Neural and Onconeural Autoantibodies and Blood–Brain Barrier Disruption Markers in Patients Undergoing Radiotherapy for High-Grade Primary Brain Tumour
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Radiotherapy
2.4. Laboratory Assay
2.4.1. BBB Biomarkers
2.4.2. Analysis of Antibodies
2.5. Functional Assessment
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
3.2. Onconeural and Anti-Neural Antibodies
3.3. Blood–Brain Barrier Biomarkers
3.4. Functional Assessment Results
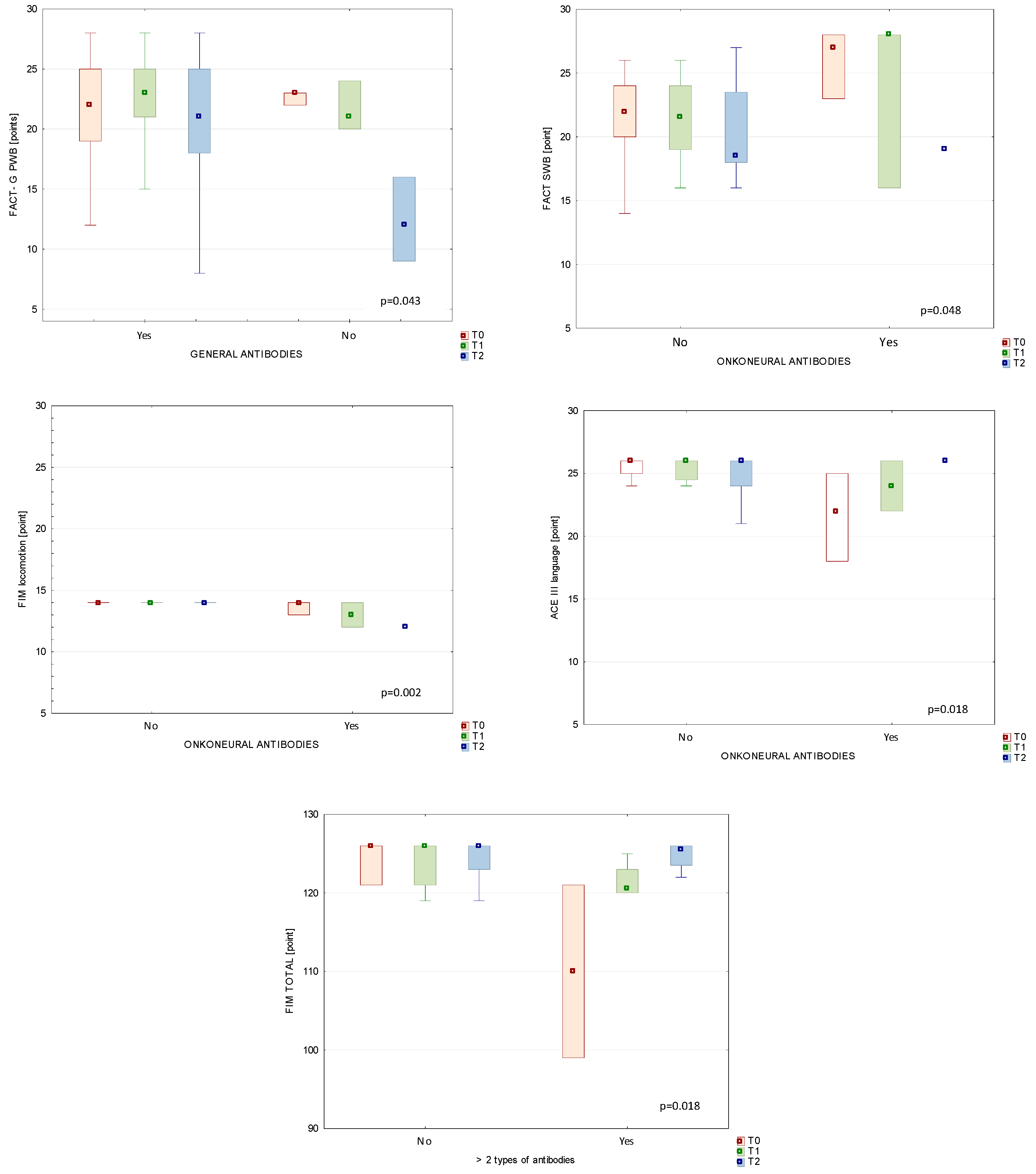
3.5. Comparison between Immunological Status and Clinical Parameters in Patients’ Subgroups
3.5.1. Antibodies and BBB Markers
3.5.2. Autoantibodies and Functional Status of BT Patients
3.6. Correlations between Blood–Brain Barrier Markers and Autoantibodies
4. Discussion
Strength and Limitations of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, J.L.; Li, G.; Shaffer, J.L.; Azoulay, M.I.; Gibbs, I.C.; Nagpal, S.; Soltys, S.G. Stereotactic Radiosurgery and Hypofractionated Radiotherapy for Glioblastoma. Neurosurgery 2018, 82, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular pathways: Radiation-induced cognitive impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef]
- Di Perri, D.; Jmil, S.; Lawson, T.M.; Van Calster, L.; Whenham, N.; Renard, L. Health-related quality of life and cognitive failures in patients with lower-grade gliomas treated with radiotherapy. Cancer Radiother. 2023, 27, 219–224. [Google Scholar] [CrossRef]
- Haldbo-Classen, L.; Amidi, A.; Wu, L.M.; Lukacova, S.; Oettingen, G.V.; Gottrup, H.; Zachariae, R.; Høyer, M. Long-term cognitive dysfunction after radiation therapy for primary brain tumors. Acta Oncol. 2019, 58, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Candia, A.; Rogers, N.K.; Castillo, R.L. Blood-Brain Barrier Dysfunction in the Detrimental Brain Function. In Connectivity and Functional Specialization in the Brain; Heinbockel, T., Zhou, Y., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, R.; Michalak, S.; Wencel-Warot, A.; Nowinski, W.L. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology 2012, 79, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Jasiak-Zatońska, M.; Michalak, S.; Osztynowicz, K.; Kozubski, W.; Kalinowska-Łyszczarz, A. Relationship between blood-brain permeability and antibodies against aquaporins in neuromyelitis optica spectrum disorders and multiple sclerosis patients. Neurol. Neurochir. Pol. 2022, 56, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Misan, N.; Michalak, S.; Kapska, K.; Osztynowicz, K.; Ropacka-Lesiak, M.; Kawka-Paciorkowska, K. Does the Blood-Brain Barrier Integrity Change in Regard to the Onset of Fetal Growth Restriction? Int. J. Mol. Sci. 2023, 24, 1965. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochem. Biophys. Acta Mol. Cell Res. 1999, 1450, 191–231. [Google Scholar] [CrossRef]
