Time-Sequential Monitoring of the Early Mesothelial Reaction in the Pleura after Cryoinjury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. OCT System
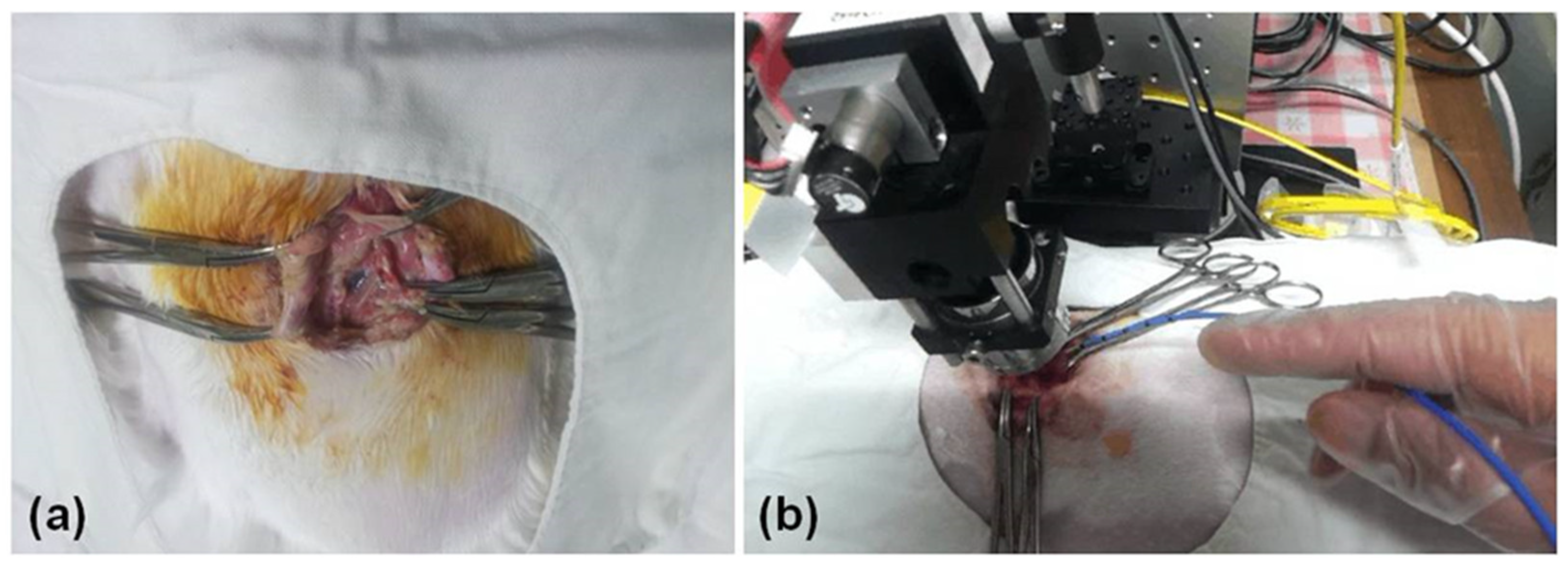
2.3. Thoracic Window
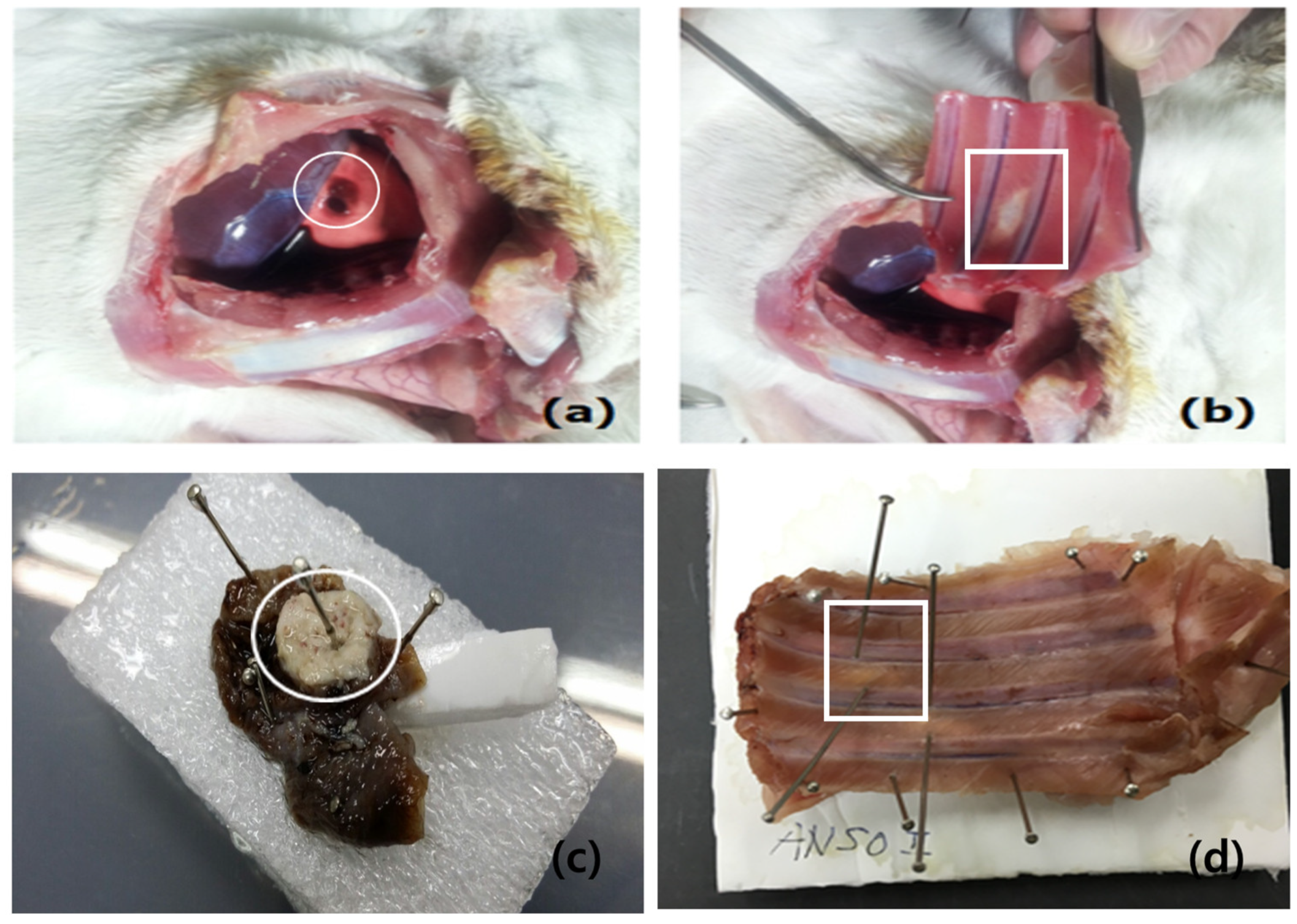
2.4. Cryoinjury to the Pleura
2.5. Hematoxylin and Eosin Staining
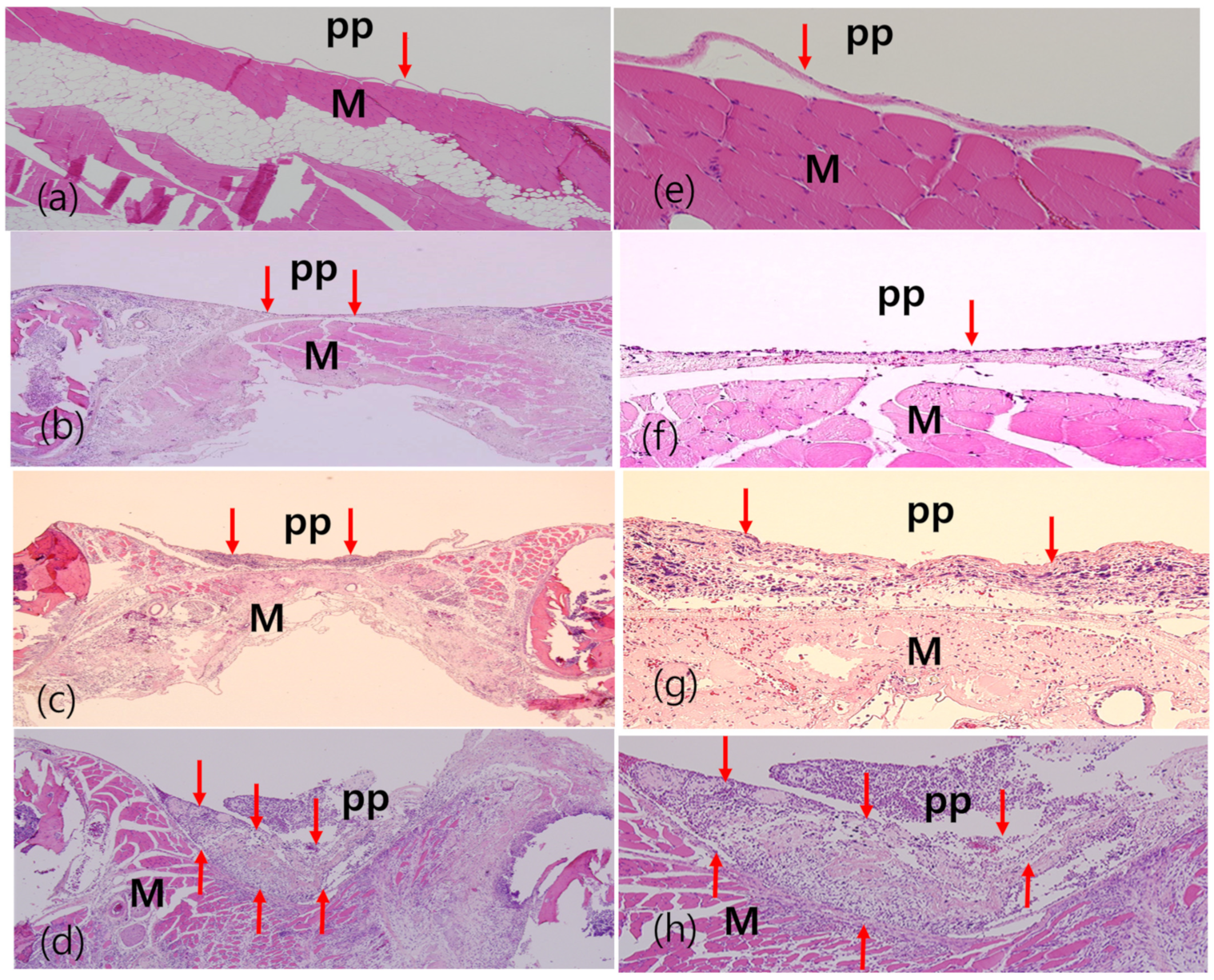
3. Results
3.1. Gross Findings of Visceral and Parietal Pleura
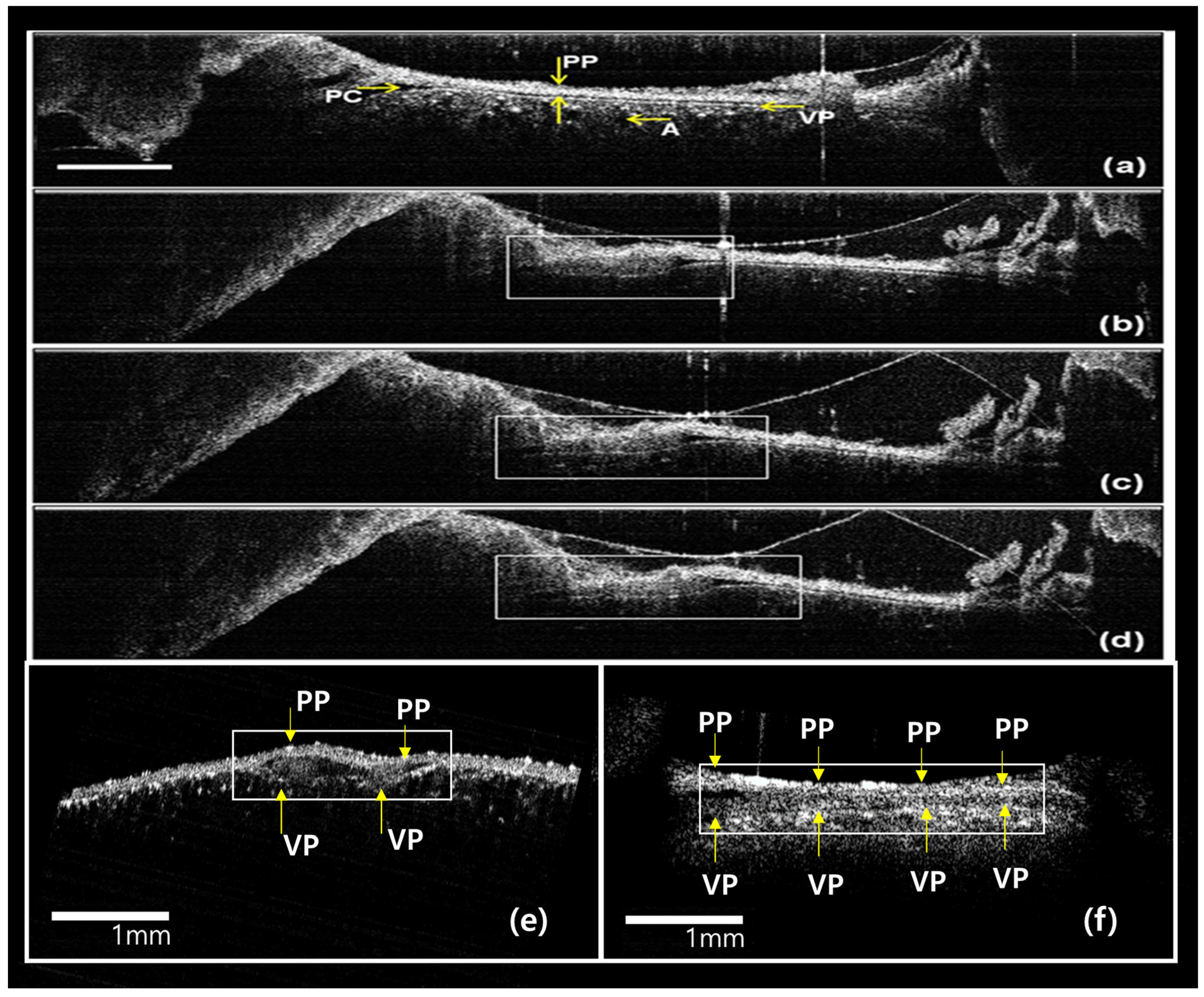
3.2. Real-Time In Vivo OCT Image
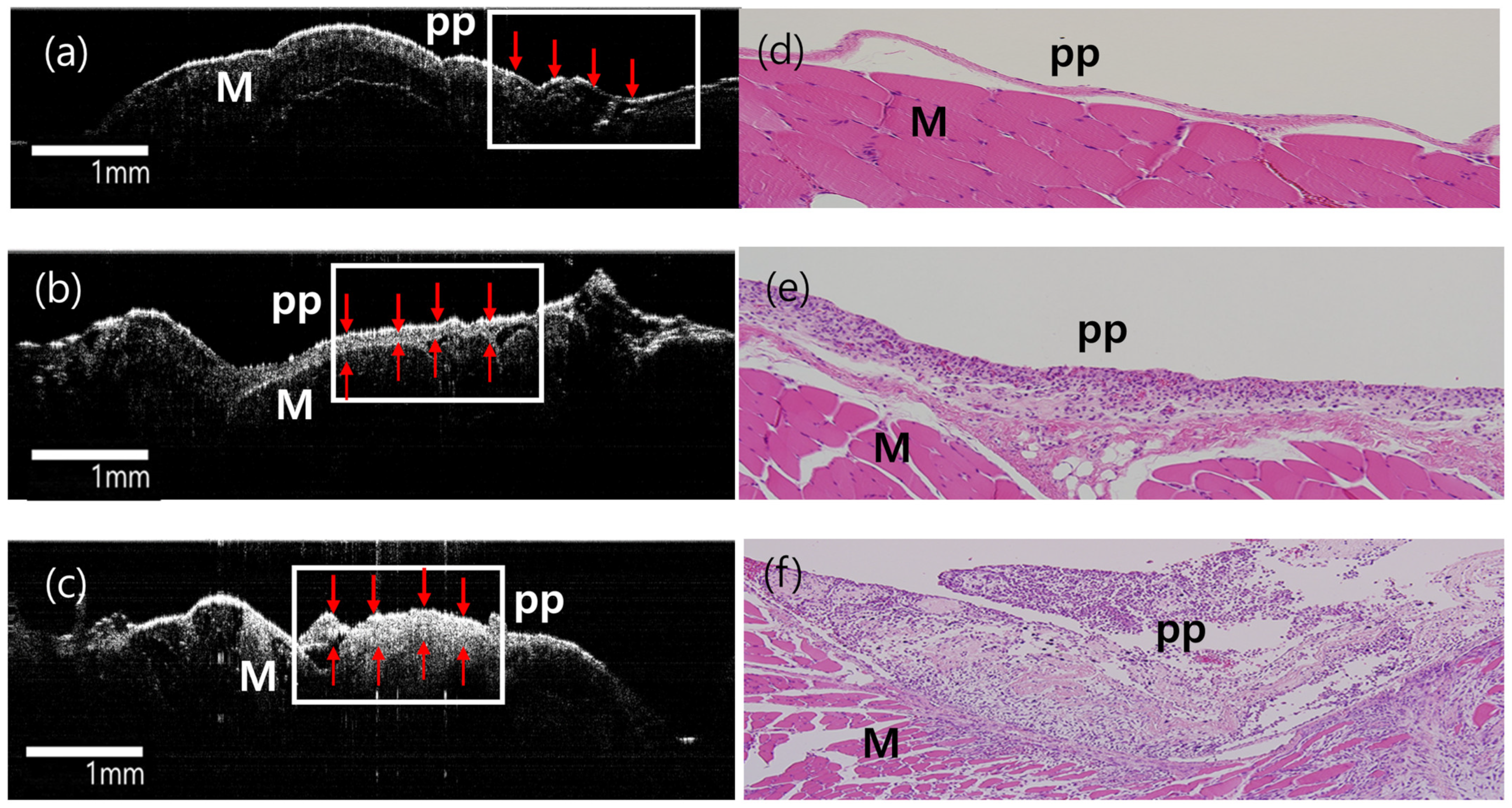
3.3. Ex Vivo Gross, Histological, and OCT Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jantz, M.A.; Antony, V.B. Pathophysiology of the pleura. Respiration 2008, 75, 121–133. [Google Scholar] [CrossRef]
- Lis, R.; Touboul, C.; Mirshahi, P.; Ali, F.; Mathew, S.; Nolan, D.J.; Maleki, M.; Abdalla, S.A.; Raynaud, C.M.; Querleu, D.; et al. Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int. J. Cancer 2011, 128, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, D.; Papadimitriou, J.M.; Walters, M.N. The mesothelium and its reactions: A review. Crit. Rev. Toxicol. 1982, 10, 81–144. [Google Scholar] [CrossRef] [PubMed]
- Adamson, I.Y.; Bakowska, J.; Bowden, D.H. Mesothelial cell proliferation: A nonspecific response to lung injury associated with fibrosis. Am. J. Respir. Cell Mol. Biol. 1994, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Marchi, E.; Vargas, F.S.; Acencio, M.M.; Antonangelo, L.; Genofre, E.H.; Teixeira, L.R. Evidence