2011, 39, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Thatrimontrichai, A.; Rujeerapaiboon, N.; Janjindamai, W.; Dissaneevate, S.; Maneenil, G.; Kritsaneepaiboon, S.; Tanaanantarak, P. Outcomes and risk factors of ventilator-associated pneumonia in neonates. World J. Pediatr. 2017, 13, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Li, X.; Zou, Y.; Wang, J.; Wang, J.; Namba, F.; Hiroyuki, Y.; Yu, J.; Yamauchi, Y.; Guo, C. Risk factors and pathogen profile of ventilator-associated pneumonia in a neonatal intensive care unit in China. Pediatr. Int. 2011, 53, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.D.; Cao, Y.; Chen, C.; Yang, Y.; Wang, C.Q.; Zhang, L.; Ding, H. Investigation of nosocomial infection in the neonatal intensive care unit. Zhongguo Dang dai er ke za zhi = Chin. J. Contemp. Pediatr. 2010, 12, 81–84. [Google Scholar]
- Bedir Demirdağ, T.; Koç, E.; Tezer, H.; Oğuz, S.; Satar, M.; Sağlam, Ö.; Uygun, S.S.; Önal, E.; Hirfanoğlu, İ.M.; Tekgündüz, K.; et al. The prevalence and diagnostic criteria of health-care associated infections in neonatal intensive care units in Turkey: A multicenter point- prevalence study. Pediatr. Neonatol. 2021, 62, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orto, V.; Raschetti, R.; Centorrino, R.; Montane, A.; Tissieres, P.; Yousef, N.; De Luca, D. Short- and long-term respiratory outcomes in neonates with ventilator-associated pneumonia. Pediatr. Pulmonol. 2019, 54, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Rangelova, V.R.; Raycheva, R.D.; Kevorkyan, A.K.; Krasteva, M.B.; Kalchev, Y.I. Ventilator-Associated Pneumonia in Neonates Admitted to a Tertiary Care NICU in Bulgaria. Front. Pediatr. 2022, 10, 909217. [Google Scholar] [CrossRef]
- Scamardo, M.S.; Dolce, P.; Esposito, E.P.; Raimondi, F.; Triassi, M.; Zarrilli, R. Trends, risk factors and outcomes of healthcare-associated infections in a neonatal intensive care unit in Italy during 2013–2017. Ital. J. Pediatr. 2020, 46, 34. [Google Scholar] [CrossRef]
- Geslain, G.; Guellec, I.; Guedj, R.; Guilbert, J.; Jean, S.; Valentin, C.; Demoulin, M.; Soreze, Y.; Carbajal, R.; Leger, P.L.; et al. Incidence and risk factors of ventilator-associated pneumonia in neonatal intensive care unit: A first French study. Minerva. Anestesiol. 2018, 84, 829–835. [Google Scholar] [CrossRef]
- Leistner, R.; Piening, B.; Gastmeier, P.; Geffers, C.; Schwab, F. Nosocomial infections in very low birthweight infants in Germany: Current data from the National Surveillance System NEO-KISS. Klin. Padiatr. 2013, 225, 75–80. [Google Scholar] [CrossRef]
- Yalaz, M.; Altun-Köroğlu, O.; Ulusoy, B.; Yildiz, B.; Akisu, M.; Vardar, F.; Ozinel, M.A.; Kültürsay, N. Evaluation of device-associated infections in a neonatal intensive care unit. Turk. J. Pediatr. 2012, 54, 128–135. [Google Scholar]
- de Mello Freitas, F.T.; Viegas, A.P.B.; Romero, G.A.S. Neonatal healthcare-associated infections in Brazil: Systematic review and meta-analysis. Arch. Public Health. 2021, 79, 89. [Google Scholar] [CrossRef]
- Hocevar, S.N.; Edwards, J.R.; Horan, T.C.; Morrell, G.C.; Iwamoto, M.; Lessa, F.C. Device-associated infections among neonatal intensive care unit patients: Incidence and associated pathogens reported to the National Healthcare Safety Network, 2006–2008. Infect. Control. Hosp. Epidemiol. 2012, 33, 1200–1206. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Al-Abdely, H.M.; El-Kholy, A.A.; AlKhawaja, S.A.A.; Leblebicioglu, H.; Mehta, Y.; Rai, V.; Hung, N.V.; Kanj, S.S.; Salama, M.F.; et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: Device-associated module. Am. J. Infect. Control 2016, 44, 1495–1504. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Maki, D.G.; Mehta, Y.; Leblebicioglu, H.; Memish, Z.A.; Al-Mousa, H.H.; Balkhy, H.; Hu, B.; Alvarez-Moreno, C.; Medeiros, E.A.; et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007–2012. Device-associated module. Am. J. Infect. Control 2014, 42, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Tablan, O.C.; Anderson, L.J.; Besser, R.; Bridges, C.; Hajjeh, R. CDC Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care—Associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm. Rep. 2004, 53, 1–36. [Google Scholar]
- WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge, Clean Care Is Safer Care. World Health Organization: Geneva, Switzerland, 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/44102/9789241597906_eng.pdf;jsessionid=DE509D0E788D33BE9CB436990FB37868?sequence=1http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf (accessed on 1 November 2023).
