The Value of Ultrasound Diagnostic Imaging of Anterior Crucial Ligament Tears Verified Using Experimental and Arthroscopic Investigations
Abstract
:1. Introduction
1.1. ACL Imaging Mmethods
1.2. ACL Clinical Evaluation
1.3. ACL Injury Types and Treatment
1.4. Aims
- To identify key parameters for ultrasound examination of ACL tears.
- To assess the accuracy of ultrasound in the evaluating of ACL tears.
- To determine ultrasound examination accuracy of anatomical parts, including arthroscopy procedure.
- To provide a statistical assessment of the characteristic features of ultrasound ACL tears for clinical application.
- An attempt at standardization of ultrasound examination of the ACL.
2. Materials and Methods
- The inclination of the ACL—patient is in supine position with a knee flexion of 90 degrees. The transducer is applied along the sagittal plane parallel to the longitudinal axis of patellar ligament. Inclination angle is the angle between base line and front line of the ACL.
- Swelling/scarifications of the ACL proximal attached to lateral femoral condyle—the patient is in prone position with knee full extension. The transducer is applied transversely to long axis of the lower limb in popliteal fossa.
- Swelling/scarifications of the ACL/posterior crucial ligament (PCL) with change of the morphology of the posterior joint capsule complex—the patient is in prone position with knee full extension. The transducer is applied parallel to the long axis of the lower limb.
- Dynamic instability—patient is in supine position with a knee flexion of 90 degrees. The transducer is applied along the sagittal plane parallel to the longitudinal axis of patellar ligament with dynamic anterior drawer test.
2.1. Part I—Anatomical Study
2.2. Part II—Clinical Study
- The inclination of the ACL.
- Swelling/scarifications of the ACL proximal attachment to lateral femoral condyle.
- Swelling/scarifications of the ACL/posterior crucial ligament (PCL) compartment with change of the morphology of the posterior joint capsule complex.
- Dynamic instability in anterior drawer test with range from 0 to 2 mm, 3 to 4 mm, and ≥5 mm.
- The following coding system was established for ACL pathologies assessment in ultrasound examination: 0/1 for parameters 1,2, and 3 and 0/1/2 for parameter no 4.
2.3. Statistical Analysis
3. Results
3.1. Part I—Anatomical Study
3.2. Part II Clinical Study
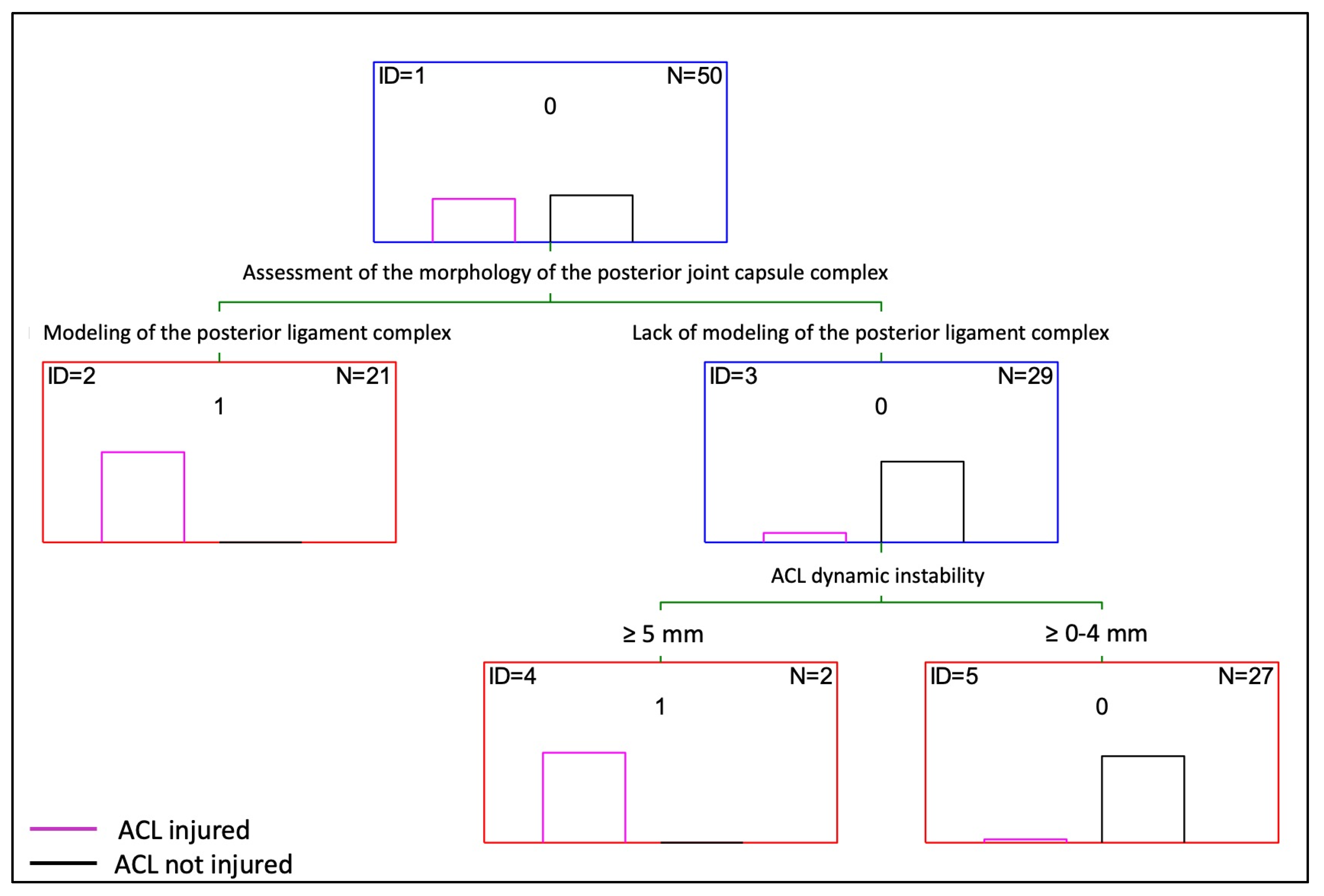
3.3. Decision Tree