that mesothelial cells regulate the acute inflammatory response in talc pleurodesis. Eur. Respir. J. 2006, 28, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Boylan, A.M.; Rüegg, C.; Kim, K.J.; Hébert, C.A.; Hoeffel, J.M.; Pytela, R.; Sheppard, D.; Goldstein, I.M.; Broaddus, V.C. Evidence of a role for mesothelial cell-derived interleukin 8 in the pathogenesis of asbestos-induced pleurisy in rabbits. J. Clin. Investig. 1992, 89, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.L.; Kane, A.B. Mesothelial cell proliferation and biopersistence of wollastonite and crocidolite asbestos fibers. Fundam. Appl. Toxicol. 1997, 38, 173–183. [Google Scholar] [CrossRef]
- Taylor, D.A.; Atkins, B.Z.; Hungspreugs, P.; Jones, T.R.; Reedy, M.C.; Hutcheson, K.A.; Glower, D.D.; Kraus, W.E. Regenerating functional myocardium: Improved performance after skeletal myoblast transplantation. Nat. Med. 1998, 4, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, E.J.; Mees, B.M.; de Waard, M.C.; de Crom, R.; Duncker, D.J. A novel model of cryoinjury-induced myocardial infarction in the mouse: A comparison with coronary artery ligation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1291–H1300. [Google Scholar] [CrossRef]
- Tanaka, S. Immunological aspects of cryosurgery in general surgery. Cryobiology 1982, 19, 247–262. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Debelak, J.P.; Venkatakrishnan, A.; Schot, D.J.; Harley, D.H.; Pinson, C.W.; Williams, P.; Washington, K.; Christman, J.W.; Chapman, W.C. Acute lung injury after hepatic cryoablation: Correlation with NF-kappa B activation and cytokine production. Surgery 1999, 126, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Washington, K.; Debelak, J.P.; Gobbell, C.; Sztipanovits, D.R.; Shyr, Y.; Olson, S.; Chapman, W.C. Hepatic cryoablation-induced acute lung injury: Histopathologic findings. J. Surg. Res. 2001, 95, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Kreuter, K.; Ju, J.; Mahon, S.; Tseng, L.; Mukai, D.; Burney, T.; Guo, S.; Su, J.; Tran, A.; et al. In vivo optical coherence tomography detection of differences in regional large airway smoke inhalation induced injury in a rabbit model. J. Biomed. Opt. 2008, 13, 034001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.W.; Heidary, A.E.; Yoon, D.; Mukai, D.; Ramalingam, T.; Mahon, S.; Yin, J.; Jing, J.; Liu, G.; Chen, Z.; et al. Quantification of airway thickness changes in smoke-inhalation injury using in-vivo 3-D endoscopic frequency-domain optical coherence tomography. Biomed. Opt. Express 2011, 2, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, K.A.; Mahon, S.B.; Mukai, D.S.; Su, J.; Jung, W.G.; Narula, N.; Guo, S.; Wakida, N.; Raub, C.; Berns, M.W.; et al. Detection and monitoring of early airway injury effects of half-mustard (2-chloroethylethylsulfide) exposure using high-resolution optical coherence tomography. J. Biomed. Opt. 2009, 14, 