- Won, S.P.; Chou, H.C.; Hsieh, W.S.; Chen, C.Y.; Huang, S.M.; Tsou, K.I.; Tsao, P.N. Handwashing program for the prevention of nosocomial infections in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2004, 25, 742–746. [Google Scholar] [CrossRef]
- Rogers, E.l.; Alderdice, F.; McCall, E.; Jenkins, J.; Craig, S. Reducing nosocomial infections in neonatal intensive care. J. Matern. Fetal. Neonatal. Med. 2010, 23, 1039–1046. [Google Scholar] [CrossRef]
- Pittet, D.; Mourouga, P.; Perneger, T.V. Compliance with handwashing in a teaching hospital. Infection Control Program. Ann Intern. Med. 1999, 130, 126–130. [Google Scholar] [CrossRef]
- Cadot, L.; Bruguière, H.; Jumas-Bilak, E.; Didelot, M.N.; Masnou, A.; de Barry, G.; Cambonie, G.; Parer, S.; Romano-Bertrand, S. Extended spectrum beta-lactamase-producing Klebsiella pneumoniae outbreak reveals incubators as pathogen reservoir in neonatal care center. Eur. J. Pediatr. 2019, 178, 505–513. [Google Scholar] [CrossRef] [PubMed]
- van Veenendaal, N.R.; Heideman, W.H.; Limpens, J.; van der Lee, J.H.; van Goudoever, J.B.; van Kempen, A.A.M.W.; van der Schoor, S.R.D. Hospitalising preterm infants in single family rooms versus open bay units: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2019, 3, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef]
- Cantey, J.B. Optimizing the Use of Antibacterial Agents in the Neonatal Period. Paediatr. Drugs. 2016, 18, 109–122. [Google Scholar] [CrossRef]
- Abdel-Gawad, T.A.; El-Hodhod, M.A.; Ibrahim, H.M.; Michael, Y.W. Gastroesophageal reflux in mechanically ventilated pediatric patients and its relation to ventilator-associated pneumonia. Crit. Care 2009, 13, R164. [Google Scholar] [CrossRef]
- Coffin, S.E.; Klompas, M.; Classen, D.; Arias, K.M.; Podgorny, K.; Anderson, D.J.; Burstin, H.; Calfee, D.P.; Dubberke, E.R.; Fraser, V.; et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect. Control Hosp. Epidemiol. 2008, 29, S31–S40. [Google Scholar] [CrossRef]
- Dutta, S.; Singh, B.; Chessell, L.; Wilson, J.; Janes, M.; McDonald, K.; Shahid, S.; Gardner, V.A.; Hjartarson, A.; Purcha, M.; et al. Guidelines for feeding very low birth weight infants. Nutrients 2015, 7, 423–442. [Google Scholar] [CrossRef]
- Kusahara, D.M.; Enz Cda, C.; Avelar, A.F.; Peterlini, M.A.; Pedreira Mda, L. Risk factors for ventilator-associated pneumonia in infants and children: A cross-sectional cohort study. Am. J. Crit. Care 2014, 23, 469–476. [Google Scholar] [CrossRef]
- Manzoni, P.; De Luca, D.; Stronati, M.; Jacqz-Aigrain, E.; Ruffinazzi, G.; Luparia, M.; Tavella, E.; Boano, E.; Castagnola, E.; Mostert, M.; et al. Prevention of nosocomial infections in neonatal intensive care units. Am. J. Perinatol. 2013, 30, 81–88. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M. ProPrems Study Group. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1056. [Google Scholar] [CrossRef]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Probiotics in Preterm Infants Study Collaborative Group. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- van den Akker, C.H.P.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R. ESPGHAN Working Group for Probiotics, Prebiotics & Committee on Nutrition. Probiotics for Preterm Infants: A Strain-Specific Systematic Review and Network Meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef]
