Diagnostic Differentiation between Pancreatitis and Pancreatic Cancer: A Scoping Review
Abstract
1. Introduction
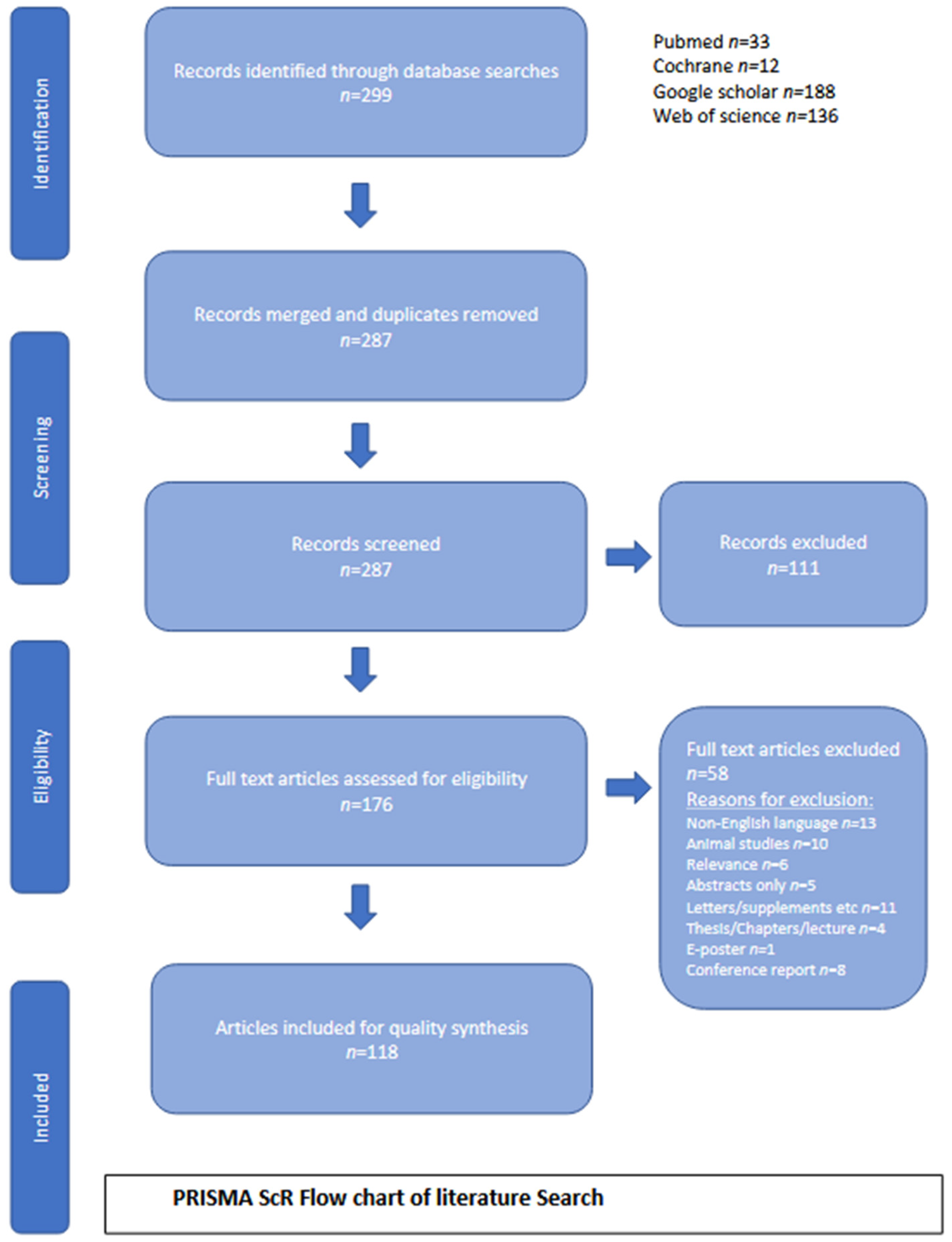
2. Methodology
2.1. Search Strategy
2.2. Study Type
- Case reports.
- Randomised controlled studies.
- Cohort studies.
- Systematic reviews and meta-analyses.
2.3. Diagnostic Differentiation
- Biomarkers.
- Imaging.
2.4. Eligibility Criteria
2.4.1. Inclusion Criteria
2.4.2. Exclusion Criteria
3. Results
3.1. Data Analysis and Presentation
3.1.1. Diagnostic Differentiation
3.1.2. Biomarkers
- Authors.
- Year of publication.
- Country of origin.
- Biomarkers identified for diagnostic differentiation.
3.1.3. Imaging
- Authors.
- Year of publication.
- Country of origin.
- Cross-sectional imaging identified for diagnostic differentiation.