- Shaaban-Ali, M.; Harmer, M.; Vaughan, R.S.; Dunne, J.A.; Latto, I.P.; Haaverstad, R.; Kulatilake, E.N.; Butchart, E.G. Changes in serum S100 beta protein and Mini-Mental State Examination after cold (28 °C) and warm (34 °C) cardiopulmonary bypass using different blood gas strategies (alpha-stat and pH-stat). Acta Anaesthesiol. Scand. 2002, 46, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Ramos, S.A.; Gruhlke, T.; Bauer, H.; Bauer, M.; Luntz, A.P.; Motsch, J.; Martin, E.; Vahl, C.F.; Missler, U.; Wiesmann, M.; et al. Cerebral and extracerebral release of protein S100B in cardiac surgical patients. Anaesthesia 2004, 59, 344–349. [Google Scholar] [CrossRef]
- Bottiger, B.W.; Mobes, S.; Glatzer, R.; Bauer, H.; Gries, A.; Bartsch, P.; Motsch, J.; Martin, E. Astroglial protein S-100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation 2001, 103, 2694–2698. [Google Scholar] [CrossRef]
- Pelinka, L.E.; Toegel, E.; Mauritz, W.; Redl, H. Serum S100B: A marker of brain damage in traumatic brain injury with and without multiple trauma. Shock 2003, 19, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.P.; Pierce, M.C.; Wisniewski, S.R.; Adelson, P.D.; Kochanek, P.M. Serum S100B concentrations are increased after closed head injury in children: A preliminary study. J. Neurotrauma 2002, 19, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, I.; Korfias, S.; Dafni, U.; Anthi, A.; Psachoulia, C.; Jullien, G.; Sakas, D.E.; Roussos, C. Protein S-100b serum levels in trauma-induced brain death. Neurology 2003, 60, 947–951. [Google Scholar] [CrossRef]
- Kanner, A.A.; Marchi, N.; Fazio, V.; Mayberg, M.R.; Koltz, M.T.; Siomin, V.; Stevens, G.H.; Masaryk, T.; Ayumar, B.; Vogelbaum, M.A.; et al. Serum S100 β a noninvasive marker of blood brain barrier function and brain lesions. Cancer 2003, 97, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Einav, S.; Shoshan, Y.; Ovadia, H.; Matot, I.; Hersch, M.; Itshayek, E. Early postoperative serum S100 beta levels predict ongoing brain damage after meningioma surgery: A prospective observational study. Crit. Care 2006, 10, R141. [Google Scholar] [CrossRef]
- Marquardt, G.; Setzer, M.; Seifert, V. Protein S-100b as serum marker for prediction of functional outcome in metastatic spinal cord compression. Acta Neurochir. 2004, 146, 449–452. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Wu, H.; Jia, Y.; Liu, J.; Mu, X.; Wu, H.; Zhao, Y. Over-expression of S100B protein as a serum marker of brain metastasis in non-small cell lung cancer and its prognostic value. Pathol. Res. Pract. 2019, 215, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, S.S.; Podlipnik, S.; Ribero, S.; Molina, R.; Rios, J.; Carrera, C.; Malvehy, J.; Puig, S. Monthly changes in serum levels of S100B protein as a predictor of metastasis development in high-risk melanoma patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1482–1488. [Google Scholar] [CrossRef]