- ELFIN Trial Investigators Group. Enteral lactoferrin supplementation for very preterm infants: A randomised placebo-controlled trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef]
- Institute for Healthcare Improvement. What is a Bundle. Available online: https://www.ihi.org/resources/Pages/ImprovementStories/WhatIsaBundle.aspx (accessed on 1 November 2023).
- Institute for Healthcare Improvement. How-to Guide: Prevent Ventilator-Associated Pneumonia. Cambridge, MA, USA. Available online: https://www.ihi.org/resources/Pages/Tools/HowtoGuidePreventVAP.aspx (accessed on 1 November 2023).
- Institute for Healthcare Improvement. Ventilator-Associated Pneumonia: Getting to Zero and Staying There. Institute of Health Improvement Stories. Available online: https://www.ihi.org/resources/Pages/ImprovementStories/VAPGettingtoZeroandStayingThere.aspx (accessed on 1 November 2023).
- Smulders, C.A.; van Gestel, J.P.; Bos, A.P. Are central line bundles and ventilator bundles effective in critically ill neonates and children? Intensive. Care Med. 2013, 39, 1352–1358. [Google Scholar] [CrossRef]
- Brennan, R.; Loughead, J.; DeJulio, P.; Leston, S.; Sosin, J. Creating and Implementing a Bundle to Reduce VAP in the NICU; Institute for Healthcare Improvement: Cambridge, MA, USA, 2006; Available online: http://www.ihi.org/resources/Pages/ImprovementStories/CreatingandImplementingaBundletoReduceVAPintheNICU.aspx (accessed on 1 November 2023).
- Brilli, R.J.; Sparling, K.W.; Lake, M.R.; Butcher, J.; Myers, S.S.; Clark, M.D.; Helpling, A.; Stutler, M.E. The business case for preventing ventilator-associated pneumonia in pediatric intensive care unit patients. Jt. Comm. J. Qual. Patient. Saf. 2008, 34, 629–638. [Google Scholar] [CrossRef]
- Jacobs Pepin, B.; Lesslie, D.; Berg, W.; Spaulding, A.B.; Pokora, T. ZAP-VAP: A Quality Improvement Initiative to Decrease Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit, 2012–2016. Adv. Neonatal. Care 2019, 19, 253–261. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Rodríguez-Calderón, M.E.; Rodríguez-Ferrer, M.; Singhal, T.; Pawar, M.; Sobreyra-Oropeza, M.; Barkat, A.; Atencio-Espinoza, T.; Berba, R.; Navoa-Ng, J.A.; et al. Findings of the International Nosocomial Infection Control Consortium (INICC), Part II: Impact of a multidimensional strategy to reduce ventilator-associated pneumonia in neonatal intensive care units in 10 developing countries. Infect. Control Hosp. Epidemiol. 2012, 33, 704–710. [Google Scholar] [CrossRef]
- Zhou, Q.; Lee, S.K.; Jiang, S.Y.; Chen, C.; Kamaluddeen, M.; Hu, X.J.; Wang, C.Q.; Cao, Y. Efficacy of an infection control program in reducing ventilator-associated pneumonia in a Chinese neonatal intensive care unit. Am. J. Infect. Control 2013, 41, 1059–1064. [Google Scholar] [CrossRef]
- Azab, S.F.; Sherbiny, H.S.; Saleh, S.H.; Elsaeed, W.F.; Elshafiey, M.M.; Siam, A.G.; Arafa, M.A.; Alghobashy, A.A.; Bendary, E.A.; Basset, M.A.; et al. Reducing ventilator-associated pneumonia in neonatal intensive care unit using “VAP prevention Bundle”: A cohort study. BMC Infect. Dis. 2015, 15, 314. [Google Scholar] [CrossRef]
- Gokce, I.K.; Kutman, H.G.K.; Uras, N.; Canpolat, F.E.; Dursun, Y.; Oguz, S.S. Successful Implementation of a Bundle Strategy to Prevent Ventilator-Associated Pneumonia in a Neonatal Intensive Care Unit. J. Trop. Pediatr. 2018, 64, 183–188. [Google Scholar] [CrossRef]