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oiestad, B.E.; Engebretsen, L.; Storheim, K.; Risberg, M.A. Knee osteoarthritis after anterior cruciate ligament injury: A systematic review. Am. J. Sports Med. 2009, 37, 1434–1443. [Google Scholar] [CrossRef]
- Spindler, K.P.; Wright, R.W. Anterior cruciate ligament tear. N. Engl. J. Med. 2008, 359, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Swenson, D.M.; Collins, C.L.; Best, T.M.; Flanigan, D.C.; Fields, S.K.; Comstock, R.D. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006–2010/2011. Med. Sci. Sports Exerc. 2013, 45, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.L.; Dunn, W.R.; Dahm, D.L.; Zeger, S.L.; Spindler, K.P. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J. Bone Jt. Surg. 2009, 91, 2242–2250. [Google Scholar] [CrossRef]
- Mountcastle, S.B.; Posner, M.; Kragh, J.F., Jr.; Taylor, D.C. Gender differences in anterior cruciate ligament injury vary with activity: Epidemiology of anterior cruciate ligament injuries in a young, athletic population. Am. J. Sports Med. 2007, 35, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Stanley, L.E.; Kerr, Z.Y.; Dompier, T.P.; Padua, D.A. Sex Differences in the Incidence of Anterior Cruciate Ligament, Medial Collateral Ligament, and Meniscal Injuries in Collegiate and High School Sports: 2009–2010 Through 2013–2014. Am. J. Sports Med. 2016, 44, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Dodson, C.C.; Secrist, E.S.; Bhat, S.B.; Woods, D.P.; Deluca, P.F. Anterior Cruciate Ligament Injuries in National Football League Athletes From 2010 to 2013: A Descriptive Epidemiology Study. Orthop. J. Sports Med. 2016, 4, 2325967116631949. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Dhillon, M.S.; Syam, K.; Prabhakar, S.; Behera, P.; Singh, H. Epidemiological profile of sports-related knee injuries in northern India: An observational study at a tertiary care center. J. Clin. Orthop. Trauma 2016, 7, 207–211. [Google Scholar] [CrossRef]
- Quiles, C.; Constantino, J.A.; Gañán, Y.; Macías, D.; Quiles, M. Stereophotogrammetric surface anatomy of the anterior cruciate ligament’s tibial footprint: Precise osseous structure and distances to arthroscopically-relevant landmarks. Knee 2018, 25, 531–544. [Google Scholar] [CrossRef]
- Abreu-e-Silva, G.M.; Oliveira, M.H.; Maranhao, G.S.; Deligne, L.M.; Pfeilsticker, R.M.; Novais, E.N.; Nunes, T.A.; de Andrade, M.A.P. Three-dimensional computed tomography evaluation of anterior cruciate ligament footprint for anatomic single-bundle reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 770–776. [Google Scholar] [CrossRef]
- Amis, A.A.; Dawkins, G.P. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J. Bone Jt. Surg. Br. 1991, 73, 260–267. [Google Scholar] [CrossRef]