044037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meissner, S.; Knels, L.; Schnabel, C.; Koch, T.; Koch, E. Three-dimensional Fourier domain optical coherence tomography in vivo imaging of alveolar tissue in the intact thorax using the parietal pleura as a window. J. Biomed. Opt. 2010, 15, 016030. [Google Scholar] [CrossRef]
- Acencio, M.M.; Vargas, F.S.; Marchi, E.; Carnevale, G.G.; Teixeira, L.R.; Antonangelo, L.; Broaddus, V.C. Pleural mesothelial cells mediate inflammatory and profibrotic responses in talc-induced pleurodesis. Lung 2007, 185, 343–348. [Google Scholar] [CrossRef]
- Jaurand, M.C.; Fleury-Feith, J. Pathogenesis of malignant pleural mesothelioma. Respirology 2005, 10, 2–8. [Google Scholar] [CrossRef]
- Kroegel, C.; Antony, V.B. Immunobiology of pleural inflammation: Potential implications for pathogenesis, diagnosis and therapy. Eur. Respir. J. 1997, 10, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Butnor, K.J.; Pavlisko, E.N.; Sporn, T.A.; Roggli, V.L. Malignant peritoneal mesothelioma and Crohn disease. J. Clin. Pathol. 2017, 70, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Whitaker, D.; Papadimitriou, J.M. Stimulation of mesothelial cell proliferation by exudate macrophages enhances serosal wound healing in a murine model. Am. J. Pathol. 2002, 160, 681–692. [Google Scholar] [CrossRef]
- Ahn, Y.-C.; Oak, C.; Park, J.-E.; Jung, M.-J.; Kim, J.-H.; Lee, H.-Y.; Kim, S.W.; Park, E.-K.; Jung, M.H. Ex vivo high-resolution optical coherence tomography (OCT) imaging of pleural reaction after pleurodesis using talc. J. Opt. Soc. Korea 2016, 20, 607–613. [Google Scholar] [CrossRef]