- Jahan, I.; Shaon, S.N.U.; Saha, D.; Moni, S.C.; Dey, S.K.; Shahidullah, M. Effectiveness of Educational Intervention in Preventing Ventilator Associated Pneumonia in Neonatal Intensive Care Unit: A Cohort Study: Prevention of ventilator associated pneumonia. Bangladesh Med. Res. Counc. Bull. 2021, 47, 143–150. [Google Scholar] [CrossRef]
- Pinilla-González, A.; Solaz-García, Á.; Parra-Llorca, A.; Lara-Cantón, I.; Gimeno, A.; Izquierdo, I.; Vento, M.; Cernada, M. Preventive bundle approach decreases the incidence of ventilator-associated pneumonia in newborn infants. J. Perinatol. 2021, 41, 1467–1473. [Google Scholar] [CrossRef]
- Menon, K.; Dundon, B.; Twolan, B.L.; AlShammari, S. Approach to unplanned extubations in a pediatric intensive care unit. Can. J. Crit. Care Nurs. 2015, 26, 25–29. [Google Scholar]
- Dezfulian, C.; Shojania, K.; Collard, H.R.; Kim, H.M.; Matthay, M.A.; Saint, S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: A meta-analysis. Am. J. Med. 2005, 118, 11–18. [Google Scholar] [CrossRef]
- Cordero, L.; Sananes, M.; Ayers, L.W. Comparison of a closed (Trach Care MAC) with an open endotracheal suction system in small premature infants. J. Perinatol. 2000, 20, 151–156. [Google Scholar] [CrossRef]
- Halm, M.A.; Krisko-Hagel, K. Instilling normal saline with suctioning: Beneficial technique or potentially harmful sacred cow? Am. J. Crit. Care 2008, 17, 469–472. [Google Scholar] [CrossRef]
- Garland, J.S. Ventilator-associated pneumonia in neonates: An update. NeoReviews 2014, 15, e225–e235. [Google Scholar] [CrossRef]
- Aly, H.; Badawy, M.; El-Kholy, A.; Nabil, R.; Mohamed, A. Randomized, controlled trial on tracheal colonization of ventilated infants: Can gravity prevent ventilator-associated pneumonia? Pediatrics 2008, 122, 770–774. [Google Scholar] [CrossRef]
- Migdał, M.; Pawińska, A.; Siekierzyńska, K.; Murawska, B.; Szreter, T.; Dzierzanowska, D. The optimum frequency of ventilator circuit changes in mechanically ventilated children treated in the paediatric intensive care unit (PICU). J. Hosp. Infect. 1999, 42, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Branson, R.D. The ventilator circuit and ventilator-associated pneumonia. Respir. Care 2005, 50, 774–785. [Google Scholar]
- Samransamruajkit, R.; Jirapaiboonsuk, S.; Siritantiwat, S.; Tungsrijitdee, O.; Deerojanawong, J.; Sritippayawan, S.; Prapphal, N. Effect of frequency of ventilator circuit changes (3 vs. 7 days) on the rate of ventilator-associated pneumonia in PICU. J. Crit. Care 2010, 25, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Ramaswamy, V.V.; Thanigainathan, S.; Pullattayil, A.K.; Kirubakaran, R. Frequency of ventilator circuit changes to prevent ventilator-associated pneumonia in neonates and children-A systematic review and meta-analysis. Pediatr. Pulmonol. 2021, 56, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.S. Critical Care Nurses’ knowledge and compliance with ventilator associated pneumonia bundle at Cairo university hospitals. Crit. Care 2013, 15, 66–78. [Google Scholar]
- Dipanjali, R.; Shivananda, P.M.; Yashoda, S. Effectiveness of an educational intervention on knowledge and practice of staff nurses on prevention of ventilator associated pneumonia among neonates in neonatal intensive care unit. Int. J. Caring Sci. 2020, 13, 1421. [Google Scholar]