- Otsubo, H.; Akatsuka, Y.; Takashima, H.; Suzuki, T.; Suzuki, D.; Kamiya, T.; Ikeda, Y.; Matsumura, T.; Yamashita, T.; Shino, K. MRI depiction and 3D visualization of three anterior cruciate ligament bundles. Clin. Anat. 2017, 30, 276–283. [Google Scholar] [CrossRef]
- Purnell, M.L.; Larson, A.I.; Clancy, W. Anterior cruciate ligament insertions on the tibia and femur and their relationships to critical bony landmarks using high-resolution volume-rendering computed tomography. Am. J. Sports Med. 2008, 36, 2083–2090. [Google Scholar] [CrossRef]
- Zantop, T.; Herbort, M.; Raschke, M.J.; Fu, F.H.; Petersen, W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am. J. Sports Med. 2007, 35, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Tampere, T.; Van Hoof, T.; Cromheecke, M.; Van der Bracht, H.; Chahla, J.; Verdonk, P.; Victor, J. The anterior cruciate ligament: A study on its bony and soft tissue anatomy using novel 3D CT technology. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Ekdahl, M.; Shen, W.; Fu, F.H. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: An anatomic study. Arthroscopy 2007, 23, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Levicoff, E.A.; Macpherson, T.A.; Moreland, M.S.; Cohen, M.; Fu, F.H. The fetal anterior cruciate ligament: An anatomic and histologic study. Arthroscopy 2007, 23, 278–283. [Google Scholar] [CrossRef]
- Helito, C.P.; Torres, J.A.; Bonadio, M.B.; Aragão, J.A.; de Oliveira, L.N.; Natalino, R.J.; Pécora, J.R.; Camanho, G.L.; Demange, M.K. Anterolateral Ligament of the Fetal Knee: An Anatomic and Histological Study. Am. J. Sports Med. 2017, 45, 91–96. [Google Scholar] [CrossRef]
- Barrett, G.R.; Thibodeaux, K.E.; Replogle, W.H.; Barrett, A.; Parks, T.; Baker, D. Body Mass Index as an Indicator of Associated Intra-articular Injuries in Patients With Anterior Cruciate Ligament Tears. J. Surg. Orthop. Adv. 2015, 24, 159–163. [Google Scholar]
- Ristić, V.; Ristić, S.; Maljanović, M.; Đan, V.; Milankov, V.; Harhaji, V. Risk factors for bilateral anterior cruciate ligament injuries. Med. Pregl. 2015, 68, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Ardern, C.L.; Webster, K.E.; Taylor, N.F.; Feller, J.A. Return to sport following anterior cruciate ligament reconstruction surgery: A systematic review and meta-analysis of the state of play. Br. J. Sports Med. 2011, 45, 596–606. [Google Scholar] [CrossRef]
- Bradley, J.; Honkamp, N.J.; Jost, P.; West, R.; Norwig, J.; Kaplan, L.D. Incidence and variance of knee injuries in elite college football players. Am. J. Orthop. 2008, 37, 310–314. [Google Scholar] [CrossRef]