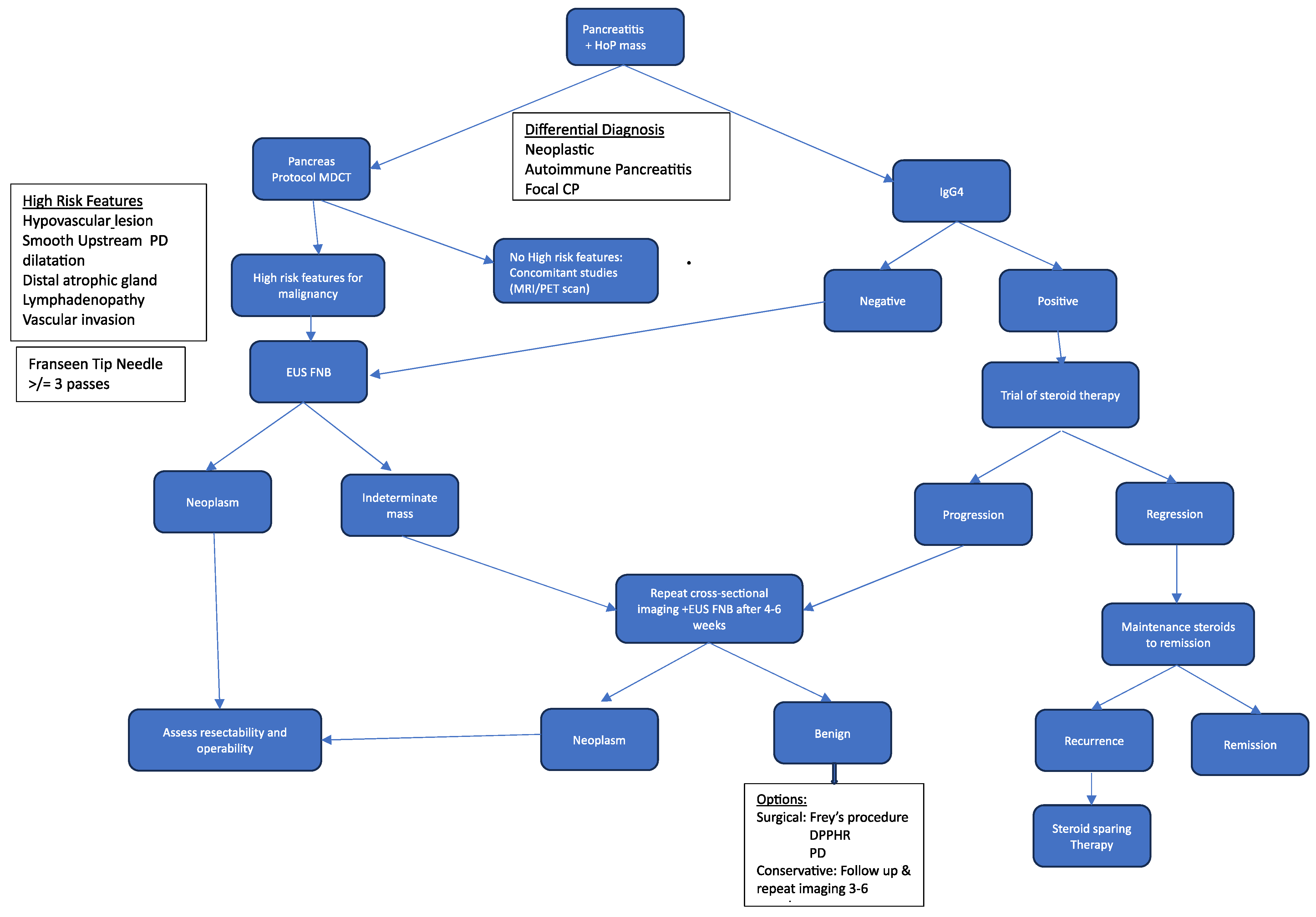
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP | Acute pancreatitis |
| AIP | Autoimmune pancreatitis |
| CA19-9 | Serum carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CEH-EUS | Contrast-enhanced harmonic endoscopic ultrasound |
| CP | Chronic pancreatitis |
| CT | Computed tomography |
| DM | Diabetes mellitus |
| DNA | Deoxyribonucleic acid |
| DPPHR | Duodenal-preserving pancreatic head resection |
| DWI-MRI | Diffusion-Weighted Imaging–Magnetic Resonance Imaging |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| EUS | Endoscopic ultrasound |
| FAP | Familial adenomatous polyps |
| 18F-FDG | 2-deoxy-2-[fluorine-18]fluoro-D-glucose |
| FNA | Fine-needle aspiration |
| FNB | Fine-needle biopsy |
| HBROCS | Hereditary breast and ovarian cancer syndrome |
| HoP Head of pancreas | |
| HP | Hereditary pancreatitis |
| HSP | Heat shock protein 70 |
| IgG4 | Immunoglobulin G4 |
| MDCT | Multi-detector computed tomography |
| MIC-1 | Macrophage inhibitor cytokine |
| MMP-9 | Matrix metalloproteinase 9 |
| MRCP | Magnetic Resonance Cholangiopancreatography |
| MRI | Magnetic resonance imaging |
| PAP | Progressive acute pancreatitis |
| PC | Pancreatic cancer |
| PD | Pancreatico-duodenectomy |
| PDAC | Pancreatic ductal adenocarcinoma |
| PET | Positron emission tomography |
| PHM | Peripancreatic hypoechoic margins |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping review |
| RAP | Recurrent acute pancreatitis |
| αSMA | α-smooth muscle actin |
| SUVmax | Maximum standardised uptake value |
| TAUS | Transabdominal ultrasound |
| TGF-1 | Transforming growth factor-Beta 1 |
| TIC | Time–intensity curves |
| TIMP 1 | Tissue inhibitor of metalloproteinase 1 |
| uPAR | Urokinase-type plasminogen activator receptor |
References
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Takeyama, Y. Long-term prognosis of acute pancreatitis in Japan. Clin. Gastroenterol. Hepatol. 2009, 7, S15–S17. [Google Scholar] [CrossRef]
- Nøjgaard, C.; Becker, U.; Matzen, P.; Andersen, J.R.; Holst, C.; Bendtsen, F. Progression from acute to chronic pancreatitis: Prognostic factors, mortality, and natural course. Pancreas 2011, 40, 1195–1200. [Google Scholar] [CrossRef]
- Tao, H.; Xu, J.; Li, N.; Chang, H.; Duan, L. Early identification of high-risk patients with acute recurrent pancreatitis progression to chronic pancreatitis. Arch. Med. Sci. 2022, 18, 535–539. [Google Scholar] [CrossRef]
- Whitcomb, D.C. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am. J. Physiol. Liver Physiol. 2004, 287, G315–G319. [Google Scholar] [CrossRef]
- Yamada, R.; Tsuboi, J.; Murashima, Y.; Tanaka, T.; Nose, K.; Nakagawa, H. Advances in the Early Diagnosis of Pancreatic Ductal Adenocarcinoma and Premalignant Pancreatic Lesions. Biomedicines 2023, 11, 1687. [Google Scholar] [CrossRef]
- Alhobayb, T.; Peravali, R.; Ashkar, M. The Relationship between Acute and Chronic Pancreatitis with Pancreatic Adenocarcinoma: Review. Diseases 2021, 9, 93. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Korpela, T.; Udd, M.; Mustonen, H.; Ristimäki, A.; Haglund, C.; Seppänen, H.; Kylänpää, L. Association between chronic pancreatitis and pancreatic cancer: A 10-year retrospective study of endoscopically treated and surgical patients. Int. J. Cancer 2020, 147, 1450–1460. [Google Scholar] [CrossRef]
- Esposito, I.; Hruban, R.H.; Verbeke, C.; Terris, B.; Zamboni, G.; Scarpa, A.; Morohoshi, T.; Suda, K.; Luchini, C.; Klimstra, D.S.; et al. Guidelines on the histopathology of chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and the European Pancreatic Club. Pancreatology 2020, 20, 586–593. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; DiMagno, E.P.; Andren-Sandberg, A.; Domellof, L. Pancreatitis and the risk of pancreatic cancer. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef]
- Perumal, S.; Palaniappan, R.; Pillai, S.A.; Velayutham, V.; Sathyanesan, J. Predictors of malignancy in chronic calcific pancreatitis with head mass. World J. Gastrointest. Surg. 2013, 5, 97–103. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Lowenfels, A.B. Chronic pancreatitis and pancreatic cancer. Dig. Dis. 2002, 20, 32–37. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, T.; Zheng, J.; Kang, W.; Xu, J.; Gao, Z.; Ma, J. Untypical autoimmune pancreatitis and pancreatic cancer: Differential diagnosis experiences extracted from misdiagnose of two cases. Orphanet J. Rare Dis. 2019, 14, 245. [Google Scholar] [CrossRef]
