Nailfold Capillaroscopy Analysis Can Add a New Perspective to Biomarker Research in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Abstract
:1. Introduction
2. Nailfold Video Capillaroscopy
3. Nailfold Video Capillaroscopy in Vasculitis
3.1. Characteristics of the Identified Articles
3.2. Capillaroscopic Phenotypes
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hagen, E.; Andrassy, K.; Csernok, E.; Daha, M.; Gaskin, G.; Gross, W.; Hansen, B.; Heigl, Z.; Hermans, J.; Jayne, D.; et al. Development and standardization of solid phase assays for the detection of anti-neutrophil cytoplasmic antibodies (ANCA) A report on the second phase of an international cooperative study on the standardization of ANCA assays. J. Immunol. Methods 1996, 196, 1–15. [Google Scholar] [CrossRef]
- Almaani, S.; Fussner, L.A.; Brodsky, S.; Meara, A.S.; Jayne, D. ANCA-Associated Vasculitis: An Update. J. Clin. Med. 2021, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Pagnoux, C. Updates in ANCA-associated vasculitis. Eur. J. Rheumatol. 2016, 3, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Kallenberg, C.G.M. Pathogenesis of ANCA-Associated Vasculitis, an Update. Clin. Rev. Allergy Immunol. 2011, 41, 224–231. [Google Scholar] [CrossRef]
- Pyo, J.Y.; Lee, L.E.; Park, Y.B.; Lee, S.W. Comparison of the 2022 ACR/EULAR Classification Criteria for Antineutrophil Cytoplasmic Antibody-Associated Vasculitis with Previous Criteria. Yonsei Med. J. 2023, 64, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J. Small-Vessel Vasculitis. N. Engl. J. Med. 1997, 337, 1512–1523. [Google Scholar] [CrossRef]
- Mueller, A.; Holl-Ulrich, K.; Gross, W.L. Granuloma in ANCA-Associated Vasculitides: Another Reason to Distinguish Between Syndromes? Curr. Rheumatol. Rep. 2013, 15, 376. [Google Scholar] [CrossRef]
- Tavakol, M.E.; Fatemi, A.; Karbalaie, A.; Emrani, Z.; Erlandsson, B.-E. Nailfold Capillaroscopy in Rheumatic Diseases: Which Parameters Should Be Evaluated? BioMed Res. Int. 2015, 2015, 974530. [Google Scholar] [CrossRef]
- Chojnowski, M.M.; Felis-Giemza, A.; Olesińska, M. Capillaroscopy—A role in modern rheumatology. Reumatologia 2016, 54, 67–72. [Google Scholar] [CrossRef]
- Cutolo, M. (Ed.) Atlas of Capillaroscopy in Rheumatic Diseases; Elsevier: Milan, Italy, 2010; pp. 103–113. [Google Scholar]
- Lee, P.; Leung, F.Y.; Alderdice, C.; Armstrong, S.K. Nailfold capillary microscopy in the connective tissue diseases: A semiquantitative assessment. J. Rheumatol. 1983, 10, 930–938. [Google Scholar]
- Keret, S.; Mazzawi, J.; Slobodin, G.; Rimar, O.; Rosner, I.; Rozenbaum, M.; Kaly, L.; Boulman, N.; Awisat, A.; Shouval, A.; et al. Nailfold video capillaroscopy as a useful diagnostic tool in systemic vasculitis. Microvasc. Res. 2022, 143, 104406. [Google Scholar] [CrossRef]
- Julia, U.M.; Rosalía, M.P.; Luisa, V.F.M.; Luis, M.d.l.F.J. Periungual Capillaroscopy Findings in a Series of Patients with Granulomatosis with Polyangiitis (Wegener): An Observational Study. Open J. Rheumatol. Autoimmune Dis. 2013, 3, 130–134. [Google Scholar] [CrossRef]