- Muta, F.; Takamori, S.; Matsuo, T.; Iwasaki, Y.; Yoshiyama, K.; Shirouzu, K. Changes in the pleural cavity by pleurodesis using talc or OK-432: An experimental study. Surg. Today 2011, 41, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, F.; Scott, J.; Grimshaw, M. Relationship between occupations and asbestos-fibre content of the lungs in patients with pleural mesothelioma, lung cancer, and other diseases. Thorax 1977, 32, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Røe, O.D.; Anderssen, E.; Sandeck, H.; Christensen, T.; Larsson, E.; Lundgren, S. Malignant pleural mesothelioma: Genome-wide expression patterns reflecting general resistance mechanisms and a proposal of novel targets. Lung Cancer 2010, 67, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Røe, O.D.; Anderssen, E.; Helge, E.; Pettersen, C.H.; Olsen, K.S.; Sandeck, H.; Haaverstad, R.; Lundgren, S.; Larsson, E. Genome-wide profile of pleural mesothelioma versus parietal and visceral pleura: The emerging gene portrait of the mesothelioma phenotype. PLoS ONE 2009, 4, e6554. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Liu, G.; Kreuter, K.; Mahon, S.; Colt, H.; Mukai, D.; Peavy, G.M.; Chen, Z.; Brenner, M. In vivo three-dimensional imaging of normal tissue and tumors in the rabbit pleural cavity using endoscopic swept source optical coherence