- Maletis, G.B.; Inacio, M.C.; Funahashi, T.T. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am. J. Sports Med. 2015, 43, 641–647. [Google Scholar] [CrossRef]
- Rossi, M.J. Editorial Commentary: Tibial Tubercle-Trochlear Groove Distance as an Independent Risk Factor for Noncontact Anterior Cruciate Ligament Injury Is Possible but remains Uncertain. Arthroscopy 2016, 32, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Degnan, A.J.; Maldjian, C.; Adam, R.J.; Fu, F.H.; Di Domenica, M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. Am. J. Roentgenol. 2015, 204, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Whatman, C. Biomechanics associated with patellofemoral pain and ACL injuries in sports. Sports Med. 2015, 45, 1325–1337. [Google Scholar] [CrossRef]
- John, R.; Dhillon, M.S.; Sharma, S.; Prabhakar, S.; Bhandari, M. Is there a genetic predisposition to anterior cruciate ligament tear? A Systematic Review. Am. J. Sports Med. 2016, 44, 3262–3269. [Google Scholar] [CrossRef]
- Dejour, D.; Ntagiopoulos, P.G.; Saggin, P.R.; Panisset, J.C. The diagnostic value of clinical tests, magnetic resonance imaging, and instrumented laxity in the differentiation of complete versus partial anterior cruciate ligament tears. Arthroscopy 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Pan, J.P.; Wang, X.C.; Huang, M.H. Analysis of the characteristics and clinical diagnosis and treatment of avulsion fracture of the lateral edge of tibial plateau. Zhongguo Gu Shang 2018, 31, 155–159. [Google Scholar]
- Kostov, H.; Stojmenski, S.; Kostova, E. Reliability Assessment of Arthroscopic Findings Versus MRI in ACL Injuries of the Knee. Acta Inform. Medica 2014, 22, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.A.; Kettering, J.M.; Towers, J.D.; Britton, C.A. MR imaging of the knee having isolated and combined ligament injuries. Am. J. Roentgenol. 1998, 170, 1207–1213. [Google Scholar] [CrossRef]
- Oei, E.H.; Nikken, J.J.; Verstijnen, A.C.; Ginai, A.Z.; Myriam Hunink, M.G. MR Imaging of the Menisci and Cruciate Ligaments: A Systematic Review. Radiology 2003, 226, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Esmaili Jah, A.A.; Keyhani, S.; Zarei, R.; Moghaddam, A.K. Accuracy of MRI in comparison with clinical and arthroscopic findings in ligamentous and meniscal injuries of the knee. Acta Orthop. Belg. 2005, 71, 189–196. [Google Scholar] [PubMed]