- Rimar, D.; Ingegnoli, F.; Rimar, O.; Rosner, I.; Rosenbaum, M.; Kaly, L.; Boulman, N.; Awisat, A.; Slobodin, G. AB0609 Nailfold video capillaroscopy as a potential diagnostic tool in systemic vasculitis. Ann. Rheum. Dis. 2019, 78, 1765. [Google Scholar] [CrossRef]
- Triggianese, P.; D’antonio, A.; Nesi, C.; Kroegler, B.; Di Marino, M.; Conigliaro, P.; Modica, S.; Greco, E.; Nucci, C.; Bergamini, A.; et al. Subclinical microvascular changes in ANCA-vasculitides: The role of optical coherence tomography angiography and nailfold capillaroscopy in the detection of disease-related damage. Orphanet J. Rare Dis. 2023, 18, 184. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kotani, T.; Wakura, R.; Suzuka, T.; Kuwabara, H.; Kiboshi, T.; Wada, Y.; Shiba, H.; Hata, K.; Shoda, T.; et al. Examination of nailfold videocapillaroscopy findings in ANCA-associated vasculitis. Rheumatology 2023, 62, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Haedecke, C.; Sigl, T.; Krüger, K. Avascular areas on nailfold capillary microscopy of patients with wegener’s granulomatosis. Clin. Rheumatol. 2000, 19, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Sendino Revuelta, A.; Barbado Hernández, F.J.; Torrijos Eslava, A.; González Anglada, I.; Peña Sánchez de Rivera, J.M.; Vázquez Rodríguez, J.J. Capilaroscopia en las vasculitis [Capillaroscopy in vasculitis]. An. Med. Interna 1991, 8, 217–220. [Google Scholar] [PubMed]
- Sullivan, M.M.; Abril, A.; Aslam, N.; Ball, C.T.; Berianu, F. Nailfold Videocapillaroscopy in Antineutrophil Cytoplasmic Antibody–Associated Vasculitis. Version 1. Available online: https://www.researchsquare.com/article/rs-3443737/v1 (accessed on 28 October 2023).
- Cutolo, M.; Grassi, W.; Cerinic, M.M. Raynaud’s phenomenon and the role of capillaroscopy. Arthritis Rheum. 2003, 48, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Bajgai, P.; Singh, R. Retinal Vasculitis in Takayasu’s Arteritis. N. Engl. J. Med. 2018, 378, e28. [Google Scholar] [CrossRef]
- Bertolazzi, C.; Gallegos-Nava, S.; Villarreal-Treviño, A.V.; Alfaro-Rodriguez, A.; Clavijo-Cornejo, D.; Gutierrez, M. The current role of capillaroscopy in vasculitides. Clin. Rheumatol. 2019, 38, 2299–2307. [Google Scholar] [CrossRef]
- Hoerth, C.; Kundi, M.; Katzenschlager, R.; Hirschl, M. Qualitative and quantitative assessment of nailfold capillaries by capillaroscopy in healthy volunteers. Vasa 2012, 41, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Flossmann, O.; Bacon, P.; de Groot, K.; Jayne, D.; Rasmussen, N.; Seo, P.; Westman, K.; Luqmani, R. Development of comprehensive disease assessment in systemic vasculitis. Ann. Rheum. Dis. 2007, 66, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef]