- Koszewicz, M.; Michalak, S.; Bilinska, M.; Budrewicz, S.; Zaborowski, M.; Slotwinski, K.; Podemski, R.; Ejma, M. Is peripheral paraneoplastic neurological syndrome possible in primary brain tumors? Brain Behav. 2016, 6, e00465. [Google Scholar] [CrossRef]
- Scaringi, C.; Agolli, L.; Minniti, G. Technical Advances in Radiation Therapy for Brain Tumors. Anticancer Res. 2018, 38, 6041–6045. [Google Scholar] [CrossRef]
- Hojan, K.; Gerreth, K. Can Multidisciplinary Inpatient and Outpatient Rehabilitation Provide Sufficient Prevention of Disability in Patients with a Brain Tumor?—A Case-Series Report of Two Programs and a Prospective, Observational Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 6488. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Byun, Y. Trajectories of Symptom Clusters, Performance Status, and Quality of Life During Concurrent Chemoradiotherapy in Patients with High-Grade Brain Cancers. Cancer Nurs. 2018, 41, E38–E47. [Google Scholar] [CrossRef] [PubMed]
- Weitzner, M.A.; Meyers, C.A.; Gelke, C.K.; Byrne, K.S.; Cella, D.F.; Levin, V.A. The functional assessment of cancer therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 1995, 75, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Maritz, R.; Tennant, A.; Fellinghauer, C.; Stucki, G.; Prodinger, B. The Functional Independence Measure 18-item version can be reported as a unidimensional interval-scaled metric: Internal construct validity revisited. J. Rehabil. Med. 2019, 51, 193–200. [Google Scholar] [CrossRef]
- Noll, K.R.; Bradshaw, M.E.; Weinberg, J.S.; Wefel, J.S. Neurocognitive functioning is associated with functional independence in newly diagnosed patients with temporal lobe glioma. Neurooncol. Pract. 2018, 5, 184–193. [Google Scholar] [CrossRef]
- Gunn, S.; Burgess, G.H.; Maltby, J. A Factor Analysis of Functional Independence and Functional Assessment Measure Scores Among Focal and Diffuse Brain Injury Patients: The Importance of Bifactor Models. Arch. Phys. Med. Rehabil. 2018, 99, 1805–1810. [Google Scholar] [CrossRef]
- Hodges, J.R.; Larner, A.J. Addenbrooke’s Cognitive Examinations: ACE, ACE-R, ACE-III, ACEapp, and M-ACE. In Cognitive Screening Instruments; Springer: Cham, Switzerland, 2017; pp. 109–137. [Google Scholar]
- Matias-Guiu, J.A.; Cortés-Martínez, A.; Valles-Salgado, M.; Rognoni, T.; Fernández-Matarrubia, M.; Moreno-Ramos, T.; Matías-Guiu, J. Addenbrooke’s cognitive examination III: Diagnostic utility for mild cognitive impairment and dementia and correlation with standardized neuropsychological tests. Int. Psychogeriatr. 2017, 29, 105–113. [Google Scholar] [CrossRef]
- Guzik, P.; Więckowska, B. Data distribution analysis—A preliminary approach to quantitative data in biomedical research. J. Med. Sci. 2023, 92, e869. [Google Scholar] [CrossRef]