- Tang, D.; Wu, Q.; Zhang, J.; Zhang, H.; Yuan, Z.; Xu, J.; Chong, Y.; Huang, Y.; Xiong, Q.; Wang, S.; et al. Galectin-1 expression in activated pancreatic satellite cells promotes fibrosis in chronic pancreatitis/pancreatic cancer via the TGF-β1/Smad pathway. Oncol. Rep. 2018, 39, 1347–1355. [Google Scholar] [CrossRef]
- Haeberle, L.; Steiger, K.; Schlitter, A.M.; Safi, S.A.; Knoefel, W.T.; Erkan, M.; Esposito, I. Stromal heterogeneity in pancreatic cancer and chronic pancreatitis. Pancreatology 2018, 18, 536–549. [Google Scholar] [CrossRef]
- Dite, P.; Novotny, I.; Dvorackova, J.; Kianicka, B.; Blaho, M.; Svoboda, P.; Uvirova, M.; Rohan, T.; Maskova, H.; Kunovsky, L. Pancreatic solid focal lesions: Differential diagnosis between autoimmune pancreatitis and pancreatic cancer. Dig. Dis. 2019, 37, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Hong, W.; Guo, Y.; Bai, Y.; Chen, B. Molecular mechanism of pancreatic stellate cells activation in chronic pancreatitis and pancreatic cancer. J. Cancer 2020, 11, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Marinho, R.; Alves, A.; Pignatelli, N.; Nunes, V. Unclassified autoimmune pancreatitis mimicking pancreatic cancer. J. Surg. Case Rep. 2019, 2019, rjy340. [Google Scholar] [CrossRef] [PubMed]
- Negoi, I.; Beuran, M.; Hostiuc, S.; Sartelli, M.; El-Hussuna, A.; De-Madaria, E. Glycosylation alterations in acute pancreatitis and pancreatic cancer: CA19-9 expression is involved in pathogenesis and maybe targeted by therapy. Ann. Transl. Med. 2019, 7 (Suppl. S8), S306. [Google Scholar] [CrossRef]
- Aronen, A.; Aittoniemi, J.; Huttunen, R.; Nikkola, A.; Rinta-Kiikka, I.; Nikkola, J.; Limnell, O.; Nordback, I.; Sand, J.; Laukkarinen, J. Plasma suPAR may help to distinguish between chronic pancreatitis and pancreatic cancer. Scand. J. Gastroenterol. 2021, 56, 81–85. [Google Scholar] [CrossRef]
- Dai, C.; Cao, Q.; Jiang, M.; Sun, M.-J. Serum immunoglobulin G4 in discriminating autoimmune pancreatitis from pancreatic cancer: A diagnostic meta-analysis. Pancreas 2018, 47, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.; Chang, C.; Chen, C. Analytically validated protein biomarkers of chronic pancreatitis and pancreatic cancer for potential clinical diagnosis with mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 34, e8580. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Li, Y.; Yue, Q.; Cui, M.; Liu, J. KRAS Mutations in Peripheral Blood (with or without CA19-9) for Differential Diagnosis of Pancreatic Cancer and Chronic Pancreatitis: A Systematic Review and Meta-analysis. Indian J. Surg. 2022, 84, 615–622. [Google Scholar] [CrossRef]
- Huang, C.; Iovanna, J.; Santofimia-Castaño, P. Targeting fibrosis: The bridge that connects pancreatitis and pancreatic cancer. Int. J. Mol. Sci. 2021, 22, 4970. [Google Scholar] [CrossRef]
- Park, W.G.; Li, L.; Appana, S.; Wei, W.; Stello, K.; Andersen, D.K.; Hughes, S.J.; Whitcomb, D.C.; Brand, R.E.; Yadav, D.; et al. Unique circulating immune signatures for recurrent acute pancreatitis, chronic pancreatitis and pancreatic cancer: A pilot study of these conditions with and without diabetes. Pancreatology 2019, 20, 51–59. [Google Scholar] [CrossRef]
- Prokopchuk, O.; Grünwald, B.; Nitsche, U.; Jäger, C.; Prokopchuk, O.L.; Schubert, E.C.; Friess, H.; Martignoni, M.E.; Krüger, A. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer 2018, 18, 128. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Werner, J.; Bazhin, A.V.; D’haese, J.G. The role of interleukin-18 in pancreatitis and pancreatic cancer. Cytokine Growth Factor Rev. 2019, 50, 1–12. [Google Scholar] [CrossRef]
- Macinga, P.; Pulkertova, A.; Bajer, L.; Maluskova, J.; Oliverius, M.; Smejkal, M.; Heczkova, M.; Spicak, J.; Hucl, T. Simultaneous occurrence of autoimmune pancreatitis and pancreatic cancer in patients resected for focal pancreatic mass. World J. Gastroenterol. 2017, 23, 2185–2193. [Google Scholar] [CrossRef]
- Hansen, S.E.J.; Langsted, A.; Varbo, A.; Madsen, C.M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Low and high pancreatic amylase is associated with pancreatic cancer and chronic pancreatitis. Eur. J. Epidemiol. 2021, 36, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Sanh, N.; Fadul, H.; Hussein, N.; Lyn-Cook, B.D.; Hammons, G.; Ramos-Cardona, X.E.; Mohamed, K.; Mohammed, S. Proteomics profiling of pancreatic cancer and pancreatitis for biomarkers discovery. J. Cell Sci. Ther. 2018, 9, 4. [Google Scholar] [CrossRef]
- Chu, C.; Lou, J.; Bao, M.; Yang, S.; Lou, J. Autoimmune pancreatitis masquerading as pancreatic cancer: Case report of a Chinese man. Int. J. Clin. Exp. Pathol. 2019, 12, 4354. [Google Scholar] [PubMed]