- Mishra, A.; Grover, C.; Singal, A.; Narang, S.; Das, G.K. Nailfold capillary changes in newly diagnosed hypertensive patients: An observational analytical study. Microvasc. Res. 2021, 136, 104173. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, E.V.; Almeida, I.N.; Gracitelli, C.P.; Agapito, C.; Zett, C.; Sant’Ana, L.; Kayser, C.; Prata, T.S.; Paranhos, A. Peripheral microvascular abnormalities Associated with Open-Angle Glaucoma. Ophthalmol. Glaucoma 2023, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Bernero, E.; Sulli, A.; Ferrari, G.; Ravera, F.; Pizzorni, C.; Ruaro, B.; Zampogna, G.; Alessandri, E.; Cutolo, M. Prospective capillaroscopy-based study on transition from primary to secondary Raynaud’s phenomenon: Preliminary results. Reumatismo 2013, 65, 186–191. [Google Scholar] [CrossRef]
- Cutolo, M.; Trombetta, A.C.; Melsens, K.; Pizzorni, C.; Sulli, A.; Ruaro, B.; Paolino, S.; Deschepper, E.; Smith, V. Automated assessment of absolute nailfold capillary number on videocapillaroscopic images: Proof of principle and validation in systemic sclerosis. Microcirculation 2018, 25, e12447. [Google Scholar] [CrossRef]
- Cutolo, M.; Vanhaecke, A.; Ruaro, B.; Deschepper, E.; Ickinger, C.; Melsens, K.; Piette, Y.; Trombetta, A.C.; De Keyser, F.; Smith, V. Is laser speckle contrast analysis (LASCA) the new kid on the block in systemic sclerosis? A systematic literature review and pilot study to evaluate reliability of LASCA to measure peripheral blood perfusion in scleroderma patients. Autoimmun. Rev. 2018, 17, 775–780. [Google Scholar] [CrossRef]
- Lambrecht, V.; Cutolo, M.; De Keyser, F.; Decuman, S.; Ruaro, B.; Sulli, A.; Deschepper, E.; Smith, V. Reliability of the quantitative assessment of peripheral blood perfusion by laser speckle contrast analysis in a systemic sclerosis cohort. Ann. Rheum. Dis. 2016, 75, 1263–1264. [Google Scholar] [CrossRef]
- Sulli, A.; Ruaro, B.; Cutolo, M. Evaluation of blood perfusion by laser speckle contrast analysis in different areas of hands and face in patients with systemic sclerosis. Ann. Rheum. Dis. 2014, 73, 2059–2061. [Google Scholar] [CrossRef]
- Gracia, T.B.; Ramos, I.E. Nailfold capillaroscopy. Med. Clin. 2023, 160, 499–500. [Google Scholar] [CrossRef]
- Movasat, A.; Shahram, F.; Carreira, P.E.; Nadji, A.; Akhlaghi, M.; Naderi, N.; Davatchi, F. Nailfold capillaroscopy in Behçet’sdisease, analysis of 128 patients. Clin. Rheumatol. 2009, 28, 603–605. [Google Scholar] [CrossRef]
- Martino, F.; Agolini, D.; Tsalikova, E.; Bederti, O.; Principessa, L.; Martino, E.; Carnevali, E.; Giardini, O. Nailfold capillaroscopy in Henoch-Schönlein purpura: A follow-up study of 31 cases. J. Pediatr. 2022, 141, 145. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, A.; Rigante, D.; Bersani, G.; Rendeli, C.; Feliciani, C.; Stabile, A. Longitudinal study of microvascular involvement by nailfold capillaroscopy in children with Henoch–Schönlein purpura. Clin. Rheumatol. 2009, 28, 1101–1105. [Google Scholar] [CrossRef]
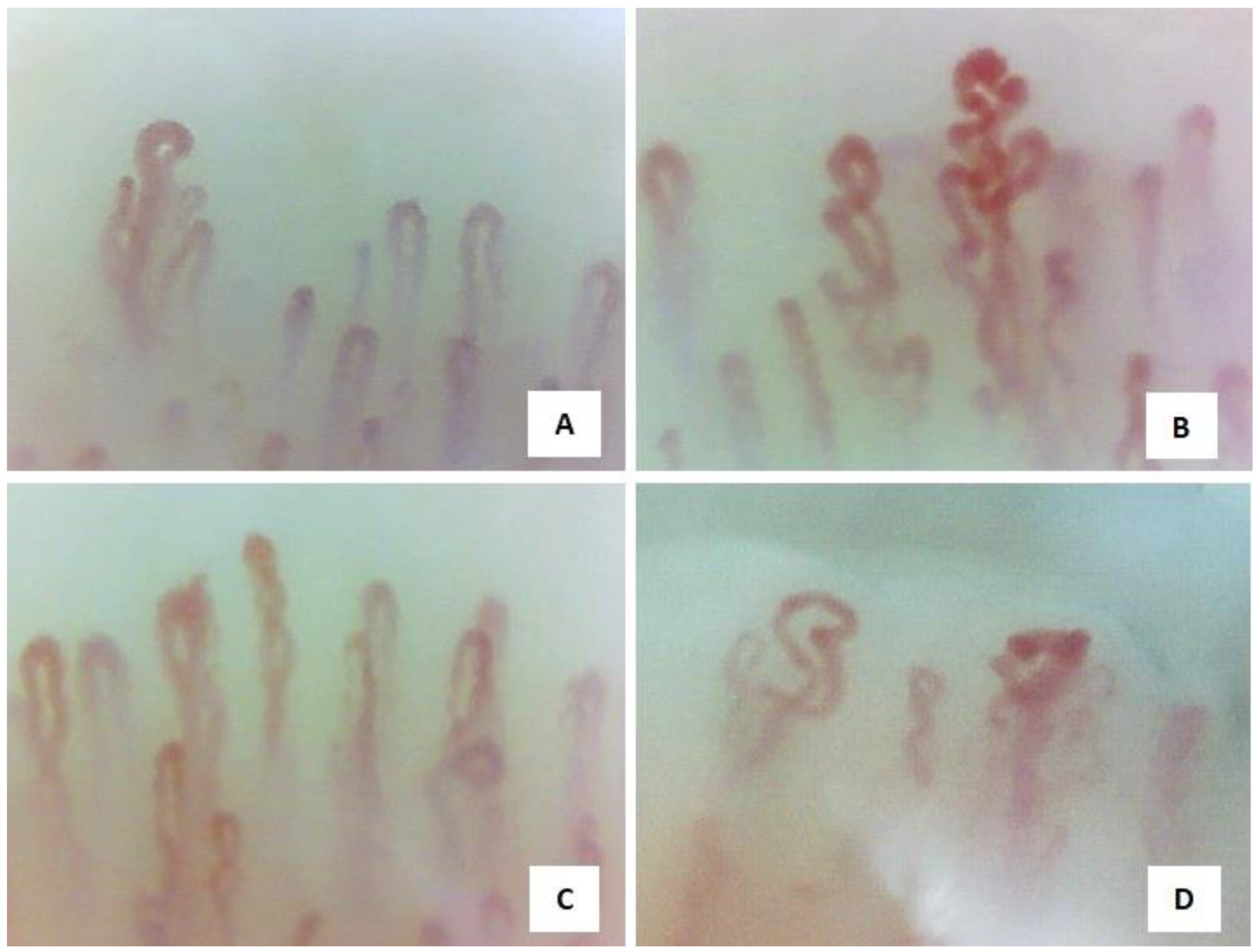
| Normal Capillaroscopic Parameters | |
| Capillary array, architecture, morphology | Homogeneous distribution of U-shaped capillaries, perpendicular to the nailfold |
| Capillary tortuosity | Usually absent |
| Dilatated and giant capillaries | Not present |
| Ramified capillaries | Not present |