tomography with thoracoscopic guidance. J. Biomed. Opt. 2009, 14, 064045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, R.; Le, T.; Murgu, S.D.; Chen, Z.; Brenner, M. Recent advances in optical coherence tomography for the diagnoses of lung disorders. Expert Rev. Respir. Med. 2011, 5, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; Sayyad, F.E.; Galor, A.; Dubovy, S.; Karp, C.L. High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology. Ocul. Surf. 2015, 13, 226–235. [Google Scholar] [CrossRef]
- Shousha, M.A.; Karp, C.L.; Canto, A.P.; Hodson, K.; Oellers, P.; Kao, A.A.; Bielory, B.; Matthews, J.; Dubovy, S.R.; Perez, V.L.; et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology 2013, 120, 883–891. [Google Scholar] [CrossRef]
- Bai, K.J.; Chuang, K.J.; Chen, J.K.; Tsai, C.Y.; Yang, Y.L.; Chang, C.C.; Chen, T.T.; Lee, C.N.; Feng, P.H.; Chen, K.Y.; et al. Alterations by Air Pollution in Inflammation and Metals in Pleural Effusion of Pneumonia Patients. Int. J. Environ. Res. Public. Health 2019, 16, 705. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.P.C.; Justo, L.T.; Dos Santos, N.V.; Marquezini, M.V.; de André, P.A.; da Rocha, F.M.M.; Pasqualucci, C.A.; Barrozo, L.V.; Singer, J.M.; De André, C.D.S.; et al. Pleural anthracosis as an indicator of lifetime exposure to urban air pollution: An autopsy-based study in Sao Paulo. Environ. Res. 2019, 173, 23–32. [Google Scholar] [CrossRef] [PubMed]



| Parietal Thickening | Visceral Thickening | ||