- Yan, T.; Ke, Y.; Chen, Y.; Xu, C.; Yu, C.; Li, Y. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19-9, globulin, eosinophils and hemoglobin. PLoS ONE 2017, 12, e0174735. [Google Scholar] [CrossRef] [PubMed]
- Kandikattu, H.K.; Venkateshaiah, S.U.; Mishra, A. Chronic pancreatitis and the development of pancreatic cancer. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1182–1210. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kawashima, H.; Ohno, E.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yamada, K.; Yashika, J.; Yoshikawa, M.; Gibo, N.; et al. Risks and characteristics of pancreatic cancer and pancreatic relapse in autoimmune pancreatitis patients. J. Gastroenterol. Hepatol. 2020, 35, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Detlefsen, S.; de Vos, J.D.; Tanassi, J.T.; Heegaard, N.H.H.; Fristrup, C.; de Muckadell, O.B.S. Value of anti-plasminogen binding peptide, anti-carbonic anhydrase II, immunoglobulin G4, and other serological markers for the differentiation of autoimmune pancreatitis and pancreatic cancer. Medicine 2018, 97, e11641. [Google Scholar] [CrossRef]
- Dranka-Bojarowska, D.; Lewinski, A.; Lekstan, A.; Gajda, M.; Ciosek, J.; Mrowiec, S. The assessment of serum and diagnostic peritoneal lavage concentration of matrix metalloproteinase-2, matrix metalloproteinase-9, carbohydrate antigen 19-9, and carcinoembryonic antigen in patients with pancreatic cancer and chronic pancreatitis. J. Physiol. Pharmacol. 2020, 71, 689–704. [Google Scholar] [CrossRef]
- Winter, K.; Dzieniecka, M.; Strzelczyk, J.; Wągrowska-Danilewicz, M.; Danilewicz, M.; Małecka-Wojciesko, E. Alpha smooth muscle actin (αSMA) immunohistochemistry use in the differentiation of pancreatic cancer from chronic pancreatitis. J. Clin. Med. 2021, 10, 5804. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Lin, T.-A.; Chen, T.-C.; Tseng, J.-H. Autoimmune pancreatitis type 2: Mimicking pancreatic cancer. Formos. J. Surg. 2020, 53, 113–116. [Google Scholar] [CrossRef]
- Saraswat, M.; Joenväärä, S.; Seppänen, H.; Mustonen, H.; Haglund, C.; Renkonen, R. Comparative proteomic profiling of the serum differentiates pancreatic cancer from chronic pancreatitis. Cancer Med. 2017, 6, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Chou, O.H.I.; Zhou, J.; Mui, J.V.; Satti, D.I.; Chung, C.T.; Lee, T.T.L.; Lee, S.; Dee, E.C.; Ng, K.; Cheung, B.M.Y.; et al. Lower risks of new-onset acute pancreatitis and pancreatic cancer in sodium glucose cotransporter 2 (SGLT2) inhibitors compared to dipeptidyl peptidase-4 (DPP4) inhibitors: A propensity score-matched study with competing risk analysis. Diabetes Epidemiol. Manag. 2023, 9, 100115. [Google Scholar] [CrossRef]
- Luo, B.; Peng, F.; Hong, M.; Su, S.; Fang, C.; Yang, X.; Xia, G.; Li, B. ERCP combined with tumor markers in differential diagnosis of pancreatic cancer and pseudotumor-like pancreatitis. J. Buon 2019, 24, 1568–1573. [Google Scholar] [PubMed]
- Wen, Y.; Cai, W.; Yang, J.; Fu, X.; Putha, L.; Xia, Q.; Windsor, J.A.; Phillips, A.R.; Tyndall, J.D.A.; Du, D.; et al. Targeting macrophage migration inhibitory factor in acute pancreatitis and pancreatic cancer. Front. Pharmacol. 2021, 12, 638950. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.-P.; Han, C.-Q.; Nie, C.; Xu, T.; Zhang, K.; Li, X.-J.; Xie, X.-R.; Lin, R.; Ding, Z. Identification of potential serum exosomal microRNAs involved in acinar-ductal metaplasia that is a precursor of pancreatic cancer associated with chronic pancreatitis. Medicine 2021, 100, e25753. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, D. Autoimmune pancreatitis and pancreatic cancer: Epidemiological aspects and immunological considerations. World J. Gastroenterol. 2021, 27, 3825–3836. [Google Scholar] [CrossRef]
- Dickerson, L.D.; Farooq, A.; Bano, F.; Kleeff, J.; Baron, R.; Raraty, M.; Ghaneh, P.; Sutton, R.; Whelan, P.; Campbell, F.; et al. Differentiation of autoimmune pancreatitis from pancreatic cancer remains challenging. Mol. Med. 2019, 43, 1604–1611. [Google Scholar] [CrossRef]
- Ghassem-Zadeh, S.; Hufnagel, K.; Bauer, A.; Frossard, J.-L.; Yoshida, M.; Kutsumi, H.; Acha-Orbea, H.; Neulinger-Muñoz, M.; Vey, J.; Eckert, C.; et al. Novel autoantibody signatures in sera of patients with pancreatic cancer, chronic pancreatitis and autoimmune pancreatitis: A protein microarray profiling approach. Int. J. Mol. Sci. 2020, 21, 2403. [Google Scholar] [CrossRef]