- Jackob, J.; Durand, T.; Feuvret, L.; Mazeron, J.J.; Delattre, J.Y.; Hoang-Xuan, K.; Psimaras, D.; Douzane, H.; Ribeiro, D.; Capelle, L.; et al. Cognitive impairment and morphological changes after radiation therapy in brain tumors: A review. Radiother. Oncol. 2018, 128, 221–228. [Google Scholar] [CrossRef]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Sun, Y.; Dong, J.; Xu, X.; Wang, H.; Zhao, X.; Zhang, J.; Yao, B.; Zhao, L.; et al. Changes in cognitive function, synaptic structure and protein expression after long-term exposure to 2.856 and 9.375 GHz microwaves. Cell Commun. Signal. 2023, 21, 34. [Google Scholar] [CrossRef]
- Pieczyńska, A.; Pilarska, A.; Hojan, K. Predictors of functional outcomes in adults with brain tumor undergoing rehabilitation treatment: A systematic review. Eur. J. Phys. Rehabil. Med. 2022, 58, 666–674. [Google Scholar] [CrossRef]
- Kramkowski, J.; Hebert, C. Neuropsychiatric sequelae of brain radiation therapy: A review of modality, symptomatology, and treatment options. Gen. Hosp. Psychiatry 2022, 74, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, L.; Ye, Z.; Shi, W.; Zhang, L.; Wang, J.; Yang, H. Radiation-induced bystander effects may contribute to radiation-induced cognitive impairment. Int. J. Radiat. Biol. 2021, 97, 329–340. [Google Scholar] [CrossRef]
- Misan, N.; Michalak, S.; Rzymski, P.; Poniedziałek, B.; Kapska, K.; Osztynowicz, K.; Ropacka-Lesiak, M. Molecular Indicators of Blood-Brain Barrier Breakdown and Neuronal Injury in Pregnancy Complicated by Fetal Growth Restriction. Int. J. Mol. Sci. 2022, 23, 13798. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.S.; In, Y.N.; Kang, C.; Park, J.S.; You, Y.; Min, J.H.; Ahn, H.J.; Yoo, I.; Kim, S.W.; Oh, S.K.; et al. Development of a strategy for assessing blood-brain barrier disruption using serum S100 calcium-binding protein B and neuron-specific enolase in early stage of neuroemergencies: A preliminary study. Medicine 2022, 101, e29644. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Fromm, M. Tight junction: Molecular structure meets function. Ann. N. Y. Acad. Sci. 2009, 1165, 1–6. [Google Scholar] [CrossRef]
- Sugiyama, S.; Sasaki, T.; Tanaka, H.; Yan, H.; Ikegami, T.; Kanki, H.; Nishiyama, K.; Beck, G.; Gon, Y.; Okazaki, S.; et al. The tight junction protein occludin modulates blood-brain barrier integrity and neurological function after ischemic stroke in mice. Sci. Rep. 2023, 13, 2892. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Vanere, P.; Gupta, T.; Munshi, A.; Jalali, R. Factors influencing activities of daily living using FIM-FAM scoring system before starting adjuvant treatment in patients with brain tumors: Results from a prospective study. J. Neurooncol. 2009, 94, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B. Factors associated with long-term functional outcomes, psychological sequelae and quality of life in persons after primary brain tumour. J. Neurooncol. 2013, 111, 355–366. [Google Scholar] [CrossRef]