| Neoangiogenesis | Not present |
| Haemorrhages, hemosiderin deposits | Usually not present, may be present after local trauma |
| Capillary number | Density of between 9 and 14 capillaries per mm |
| Avascular areas | Not present |
| Capillary blood flow | Dynamic, without stasis of red blood cells |
| Selected Articles Reporting any Possible Capillaroscopy Alterations Observed | ||||
|---|---|---|---|---|
| Articles | Study Methods | Exclusion Criteria in the Vasculitis Group | Capillaroscopic Strategy and Methods | Capillaroscopy Findings |
| Keret et al. [12] | 25 patients were examined via NVC, 17 with active vasculitis and 8 with vasculitis in remission. Among them, only 1 patient with active GPA, 3 with active EGPA, and 2 with active MPA. | Patients were excluded if they had history of onychophagia, recurrent trauma to fingers, severe metabolic vasculopathy, and CTDs. | Capillaroscopic changes were evaluated using Optilia Mediscope. NVC was performed on fingers 2, 3, 4, 5 in both hands and by only experienced examiners in all vasculitis patients. | Regarding the NVC abnormalities, they were significantly more common in patients with vasculitis in comparison with healthy controls. |
| Julia et al [13] | 10 patients with GPA were studied using periungual capillaroscopy. All were under treatment and with active vasculitis. | Not defined. | The capillaroscope used was a stereomicroscope and perilingual capillaroscopy was performed at the level of the 3rd, 4th and 5th fingers of the right and left hands, always by the same operator. | Capillaroscopic alterations were found in 8 out of 10 patients enrolled. |
| Rimar et al. [14] | 25 patients were evaluated by NVC, among whom there were 8 with vasculitis in remission and 7 with AAV (2 GPA, 3 EGPA, 2 MPA). | Subjects affected by peripheral artery disease and ischemic heart disease were excluded. | Capillaroscopic changes were evaluated using Optilia Mediscope. | Higher rates of non-specific capillaroscopic abnormalities were detected in the majority of study cohort. |
| Triggianese et al. [15] | Observational study was realised on 23 patients affected by AAV. They were analysed via NVC. | Exclusion criteria were systemic disorders such as diabetes or patients with severe renal dysfunction and other autoimmune systemic diseases (recent and previous history), neoplasia, pregnancy or lactation. | Nailfold vessel examination was performed using the NVC (Inspectis Digital Capillaroscope Light CAP-1). | 82% of AAV cohort showed non-specific NVC abnormalities, which were found with similar rates compared with healthy controls. |
| Matsuda et al. [16] | 51 patients were examined by NVC, all were newly diagnosed with AAV. NVC was performed at the first visit and was repeatedly evaluated 3 months after immunosuppressive therapy. | Patients with infectious vasculitis, drug-induced or secondary vasculitis, sarcoidosis or other CTDs and malignancy were excluded. | NVC was assessed using a Dino-lite capillaroscopy device. NVC was evaluated on fingers 2, 3, 4, 5 in both hands. | 70.6% of the enrolled patients showed significant NVC changes; 4 patients even showed a scleroderma pattern. |