- Navali, A.M.; Bazavar, M.; Mohseni, M.A.; Safari, B.; Tabrizi, A. Arthroscopic evaluation of the accuracy of clinical examination versus MRI in diagnosing meniscus tears and cruciate ligament ruptures. Arch. Iran. Med. 2013, 16, 229–232. [Google Scholar]
- Khanda, G.; Akhtar, W.; Ahsan, H.; Ahmad, N. Assessment of menisci and ligamentous injuries of the knee on magnetic resonance imaging: Correlation with arthroscopy. J. Pak. Med. Assoc. 2008, 58, 537–540. [Google Scholar] [PubMed]
- Cheng, X.Y.; Feng, J.F.; Lu, Y.H.; Zhao, Y.L.; Yang, Z.Q. Diagnostic value of Blumensaat angle for anterior cruciate ligament injury. Zhongguo Gu Shang 2017, 30, 726–730. [Google Scholar] [PubMed]
- Wang, J.; Wu, H.; Dong, F.; Li, B.; Wei, Z.; Peng, Q.; Dong, D.; Li, M.; Xu, J. The role of ultrasonography in the diagnosis of anterior cruciate ligament injury: A systematic review and meta-analysis. Eur. J. Sport Sci. 2018, 18, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Puzzitiello, R.N.; Agarwalla, A.; Zuke, W.A.; Garcia, G.H.; Forsythe, B. Imaging Diagnosis of Injury to the Anterolateral Ligament in Patients With Anterior Cruciate Ligaments: Association of Anterolateral Ligament Injury With Other Types of Knee Pathology and Grade of Pivot-Shift Examination: A Systematic Review. Arthroscopy 2018, 34, 2728–2738. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.A.; Tindall, A.J.; James, K.D.; Relwani, J.; Fernando, K.W. Accuracy of handheld ultrasound scanning in detecting meniscal tears. J. Bone Jt. Surg. Br. 2008, 90, 1045–1048. [Google Scholar] [CrossRef]
- Timotijević, S.; Vukasinović, Z.; Bascavević, Z. Validity of clinical and ultrasound examination related to arthroscopy in acute injury of the medial meniscus of the knee. Srp. Arh. Za Celok. Lek. 2008, 136, 28–32. [Google Scholar] [CrossRef]
- Najafi, J.; Bagheri, S.; Lahiji, F.A. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J. Ultrasound Med. 2006, 25, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Tauscher, A.; Kühner, C.; Göhring, U.; Sohn, C.; Meeder, P.J. 3-dimensional ultrasound in clinical diagnosis of meniscus lesions. Ultraschall Med. 1998, 19, 28–33. [Google Scholar] [CrossRef]
- Khan, Z.; Faruqui, Z.; Ogyunbiyi, O.; Rosset, G.; Iqbal, J. Ultrasound assessment of internal derangement of the knee. Acta Orthop. Belg. 2006, 72, 72–76. [Google Scholar]
- Van Oudenaarde, K.; Swart, N.M.; Bloem, J.L.; Bierma-Zeinstra, S.M.; Algra, P.R.; Koes, B.; Verhaar, J.; Nelissen, R.G.; Bindels, P.J.; Luijsterburg, P.A.; et al. Post-traumatic knee MRI findings and associations with patient, trauma, and clinical characteristics: A subgroup analysis in primary care in the Netherlands. Br. J. Gen. Pract. 2017, 67, e851–e858. [Google Scholar] [CrossRef]