- Danchaivijitr, S.; Assanasen, S.; Apisarnthanarak, A.; Judaeng, T.; Pumsuwan, V. Effect of an Education Program on the Prevention of Ventilator-Associated Pneumonia: A Multicenter Study. J. Med. Assoc. Thail. 2005, 88, 36–41. [Google Scholar]
| Neo-KISS Module [14] | Van der Zwett et al. [7] |
|---|---|
Radiological findings
| Clinical findings One of the following:
|
| AND Deterioration of gas exchange, drop in saturation | Radiological findings New emergence or progression of the following
|
AND FOUR of the following criteria
| Microbiological findings *
|
| Study | Study Design | Diagnostic Criteria | Incidence | Most Common Pathogen | Risk Factors |
|---|---|---|---|---|---|
| African (AFR) and Eastern Mediterranean Region (EMR) | |||||
| Gohr et al. [30] | Prospective observational cohort 140 neonates | Radiographic Clinical Laboratory Microbiologic | 27.2% | Klebsiella spp. S. aureus Candida spp. | Reintubation Use of sedatives |
| Khattab et al. [16] | Prospective observational 85 neonates | Radiographic Clinical Microbiological | 55.2% | S. aureus Klebsiella spp. Candida spp. | Low birth weight MV |
| Afjeh et al. [31] | Prospective cohort81 neonates | Radiographic Clinical | 11.6 episodes | E. coli K. pneumoniae | Purulent sputum |
| Badr et al. [14] | Prospective observational 56 neonates | Radiographic Clinical Laboratory Microbiologic | 57.1% | Klebsiella spp. S. aureus Pseudomonas spp. E. coli | Low birth weight Duration of MV Prematurity |
| ELMeneza et al. [32] | Prospective observational 91 neonates | Radiographic Clinical Microbiological | 43.2 episodes/1000 ventilator days | Klebsiella spp. Pseudomonas Staph aureus | Prematurity RDS Reintubation Duration of MV |
| Region of the Americas (AMR) | |||||
| Dudeck et al. [33] | Prospective observational 137 NICUs | CDC/NNIS definition | 1.0 episodes/1000 ventilator days for neonates < 750 g 0.1 episodes/1000 ventilator days for neonates > 2500 g | Not reported | Not reported |
| Patrick et al. [34] | Prospective cohort 173 NICUs | CDC/NNIS definition | 1.6 episodes/1000 ventilator days (2007) 0.6 episodes/1000 ventilator days (2012) | Not reported | VLBW |
| Urzedo et al. [35] | Prospective cohort 4615 neonates | Radiographic Clinical Microbiological | 3.2 episodes/1000 ventilator days | Coagulase (-) Staphylococcus | Not reported |
| Romanelli et al. [36] | Prospective observational 886 neonates | CDC/NNIS definition | 5.7 episodes/1000 ventilator days | Staphylococcus aureus Klebsiella spp. Enterobacter cloacae | Not reported |
| South East Asian Region (EASR) and Western Pacific Region (WPR) | |||||
| Mir ZH et al. [37] | Prospective observational 96 neonates | Radiographic Clinical microbiological | 68.96 episodes/1000 ventilator days | Klebsiella spp. E. coli Acinetobacter | Birth weight <1500 g Duration of MV |
| Vijayakanthi et al. [38] | Retrospective observational 265 neonates | Radiographic Clinical Microbiological | 22.2 episodes/1000 ventilator days | Klebsiella spp. | Repeated intubations Unstable initial Cardiopulmonary assessment |
| Ibrahim et al. [39] | Descriptive correlational 1090 neonates | CDC/NNIS definition | 5.7 episodes/1000 ventilator days | S. aureus Klebsiella spp. Acinetobacter spp. | Birth weight Gestational age |
| Katoch et al. [40] | Prospective observational 37 neonates | CDC/NNIS definition | 23.3% | Not reported | Preterm birth (<37 g.w.) |