| Anders et al. [17] | They prospectively analysed of 116 patients by reviewing nailfold capillary microscopy records and made the final diagnosis of GPA for 12 patients, such that they were all newly diagnosed. | Not defined. | NCM was performed with a bifocal stereomicroscope. All fingers were examined in both hands in each patient. | Avascular areas were exclusively found in patients with GPA, in 92% of total GPA patients. |
| Sendino et al. [18] | Light microscopy was used to report capillary alterations in 15 patients with various vasculitis (i.e. ANCA-associated vasculitis, giant cell arteritis (GCA) and polyarteritis nodosa (PAN)). | Not defined. | Not defined. | Capillaroscopy showed changes in 11 patients, with higher rates in the active phase of the diseases. |
| Sullivan et al. [19] | 33 patients affected by small-vessel vasculitis were recruited and they were analysed using NVC. | Patients were excluded if they had history of CTDs, type 2 diabetes, dialysis dependence, antiphospholipid syndrome, bleeding or recent hand trauma, among others. | Capillaroscopy was performed using the NVC and a minimum of 2 images per nailfold was evaluated for 8 digits (excluding thumbs) for each patient. | NVC abnormalities were detected only in 54.5% of AAV group (18/33). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Screm, G.; Mondini, L.; Confalonieri, P.; Salton, F.; Trotta, L.; Barbieri, M.; Mari, M.; Reccardini, N.; Della Porta, R.; Kodric, M.; et al. Nailfold Capillaroscopy Analysis Can Add a New Perspective to Biomarker Research in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Diagnostics 2024, 14, 254. https://doi.org/10.3390/diagnostics14030254
Screm G, Mondini L, Confalonieri P, Salton F, Trotta L, Barbieri M, Mari M, Reccardini N, Della Porta R, Kodric M, et al. Nailfold Capillaroscopy Analysis Can Add a New Perspective to Biomarker Research in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Diagnostics. 2024; 14(3):254. https://doi.org/10.3390/diagnostics14030254
Chicago/Turabian StyleScrem, Gianluca, Lucrezia Mondini, Paola Confalonieri, Francesco Salton, Liliana Trotta, Mariangela Barbieri, Marco Mari, Nicolò Reccardini, Rossana Della Porta, Metka Kodric, and et al. 2024. "Nailfold Capillaroscopy Analysis Can Add a New Perspective to Biomarker Research in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis" Diagnostics 14, no. 3: 254. https://doi.org/10.3390/diagnostics14030254
APA StyleScrem, G., Mondini, L., Confalonieri, P., Salton, F., Trotta, L., Barbieri, M., Mari, M., Reccardini, N., Della Porta, R., Kodric, M., Bandini, G., Hughes, M., Bellan, M., Lerda, S., Confalonieri, M., & Ruaro, B. (2024). Nailfold Capillaroscopy Analysis Can Add a New Perspective to Biomarker Research in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Diagnostics, 14(3), 254. https://doi.org/10.3390/diagnostics14030254