- Floyd, R.T.; Peery, D.S.; Andrews, J.R. Advantages of the prone Lachman versus the traditional Lachman. Orthopedics 2008, 31, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Malanga, G.A.; Andrus, S.; Nadler, S.F.; McLean, J. Physical examination of the knee: A review of the original test description and scientific validity of common orthopedic tests. Arch. Phys. Med. Rehabil. 2003, 84, 592–603. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.K. Reliability of the anterior drawer test, the pivot shift test, and the Lachman test. Clin. Orthop. Relat. Res. 1995, 317, 237–242. [Google Scholar]
- Cimino, F.; Volk, B.S.; Setter, D. Anterior cruciate ligament injury: Diagnosis, management, and prevention. Am. Fam. Physician 2010, 82, 917–922. [Google Scholar]
- Mulligan, E.P.; McGuffie, D.Q.; Coyner, K.; Khazzam, M. The reliability and diagnostic accuracy of assessing the translation endpoint during the Lachman test. Int. J. Sports Phys. Ther. 2015, 10, 52–61. [Google Scholar] [PubMed]
- Bach, B.R., Jr.; Warren, R.F.; Wickiewicz, T.L. The pivot shift phenomenon: Results and description of a modified clinical test for anterior cruciate ligament insufficiency. Am. J. Sports Med. 1988, 16, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Gollehon, D.L.; Torzilli, P.A.; Warren, R.F. The role of posterolateral and cruciate ligaments in the stability of the human knee: A biomechanical study. J. Bone Jt. Surg. 1987, 69, 233–242. [Google Scholar] [CrossRef]
- Jakob, R.P.; Staubli, H.U.; Deland, J.T. Grading the pivot shift; objective tests with implications for treatment. J. Bone Jt. Surg. 1987, 69, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Makhmalbaf, H.; Moradi, A.; Ganji, S.; Omidi-Kashani, F. Accuracy of Lachman and Anterior Drawer Tests for Anterior Cruciate Ligament Injuries. Arch. Bone Jt. Surg. 2013, 1, 94–97. [Google Scholar] [PubMed]
- Colombet, P.; Dejour, D.; Panisset, J.C.; Siebold, R. Current concept of partial anterior cruciate ligament ruptures. Orthop. Traumatol. Surg. Res. 2010, 96 (Suppl. 8), S109–S118. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Oda, T.; Tsukazaki, S.; Kinugasa, R.; Hirose, N.; Fukubayashi, T. Relationship between quadriceps femoris muscle volume and muscle torque after anterior cruciate ligament rupture. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 641–645. [Google Scholar] [CrossRef]