| Kawanishi et al. [41] | Retrospective observational 71 neonates ≤2000 g | RadiographicClinical MicrobiologicalFoglia criteria | 8.44 episodes/1000 ventilator days | S. aureus, P. aeruginosa | BW < 626 g |
| Lee et al. [42] | Retrospective observational 605 neonates | RadiographicClinical Microbiological | 7.1 episodes/1000 ventilator days | K. pneumoniae B. cepacia | Longer duration of intubation TPN |
| Navoa et al. [43] | Prospective observational 1813 neonates | RadiographicClinical Microbiological | 9.6 episodes/1000 ventilator days | Acinetobacter spp. Pseudomonas spp. Enterobacter spp. | Not reported |
| Thatrimontrichai et al. [44] | Prospective cohort 128 neonates | CDC/NNIS definition | 10.1 episodes/1000 ventilator days | Acinetobacter baumannii Stenotrophomonas maltophilia | BW ≤ 750 gsedative medication use |
| Deng et al. [45] | Retrospective case–control 349 patients | Foglia definition | 25.6 episodes/1000 ventilator days | Klebsiella spp. Acinetobacter baumannii | Birth weight reintubation Respiratory symptoms |
| Cai et al. [46] | Prospective observational 1159 neonates | CDC/NNIS definition | 48.8 episodes/1000 ventilator days | Acinetobacter baumannii Klebsiella pneumoniae Coagulase negative staphylococcus | Not reported |
| European region (EUR) | |||||
| Demirbag et al. [47] | Point-prevalence 47 neonates | CDC/NNIS definition | 38.2% | Klebsiella spp. MRSA | Not reported |
| Dell Orto et al. [48] | Prospective, population-based cohort 199 neonates | CDC/NNIS definition | 17.1 episodes/1000 ventilator days | E. coli Klebsiella spp. Staph haem. | Not reported |
| Wojkowska et al. [49] | Prospective cohort 1695 neonates | NEO-KISS definition | 18.2 episodes/1000 ventilator days | CONS P. aeruginosa A. baumannii | Duration of MV |
| Cernada et al. [19] | Prospective observational cohort | CDC/NNIS definition | 10.9 episodes/1000 ventilator days | P. aeruginosa S. aureus Polymicrobial (16.7%) | Duration of MV |
| Scamardo et al. [50] | Prospective observational 1265 neonates | CDC/NNIS definition | 20% | P. aeruginosa (28%), Stenotrophomonas maltophilia (20%) CONS (20%) | Not reported |
| Geslain et al. [51] | Prospective observational 381 neonates | CDC/NNIS definition | 8.8 episodes/1000 ventilator days | Enterobacter cloacae Staph. spp. Klebsiella spp. | BW < 1000 g Higher SNAPP score |
| Leistner et al. [52] | Patient-based prospective 33 048 VLBW neonates | NEO-KISS definition | 2.3 episodes/1000 ventilator days | Staph aureus CONS Klebsiella spp. | Not reported |
| Yalaz et al. [53] | Prospective cohort 600 neonates | CDC/NNIS definition | 13.76 episodes/1000 ventilator days | Stenotrophomonas maltophilia Kl. pneumoniae Pseudomonas aeruginosa | Not reported |
| Study | Interventions Included in the Bundle | VAP Rates | Mortality Rates |
|---|---|---|---|
| Brilli et al. [83] 2008 | Head of bed elevation, daily assessment of readiness for extubation while providing oral care, administering medication to prevent peptic ulcers, practicing proper hand hygiene, changing ventilator circuit if visibly soiled or malfunctioning | Pre-intervention 7.8 episodes/1000 ventilator days | Not reported |
| Post-intervention 0.5 episodes/1000 ventilator days | |||
| Pepin et al. [84] 2012 | Proper hand hygiene, meticulous intubation technique, assessment of readiness for extubation, thorough disinfection of the environment and equipment, effective management of bedside patient care routines | Pre-intervention 8.5 episodes/1000 ventilator days | Not reported |