- Mungamuri, S.K.; Pasupulati, A.K.; Mavuduru, V.A. Immunotherapy for Diabetogenic Pancreatitis and Pancreatic Cancer: An Update. In Exploring Pancreatic Metabolism and Malignancy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 215–236. [Google Scholar] [CrossRef]
- Sunami, Y.; Chen, Y.; Trojanowicz, B.; Sommerer, M.; Hämmerle, M.; Eils, R.; Kleeff, J. Single Cell Analysis of Cultivated Fibroblasts from Chronic Pancreatitis and Pancreatic Cancer Patients. Cells 2022, 11, 2583. [Google Scholar] [CrossRef]
- Lindahl, A.; Heuchel, R.; Forshed, J.; Lehtiö, J.; Löhr, M.; Nordström, A. Discrimination of pancreatic cancer and pancreatitis by LC-MS metabolomics. Metabolomics 2017, 13, 61. [Google Scholar] [CrossRef]
- Gluszek, S.; Matykiewicz, J.; Grabowska, U.; Chrapek, M.; Nawacki, L.; Wawrzycka, I.; Gluszek-Osuch, M.; Kozieł, D. Clinical usefulness of pentraxin 3 (PTX3) as a biomarker of acute pancreatitis and pancreatic cancer. Med. Stud. 2020, 36, 6–13. [Google Scholar] [CrossRef]
- Bang, U.C.; Watanabe, T.; Bendtsen, F. The relationship between the use of statins and mortality, severity, and pancreatic cancer in Danish patients with chronic pancreatitis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, R.; Zhang, Z.; Zhao, W.; Ji, Z.; Tan, H.; Zhou, X. Exploring and validating the clinical risk factors for pancreatic cancer in chronic pancreatitis patients using electronic medical records datasets: Three cohorts comprising 2,960 patients. Transl. Cancer Res. 2020, 9, 629–638. [Google Scholar] [CrossRef]
- Nissinen, S.I.; Venäläinen, M.; Kumpulainen, P.; Roine, A.; Häkkinen, M.R.; Vepsäläinen, J.; Oksala, N.; Rantanen, T. Discrimination between pancreatic cancer, pancreatitis and healthy controls using urinary polyamine panel. Cancer Control 2021, 29, 1–8. [Google Scholar] [CrossRef]
- Agarwal, K.K.; Jassal, R.; Browne, A.; Hossain, M.; Akhtar, R. Autoimmune Pancreatitis Masquerading as Pancreatic Cancer: A Case Report and Literature Review. Cureus 2022, 14, e21900. [Google Scholar] [CrossRef]
- Zhang, H.; Han, W.; Jin, M.; Lai, Y.; Wang, X.; Wang, J.; Yao, Y.; Wu, D.; Qian, J.; Yang, H. Establishment and verification of a scoring model for the differential diagnosis of pancreatic cancer and chronic pancreatitis. Pancreas 2018, 47, 459–465. [Google Scholar] [CrossRef]
- Kunovsky, L.; Dite, P.; Jabandziev, P.; Dolina, J.; Vaculova, J.; Blaho, M.; Bojkova, M.; Dvorackova, J.; Uvirova, M.; Kala, Z.; et al. Helicobacter pylori infection and other bacteria in pancreatic cancer and autoimmune pancreatitis. World J. Gastrointest. Oncol. 2021, 13, 835–844. [Google Scholar] [CrossRef]
- Macinga, P.; Bajer, L.; Del Chiaro, M.; Chari, S.T.; Dite, P.; Frulloni, L.; Ikeura, T.; Kamisawa, T.; Kubota, K.; Naitoh, I.; et al. Pancreatic cancer in patients with autoimmune pancreatitis: A scoping review. Pancreatology 2021, 21, 928–937. [Google Scholar] [CrossRef]
- Grassia, R.; Imperatore, N.; Capone, P.; Cereatti, F.; Forti, E.; Antonini, F.; Tanzi, G.P.; Martinotti, M.; Buffoli, F.; Mutignani, M.; et al. EUS-guided tissue acquisition in chronic pancreatitis: Differential diagnosis between pancreatic cancer and pseudotumoral masses using EUS-FNA or core biopsy. Endosc. Ultrasound 2020, 9, 122–129. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Xia, S.; Guo, F.; Pan, C.; Xiang, H.; Shang, D. T Lymphocytes: A promising immunotherapeutic target for pancreatitis and pancreatic cancer? Front. Oncol. 2020, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Walling, A.; Freelove, R. Pancreatitis and pancreatic cancer. Prim. Care Clin. Off. Pract. 2017, 44, 609–620. [Google Scholar] [CrossRef]
- Rana, S.S.; Gorsi, U.; Gupta, P.; Sharma, R.; Basher, R.; Dhalaria, L.; Gupta, R. Pancreatic cancer masked by acute pancreatitis as well as an unusual iatrogenic complication. J. Dig. Endosc. 2018, 09, 088–091. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Chang, S.-M.; Wu, P.-Y.; Chang, C.-C. Localized autoimmune pancreatitis mimicking pancreatic cancer: Case report and literature review. J. Int. Med. Res. 2018, 46, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Kalayarasan, R.; Narayanan, S.; Sahoo, J.; Mohan, P. Impact of surgery for chronic pancreatitis on the risk of pancreatic cancer: Untying the Gordian knot. World J. Gastroenterol. 2021, 27, 4371–4382. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Kohtakangas, E.L.; Mitrovic, B.; Asai, K.; Shum, J.B. Autoimmune pancreatitis masquerading as pancreatic cancer: When in doubt, cut it out. J. Gastrointest. Cancer 2017, 49, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Ofuji, K.; Akazawa, Y.; Saito, Y.; Nosaka, T.; Ozaki, Y.; Takahashi, K.; Naito, T.; Matsuda, H.; Hiramatsu, K.; et al. Clinical Usefulness of [18F]-Fluoro-2-Deoxy-d-Glucose–Positron Emission Tomography/Computed Tomography for Distinguishing Between Autoimmune Pancreatitis and Pancreatic Cancer. Pancreas 2021, 50, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Gweon, T.-G.; Park, S.H.; Kim, T.H.; Kim, C.W.; Chang, J.H. Incidence and risk of pancreatic cancer in patients with chronic pancreatitis: Defining the optimal subgroup for surveillance. Sci. Rep. 2023, 13, 106. [Google Scholar] [CrossRef]