- Hardaker, W.T., Jr.; Garrett, W.E., Jr.; Bassett, F.H., 3rd. Evaluation of acute traumatic hemarthrosis of the knee joint. South. Med. J. 1990, 83, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, M.C.; Kowalczuk, M.; Andruszkiewicz, N.; Simunovic, N.; Farrokhyar, F.; Turnbull, T.L.; Debski, R.E.; Ayeni, O.R. Diagnostic accuracy of physical examination for anterior knee instability: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2805–2813. [Google Scholar] [CrossRef]
- Luhmann, S.J. Acute traumatic knee effusions in children and adolescents. J. Pediatr. Orthop. 2003, 23, 199–202. [Google Scholar] [CrossRef]
- Temponi, E.F.; de Carvalho Júnior, L.H.; Sonnery-Cottet, B.; Chambat, P. Partial tearing of the anterior cruciate ligament: Diagnosis and treatment. Rev. Bras. De Ortop. Engl. Ed. 2015, 50, 9–15. [Google Scholar] [CrossRef]
- Panisset, J.C.; Ntagiopoulos, P.G.; Saggin, P.R.; Dejour, D. A comparison of Telos™ stress radiography versus Rolimeter™ in the diagnosis of different patterns of anterior cruciate ligament tears. Orthop. Traumatol. Surg. Res. 2012, 98, 751–758. [Google Scholar] [CrossRef]
- Siegel, L.; Vandenakker-Albanese, C.; Siegel, D. Anterior cruciate ligament injuries: Anatomy, physiology, biomechanics, and management. Clin. J. Sport Med. 2012, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Noyes, F.R.; Mooar, L.A.; Moorman, C.T., 3rd; McGinniss, G.H. Partial tears of the anterior cruciate ligament. Progression to complete ligament deficiency. J. Bone Jt. Surg. Br. 1989, 71, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Choi, J.Y.; Lee, G.K.; Choi, J.A.; Chung, H.W.; Kang, H.S. Grading of anterior cruciate ligament injury. Diagnostic efficacy of oblique coronal magnetic resonance imaging of the knee. Comput. Assist. Tomogr. 2003, 27, 814–819. [Google Scholar] [CrossRef] [PubMed]
- DeFranco, M.J.; Bach, B.R., Jr. A comprehensive review of partial anterior cruciate ligament tears. J. Bone Jt. Surg. Am. 2009, 91, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Crain, E.H.; Fithian, D.C.; Paxton, E.W.; Luetzow, W.F. Variation in anterior cruciate ligament scar pattern: Does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy 2005, 21, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sievert, Z.A.; Bennett, H.J.; Weinhandl, J.T. Intra- and inter-rater reliability of ultrasound measures of the anterior cruciate ligament. J. Ultrasound 2021, 24, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-T.; Wu, C.-H.; Yu, C.-W.; Wang, J.-H.; Shih, T.-F.; Wang, T.-G.; Chen, W.-S. Sonography of the Normal Anterior Cruciate Ligament: A Preliminary Report. J. Med. Ultrasound 2013, 21, 16–20. [Google Scholar] [CrossRef]