| Post-intervention 2.5 episodes/1000 ventilator days | |||
| Rosenthal et al. [85] 2012 | Hand hygiene, daily assessment of readiness for extubation, oral care with an antiseptic solution, use of noninvasive ventilation when possible, change in ventilator circuit only when visibly soiled | Pre-intervention 17.8 episodes/1000 ventilator days | Not reported |
| Post-intervention 12.0 episodes/1000 ventilator days | |||
| Zhou et al. [86] 2013 | Hand hygiene, assessment of readiness for extubation, closed endotracheal suctioning, educational activities, weekly changing of the ventilator circuit, rational use of antibiotics | Pre-intervention 48.8 episodes/1000 ventilator days | Pre-intervention 14.0% |
| Post-intervention 20.8 episodes/1000 ventilator days | Post-intervention 2.7% | ||
| Azab et al. [87] 2015 | Head of bed elevation, daily assessment of readiness for extubation, oral care, peptic ulcer prophylaxis, hand hygiene, changing ventilator circuit if visibly soiled or malfunctioning | Pre-intervention 36.4 episodes/1000 ventilator days | Pre-intervention 25.8 % |
| Post-intervention 23.0 episodes/1000 ventilator days | Post-intervention 17.3% | ||
| Gocke et al. [88] 2018 | Adherence to hand hygiene guidelines, readiness to wean assessment, ventilator circuit evaluation and changing the circuit only when visibly soiled or malfunctioning, periodic draining and discarding of ventilator circuit condensate, bed head elevation to 10–13 degrees, oral care | Pre-intervention 7.3 episodes/1000 ventilator days | Not reported |
| Post-intervention 2.7 episodes/1000 ventilator days | |||
| Jahan et al. [89] 2018 | Hand hygiene, daily assessment of extubation readiness, use of non-invasive ventilation when possible, head of bed elevation, oral care, changing ventilator circuit when visibly soiled | Pre-intervention 59% | Pre-intervention 68.2% |
| Pre-intervention 26.3% | Post-intervention 52.6% | ||
| Pinilla-González et al. [90] 2021 | Healthcare training, hand hygiene, sterile management of airways, avoiding reintubation, oral care, head of bed elevation, changing ventilator circuit only when visibly soiled, tube feeding for 60–120 min | Pre-intervention 11.8 episodes/1000 ventilator days | Pre-intervention 21.3% |
| Post-intervention 1.9 episodes/1000 ventilator days | Post-intervention 13.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangelova, V.; Kevorkyan, A.; Raycheva, R.; Krasteva, M. Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit—Incidence and Strategies for Prevention. Diagnostics 2024, 14, 240. https://doi.org/10.3390/diagnostics14030240
Rangelova V, Kevorkyan A, Raycheva R, Krasteva M. Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit—Incidence and Strategies for Prevention. Diagnostics. 2024; 14(3):240. https://doi.org/10.3390/diagnostics14030240
Chicago/Turabian StyleRangelova, Vanya, Ani Kevorkyan, Ralitsa Raycheva, and Maya Krasteva. 2024. "Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit—Incidence and Strategies for Prevention" Diagnostics 14, no. 3: 240. https://doi.org/10.3390/diagnostics14030240
APA StyleRangelova, V., Kevorkyan, A., Raycheva, R., & Krasteva, M. (2024). Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit—Incidence and Strategies for Prevention. Diagnostics, 14(3), 240. https://doi.org/10.3390/diagnostics14030240