- Li, S.; Tian, B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine 2017, 96, e5908. [Google Scholar] [CrossRef]
- Bieliuniene, E.; Frøkjær, J.B.; Pockevicius, A.; Kemesiene, J.; Lukosevicius, S.; Basevicius, A.; Atstupenaite, V.; Barauskas, G.; Ignatavicius, P.; Gulbinas, A.; et al. CT- and MRI-based assessment of body composition and pancreatic fibrosis reveals high incidence of clinically significant metabolic changes that affect the quality of life and treatment outcomes of patients with chronic pancreatitis and pancreatic cancer. Medicina 2019, 55, 649. [Google Scholar] [CrossRef]
- Umans, D.S.; A Hoogenboom, S.; Sissingh, N.J.; Lekkerkerker, S.J.; Verdonk, R.C.; E van Hooft, J. Pancreatitis and pancreatic cancer: A case of the chicken or the egg. World J. Gastroenterol. 2021, 27, 3148–3157. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Satoh, T.; Ishikawa, K.; Ishiwatari, H.; Endo, M.; Urikura, A.; Kishida, Y.; Imai, K.; Hotta, K.; Yabuuchi, Y.; et al. Comparison of five-phase computed tomography images of type 1 autoimmune pancreatitis and pancreatic cancer: Emphasis on cases with atypical images. Pancreatology 2021, 21, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Kano, M.; Kobayashi, S.; Ito, T.; Masuda, M.; Mitsuyama, T.; Nakayama, S.; Ikeura, T.; Shimatani, M.; Uchida, K.; et al. Diffuse pancreatic cancer mimicking autoimmune pancreatitis. Intern. Med. 2019, 58, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, G.; Zuo, C.; Jia, N.; Wang, H. 18F- FDG PET/CT helps differentiate autoimmune pancreatitis from pancreatic cancer. BMC Cancer 2017, 17, 695. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, Y. A comparative analysis of CT and MRI in differentiating pancreatic cancer from mass pancreatitis. Am. J. Transl. Res. 2021, 13, 6431–6438. [Google Scholar]
- Zhao, Y.; Li, F.; An, N.; Peng, Z. Atypical enhanced computed tomography signs of pancreatic cancer and its differential diagnosis from autoimmune pancreatitis. Gland. Surg. 2021, 10, 347–354. [Google Scholar] [CrossRef]
- Ergin, E.; Oruc, N.; Özütemiz, Ö. Autoimmune Pancreatitis after a Seven-Year History of Suspicious Pancreatic Cancer. Case Rep. Gastroenterol. 2021, 15, 195–201. [Google Scholar] [CrossRef]
- Cho, M.K.; Moon, S.-H.; Song, T.J.; Kim, R.E.; Oh, D.W.; Park, D.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.-H. Contrast-enhanced endoscopic ultrasound for differentially diagnosing autoimmune pancreatitis and pancreatic cancer. Gut Liver 2018, 12, 591–596. [Google Scholar] [CrossRef]
- Srisajjakul, S.; Prapaisilp, P.; Bangchokdee, S. CT and MR features that can help to differentiate between focal chronic pancreatitis and pancreatic cancer. Radiol. Medica 2020, 125, 356–364. [Google Scholar] [CrossRef]
- Tacelli, M.; Zaccari, P.; Petrone, M.; Della Torre, E.; Lanzillotta, M.; Falconi, M.; Doglioni, C.; Capurso, G.; Arcidiacono, P. Differential EUS findings in focal type 1 autoimmune pancreatitis and pancreatic cancer: A proof-of-concept study. Endosc. Ultrasound 2022, 11, 216–222. [Google Scholar] [CrossRef]
- Harmsen, F.-J.; Domagk, D.; Dietrich, C.; Hocke, M. Discriminating chronic pancreatitis from pancreatic cancer: Contrast-enhanced EUS and multidetector computed tomography in direct comparison. Endosc. Ultrasound 2018, 7, 395–403. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, J.; Jin, Z.; Xue, H.; Dai, M.; Zhang, W.; Sun, Z.; Xu, J.; Garcia, S.R.M.; Asbach, P.; et al. Distinguishing pancreatic cancer and autoimmune pancreatitis with in vivo tomoelastography. Eur. Radiol. 2020, 31, 3366–3374. [Google Scholar] [CrossRef]
- Wyse, J.M.; Sahai, A.V. Endoscopic ultrasound-guided management of pain in chronic pancreatitis and pancreatic cancer: An update. Current Treatment Options in Gastroenterology. Curr. Treat. Options Gastroenterol. 2018, 16, 417–427. [Google Scholar] [CrossRef]