|---|---|---|---|
| Pathology | Cryoinjury | 150 ± 8.5 μm | 30 ± 10.5 μm |
| Sham | 95 ± 7.6 μm | 13 ± 5.5 μm | |
| Normal | 12 ± 3.5 μm | 11 ± 2.5 μm | |
| p-Value | 0.02 | 0.04 | |
| Ex vivo OCT image | Cryoinjury | 155 ± 10.7 μm | 45 ± 12.5 μm |
| Sham | 85 ± 5.5 μm | 14 ± 7.5 μm | |
| Normal | 13 ± 2.5 μm | 12 ± 3.5 μm | |
| p-Value | 0.03 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Chae, Y.-K.; Nam, S.-J.; Lee, H.; Hwang, S.-S.; Park, E.-K.; Ahn, Y.-C.; Oak, C. Time-Sequential Monitoring of the Early Mesothelial Reaction in the Pleura after Cryoinjury. Diagnostics 2024, 14, 292. https://doi.org/10.3390/diagnostics14030292
Kim T, Chae Y-K, Nam S-J, Lee H, Hwang S-S, Park E-K, Ahn Y-C, Oak C. Time-Sequential Monitoring of the Early Mesothelial Reaction in the Pleura after Cryoinjury. Diagnostics. 2024; 14(3):292. https://doi.org/10.3390/diagnostics14030292
Chicago/Turabian StyleKim, Taeyun, Yu-Kyung Chae, Sung-Jin Nam, Haeyoung Lee, Sang-Suk Hwang, Eun-Kee Park, Yeh-Chan Ahn, and Chulho Oak. 2024. "Time-Sequential Monitoring of the Early Mesothelial Reaction in the Pleura after Cryoinjury" Diagnostics 14, no. 3: 292. https://doi.org/10.3390/diagnostics14030292
APA StyleKim, T., Chae, Y.-K., Nam, S.-J., Lee, H., Hwang, S.-S., Park, E.-K., Ahn, Y.-C., & Oak, C. (2024). Time-Sequential Monitoring of the Early Mesothelial Reaction in the Pleura after Cryoinjury. Diagnostics, 14(3), 292. https://doi.org/10.3390/diagnostics14030292