- Skovgaard Larsen, L.P.; Rasmussen, O.S. Diagnosis of acute rupture of the anterior cruciate ligament of the knee by sonography. Eur. J. Ultrasound 2000, 12, 163–167. [Google Scholar] [CrossRef]
- Suzuki, S.; Kasahara, K.; Futami, T.; Iwasaki, R.; Ueo, T.; Yamamuro, T. Ultrasound diagnosis of pathology of the anterior and posterior cruciate ligaments of the knee joint. Arch. Orthop. Trauma Surg. 1991, 110, 200–203. [Google Scholar] [CrossRef]
- Chylarecki, C.; Hierholzer, G.; Tabertshofer, H. Tabertshofer. Sonographische Kriterien der frischen Ruptur des vorderen Kreuzbandes Anatomisch-experimentelle und klinische Studie. Unfallchirurg 1995, 21, 109–117. [Google Scholar] [CrossRef]
- Ptasznik, R.; Feller, J.; Bartlett, J.; Fitt, G.; Mitchell, A.; Hennessy, O. The value of sonography in the diagnosis of traumatic rupture of the anterior cruciate ligament of the knee. Am. J. Roentgenol. 1995, 164, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
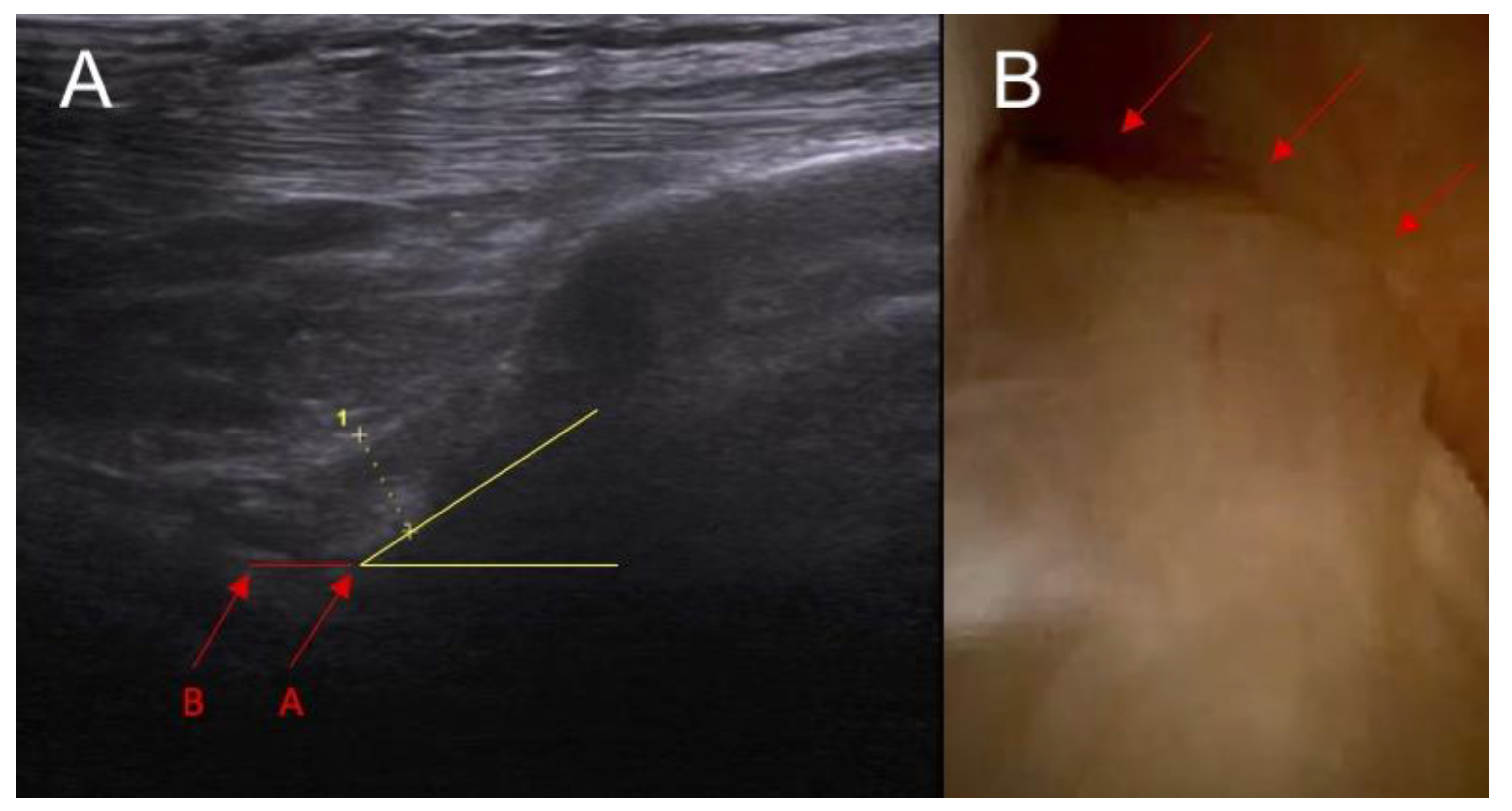


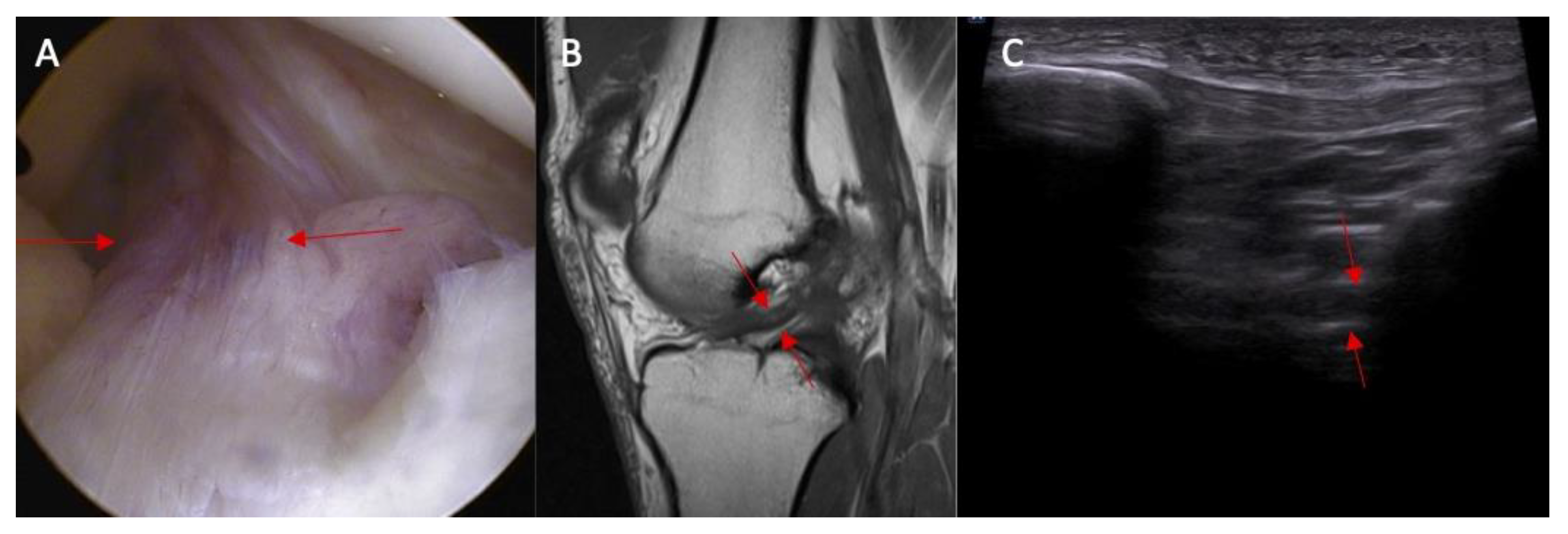
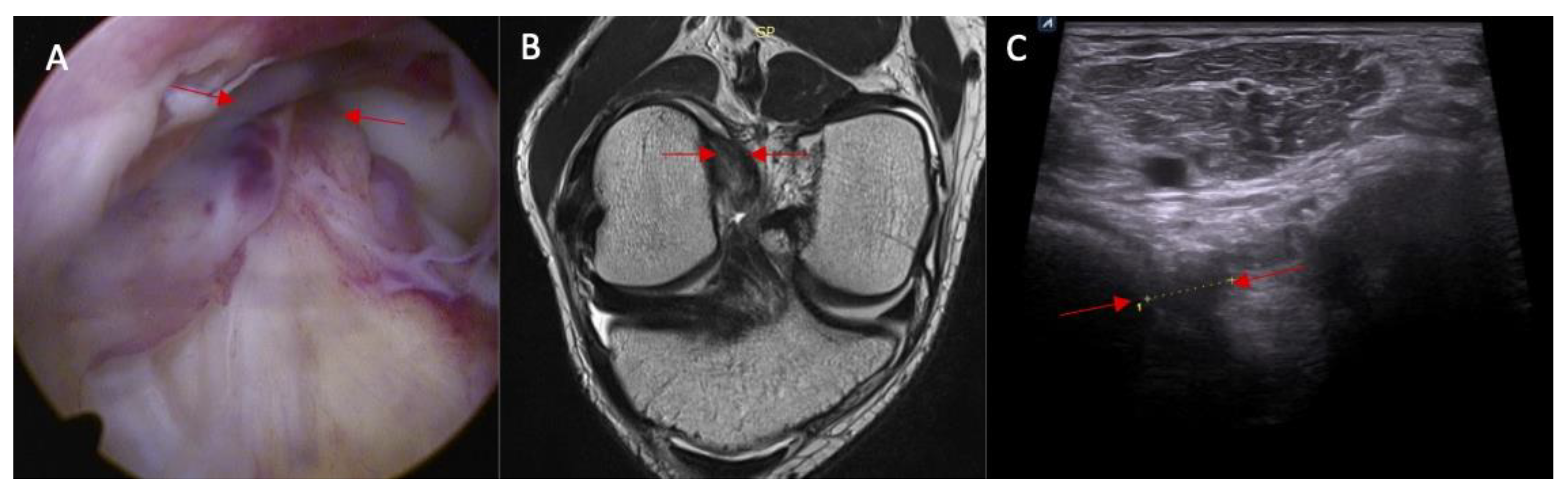
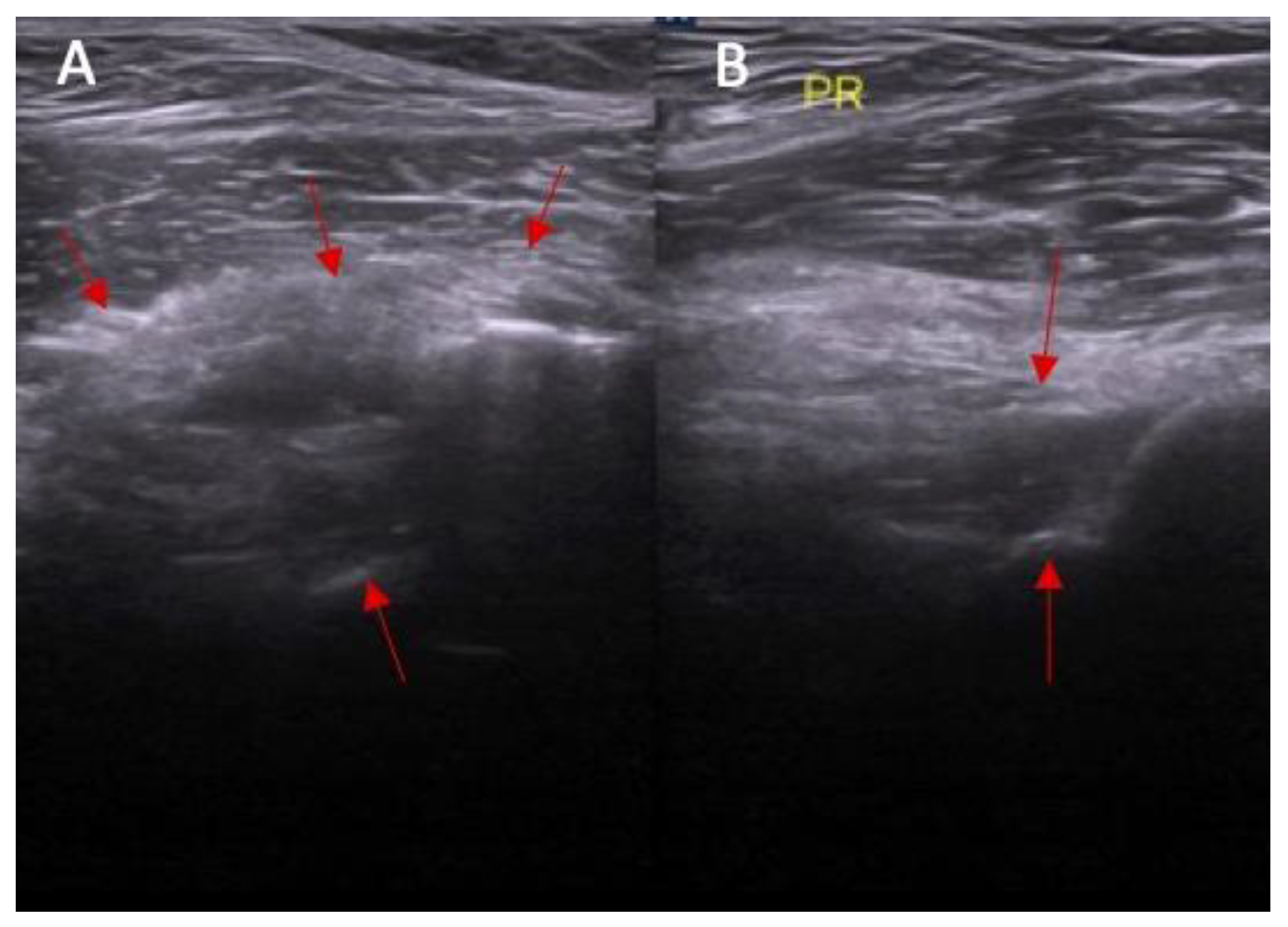
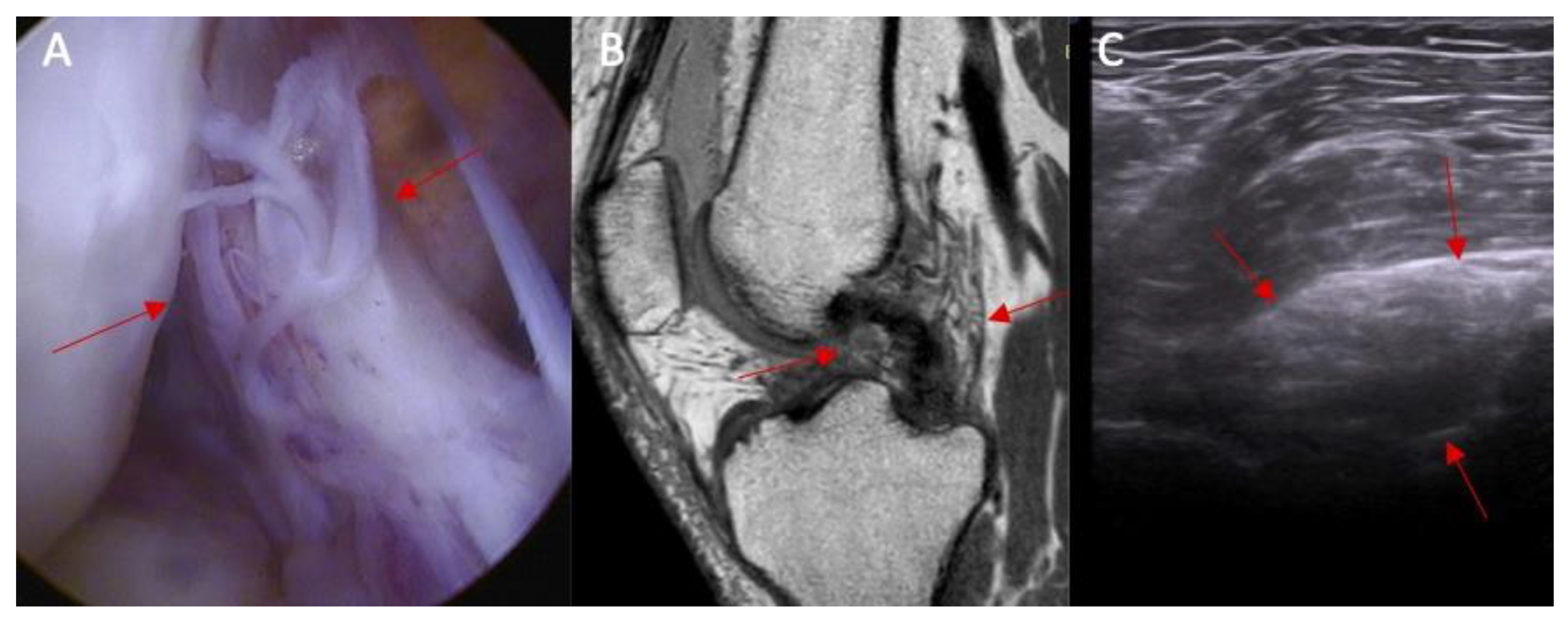
| Parameter | Study Group (n = 25) | Control Group (n = 25) | p |
|---|---|---|---|
| Abnormal inclination of the ACL (n, %) | 17 (67%) | 2 (8%) | <0.0001 |
| Swelling of the ACL intact to femur condyle | 21 (83%) | 0 | <0.0001 |
| Swelling/scarifications of the ACL/PCL | 22 (88%) | 0 | <0.0001 |
| Dynamic instability: | <0.0001 | ||
| 0–2 mm | 0 | 19 (76%) | |
| 3–4 mm | 8 (33%) | 6 (23%) | |
| ≥5 mm | 17 (67%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilczyk, C. The Value of Ultrasound Diagnostic Imaging of Anterior Crucial Ligament Tears Verified Using Experimental and Arthroscopic Investigations. Diagnostics 2024, 14, 305. https://doi.org/10.3390/diagnostics14030305
Wasilczyk C. The Value of Ultrasound Diagnostic Imaging of Anterior Crucial Ligament Tears Verified Using Experimental and Arthroscopic Investigations. Diagnostics. 2024; 14(3):305. https://doi.org/10.3390/diagnostics14030305
Chicago/Turabian StyleWasilczyk, Cezary. 2024. "The Value of Ultrasound Diagnostic Imaging of Anterior Crucial Ligament Tears Verified Using Experimental and Arthroscopic Investigations" Diagnostics 14, no. 3: 305. https://doi.org/10.3390/diagnostics14030305
APA StyleWasilczyk, C. (2024). The Value of Ultrasound Diagnostic Imaging of Anterior Crucial Ligament Tears Verified Using Experimental and Arthroscopic Investigations. Diagnostics, 14(3), 305. https://doi.org/10.3390/diagnostics14030305