- Konings, I.C.; Cahen, D.L.; Harinck, F.; Fockens, P.; van Hooft, J.E.; Poley, J.-W.; Bruno, M.J. Evolution of features of chronic pancreatitis during endoscopic ultrasound-based surveillance of individuals at high risk for pancreatic cancer. Endosc. Int. Open 2018, 6, E541–E548. [Google Scholar] [CrossRef]
- Enjuto, D.T.; Herrera, N.; Ceinos, C.J.; Bonilla, A.R.; Llorente-Lázaro, R.; Guerreiro, J.G.; Castro-Carbajo, P. Hereditary pancreatitis related to SPINK-1 mutation. Is there an increased risk of developing pancreatic cancer? J. Gastrointest. Cancer 2021, 54, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.Y.; Chen, Q.; Yu, W.; Dong, E.Y.; Chung, J.; Pandol, S.J.; Yadav, D.; Conwell, D.L.; Wu, B.U. Identification of individuals at increased risk for pancreatic cancer in a community-based cohort of patients with suspected chronic pancreatitis. Clinical and Translational Gastroenterology. Clin. Transl. Gastroenterol. 2020, 11, e00147. [Google Scholar] [CrossRef] [PubMed]
- Teske, C.; Kahlert, C.; Welsch, T.; Liedel, K.; Weitz, J.; Uckermann, O.; Steiner, G. Label-free differentiation of human pancreatic cancer, pancreatitis, and normal pancreatic tissue by molecular spectroscopy. J. Biomed. Opt. 2022, 27, 075001. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Ayav, A.; Belle, A.; Orry, X.; Chevaux, J.-B.; Laurent, V. Pancreatic cancer in patients with chronic calcifying pancreatitis: Computed tomography findings—A retrospective analysis of 48 patients. Eur. J. Radiol. 2016, 86, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Singh, N.; Gupta, S.; Rashid, S.; Nalika, N.; Sachdev, V.; Bal, C.S.; Datta Gupta, S.; Chauhan, S.S.; Saraya, A. Progression of chronic pancreatitis to pancreatic cancer: Is there a role of gene mutations as a screening tool? Pancreas 2018, 47, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tirkes, T.; Shah, Z.K.; Takahashi, N.; Grajo, J.R.; Chang, S.T.; Venkatesh, S.K.; Conwell, D.L.; Fogel, E.L.; Park, W.; Topazian, M.; et al. Reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography: The consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Radiology 2019, 290, 207–215. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.-R.; Zhuo, L.-Y.; Yin, X.-P.; Ren, J.-L.; Li, C.-Y.; Xing, L.-H.; Zheng, T.-T. Retrospective Analysis of the Value of Enhanced CT radiomics analysis in the differential diagnosis between pancreatic cancer and chronic pancreatitis. Int. J. Gen. Med. 2022, 15, 233–241. [Google Scholar] [CrossRef]
- Bartell, N.; Bittner, K.; Vetter, M.S.; Kothari, T.; Kaul, V.; Kothari, S. Role of endoscopic ultrasound in detecting pancreatic cancer missed on cross-sectional imaging in patients presenting with pancreatitis: A retrospective review. Dig. Dis. Sci. 2019, 64, 3623–3629. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W. The advantage of using CEUS time-intensity curves vs. conventional ultrasound in the differential diagnosis of mass pancreatitis and pancreatic cancer. Int. J. Clin. Exp. Med. 2020, 13, 8578–8584. [Google Scholar]
- Yang, A.; Guo, T.; Xu, T.; Zhang, S.; Lai, Y.; Wu, X.; Wu, D.; Feng, Y.; Jiang, Q.; Wang, Q.; et al. The role of EUS in diagnosing focal autoimmune pancreatitis and differentiating it from pancreatic cancer. Endosc. Ultrasound 2021, 10, 280–287. [Google Scholar] [CrossRef]
- Konur, S.; Ozkahraman, A.; Surmeli, N.; Gunduz, I.; Iliklerden, U.H.; Dertli, R.; Kayar, Y. The severity of acute pancreatitis according to modified balthazar classification in patients with pancreatic cancer. Tumori J. 2020, 106, 356–361. [Google Scholar] [CrossRef]


| First Author | Year | Country | Biomarkers |
|---|---|---|---|
| 2019 | China | IgG4+/IgG+ plasma cell ratio > 40% |
| 2018 | China | overexpression of galectin-1 promotes PSC activity |
| 2018 | Germany | PDAC stroma > mucin content than CP |
| 2019 | Czech Republic | Plasmatic IgG4 levels >135 mg/dL in PC |
| 2020 | China | Pancreatic stellate cell (PSC)-stimulating factors |
| 2019 | Portugal | CA19-9 and IgG4 |
| 2019 | Romania | CA 199 and glycosylation alterations |
| 2017 | Finland | P-suPAR was significantly higher in PC |
| 2018 | China | IgG4 high specificity and low sensitivity (AIPvsPC) |
| 2020 | Taiwan | use of mass spectrometry for protein biomarkers |
| 2022 | China | CA19-9 and KRAS mutations in blood |
| 2021 | France | nuclear protein 1 (NUPR1 |
| 2020 | USA | unique immune signature panels |
| 2018 | Germany | matrix metalloproteinase inhibitor TIMP1 |
| 2019 | Germany | Interleukin-18 |
| 2017 | Czech Republic | IgG4 levels |
| 2021 | Denmark | Low and high amylase is associated with PC and CP |
| 2018 | USA | transferrin, ER-60 protein, proapolipoprotein, tropomyosin 1, alpha 1 actin precursor, ACTB protein, and gamma 2 propeptide, aldehyde dehydrogenase 1A1, pancreatic lipase, and annexin A1 |
| 2019 | China | ratio of IgG4/IgG and CA 19-9 |
| 2017 | China | Ca19-9 |
| 2020 | USA | Cytokines IL-4, IL-5, IL-6, IL-13, IL-15, IL-17, IL-18, IFN-γ, TNF-α, and chemokines |
| 2020 | Japan | serum IgG4 and CA19-9 |
| 2018 | Denmark | anti-plasminogen binding peptide, anticarbonic anhydrase II, IgG4 |
| 2020 | Poland | MMP-2, MMP-9, CA19-9, and CEA |
| 2021 | Poland | αSMA expression higher in tumours > than 3 cm |
| 2020 | Taiwan | CA19-9, CEA, CRP and IgG4 |
| 2017 | Finland | Proteomics |
| 2022 | China | sodium–glucose cotransporter 2 inhibitors vs. dipeptidyl peptidase-4 inhibitors |
| 2019 | China | serum CEA and CA19-9 |
| 2021 | China | Macrophage Migration Inhibitory Factor |
| 2021 | China | serum exosomal microRNAs |
| 2021 | Kazakhstan | IgG4, IL-4, IL-5, IL-13, IL-10, and TGF-β |
| 2019 | UK | IgG4 immunohistochemistry |
| 2020 | Germany | Novel Autoantibody Signatures |
| 2019 | India | cytokines |
| 2022 | Germany | scRNAseq and bioinformatics analyses |
| 2017 | Sweden | Glycocholic acid, N-palmitoyl glutamic acid and hexanoylcarnitine |
| 2020 | Poland | pentraxin 3 (PTX3) |
| 2018 | Denmark | cytokines and chemokines |
| 2020 | China | CA19-9 |
| 2021 | Finland | polyamines—acetylputrescine, diacetylspermidine, N8-acetylspermidine and diacetylputrescine |
| 2022 | USA | CA19-9, IgG4 |
| 2018 | China | CA19-9 |
| 2021 | Czech Republic | cytotoxin-associated gene A |
| 2021 | Czech Republic | IgG4 |
| 2020 | Finland | CA19-9, CEA |
| 2020 | Italy | CA19-9 |
| 2020 | China | T Lymphocytes |
| 2017 | USA | CA19-9 |
| 2018 | India | CA19-9 |
| 2018 | Taiwan | IgG and IgG4 levels |
| 2021 | India | Cytokines and chemokines |
| 2018 | Canada | CA19-9 and IgG4/IgG |
| 2021 | Japan | CA19-9 and IgG4/IgG |
| 2022 | South Korea | CA19-9 |
| 2017 | China | CA19-9 |
| 2019 | Lithuania | Faecal elastase-1 |
| 2021 | Netherlands | CA19-9 |
| 2021 | Japan | CEA, CA19-9, IgG4 |
| 2019 | Japan | CEA, CA19-9, IgG4 |
| First Author | Year of Publication | Country | Modalities |
|---|---|---|---|
| 2017 | China | 18F-FDG PET/CT |
| 2021 | Netherlands | CT, EUS, MRI |
| 2021 | China | CT and MRI |
| 2020 | Finland | CT, MRCP, US, FDG PET/CT, EUS |
| 2021 | China | CT |
| 2021 | Turkey | CT, ERCP |
| 2022 | USA | CT, ERCP, EUS |
| 2018 | Canada | US, MRI, CT, ERCP |
| 2020 | Taiwan | CT, MRCP |
| 2019 | China | CT |
| 2021 | Japan | 18F- FDG PET/CT |
| 2021 | Japan | CT |
| 2018 | South Korea | CEUS |
| 2020 | Thailand | CT and MRI |
| 2020 | Lithuania | CT- and MRI |
| 2022 | Italy | EUS |
| 2019 | UK | CT |
| 2019 | Japan | CT, MRI, FDG-PET |
| 2018 | Germany | MDCT, B-mode EUS, ESE, CELMI-EUS, EUS-FNA |
| 2021 | China | MR elastography and tomoelastography |
| 2018 | Canada | EUS |
| 2019 | China | ERCP |
| 2018 | China | CT |
| 2020 | Italy | EUS-FNB and EUS-FNA |
| 2018 | Netherlands | EUS |
| 2021 | Spain | CT |
| 2020 | USA | CT |
| 2022 | South Korea | CT and MRI |
| 2022 | Germany | infrared spectroscopy |
| 2018 | Taiwan | CT, MRI, FDG-PET, EUS |
| 2017 | France | CT |
| 2018 | India | CT, MRI, FDG-PET, EUS, CE-EUS |
| 2019 | Czech Republic | US, EUS |
| 2017 | USA | CT, MRCP, EUS, ERCP |
| 2018 | India | FDG-PET, EUS |
| 2019 | USA | CT and MRI, and MRCP |
| 2022 | China | Enhanced CT Radiomics |
| 2020 | Japan | EUS-FNA, CE-EUS, CT |
| 2019 | USA | EUS-FNA, MRI, CT |
| 2017 | Czech Republic | CT, EUS, ERCP |
| 2020 | China | US, CEUS |
| 2021 | China | EUS |
| 2020 | Turkey | CT |
| 2019 | Portugal | CT, ERCP |
| 2019 | China | CT, MRI, FDG-PET |
| 2018 | Denmark | EUS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madela, F.; Ferndale, L.; Aldous, C. Diagnostic Differentiation between Pancreatitis and Pancreatic Cancer: A Scoping Review. Diagnostics 2024, 14, 290. https://doi.org/10.3390/diagnostics14030290
Madela F, Ferndale L, Aldous C. Diagnostic Differentiation between Pancreatitis and Pancreatic Cancer: A Scoping Review. Diagnostics. 2024; 14(3):290. https://doi.org/10.3390/diagnostics14030290
Chicago/Turabian StyleMadela, Fusi, Lucien Ferndale, and Colleen Aldous. 2024. "Diagnostic Differentiation between Pancreatitis and Pancreatic Cancer: A Scoping Review" Diagnostics 14, no. 3: 290. https://doi.org/10.3390/diagnostics14030290
APA StyleMadela, F., Ferndale, L., & Aldous, C. (2024). Diagnostic Differentiation between Pancreatitis and Pancreatic Cancer: A Scoping Review. Diagnostics, 14(3), 290. https://doi.org/10.3390/diagnostics14030290