- Valiyaveettil, D.G.A.; Malik, M.; Eaga, P.; Ahmed, S.F.; Joseph, D. A prospective study of assessment of neurocognitive function in illiterate patients with gliomas treated with chemoradiation: Assessment of neurocognitive function in gliomas. Cancer Treat. Res. Commun. 2021, 26, 100288. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, Z.; Amouheidari, A.; Hassanpour, M.; Davanian, F.; Iraji, S.; Shokrani, P.; Nazem-Zadeh, M.R. Early Detection of Radiation-Induced Injury and Prediction of Cognitive Deficit by MRS Metabolites in Radiotherapy of Low-Grade Glioma. BioMed Res. Int. 2021, 2021, 6616992. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Participants (n = 34), n (%) or Mean ± SD | |
|---|---|---|
| Age | Years | 48.80 ± 14.70 |
| Years | Male | 19 (55.9) |
| Female | 15 (44.1) | |
| Education | Primary | 0 (0.0) |
| Vocational | 8 (23.5) | |
| Secondary | 17 (50.0) | |
| High | 9 (26.5) | |
| Type of tumour | Glioblastoma, grade 4 | 17 (50.0) |
| Anaplastic astrocytoma | 6 (17.6) | |
| Oligodendroglioma, grade 3 | 1 (2.9) | |
| Total resection | Yes | 18 (82.4) |
| No | 16 (17.6) | |
| Tumour location, hemisphere | Right | 20 (58.8) |
| Left | 14 (41.2) | |
| Chemotherapy | Yes | 29 (85.3) |
| No | 5 (14.7) | |
| Steroids | Yes | 34 (100.0) |
| No | 0 (0) | |
| T0 | T1 | T2 | p Value | |
|---|---|---|---|---|
| OCLN [pg/mL] | 1.18 | 0.00 | 0.00 | 0.3454 |
| median (IQR) | (0.0–5.96) | (0.00–7.57) | (0.00–3.94) | |
| CLN5 [pg/mL] | 0.99 | 0.87 | 0.77 | 0.5857 |
| median (IQR) | (0.37–2.48) | (0.24 –2.34) | (0.00–1.78) | |
| Zo-1 [RU/mL] | 25.81 | 25.55 | 20.03 | 0.3944 |
| median (IQR) | (12.51–50.74) | (13.47–50.83) | (11.99–48.14) | |
| S100β [pg/mL] | 0.03 | 0.04 | 0.05 | 0.0531 |
| median (IQR) | (0.01–0.63) | (0.01–0.2) | (0.02–0.14) |
| Study Time Points | T0 | T1 | T2 | p Value |
|---|---|---|---|---|
| Median (IQR) | ||||
| Scale | FACT-G | |||
| Total score | 80.5 (46–107) | 81 (43–108) | 72 (43–103) | 0.1482 |
| PWB | 23 (10–28) | 24 (10–28) | 21 (5–28) | 0.1969 |
| EWB | 17 (4–24) | 16 (7–24) | 15 (6–24) | 0.5982 |
| SWB | 24 (4–28) | 24 (4–28) | 23 (4–28) | 0.0682 |
| FWB | 19 (10–28) | 19 (6–28) | 17 (6–27) | 0.4429 |
| Scale | ACE III | |||
| Total score | 88.4 (60–100) | 88.82 (55–100) | 87.7 (55–100) | 0.6368 |
| Attention | 18 (8–18) | 17.36 (13–18) | 16.96 (11–18) | 0.0043 * |
| Memory | 21.8 (12–26) | 21.5 (9–26) | 21.3 (10–26) | 0.5378 |
| Language | 24.9 (17–26) | 24.7 (17–26) | 24.9 (20–26) | 0.3777 |
| Fluency | 10.2 (1–14) | 10.18 (1–14) | 10.4 (1–14) | 0.1777 |
| Visual–spatial | 14.8 (8–17) | 14.94 (6–17) | 14.46 (8–16) | 0.2709 |
| Scale | FIM | |||
| TOTAL score | 124.8 (111–126) | 124.1 (119–126) | 118.3 (68–126) | 0.0523 |
| Self-care | 41.7 (30–42) | 41.9 (14–42) | 39.5 (12–42) | 0.0231 * |
| Mobility | 21.1 (21–28) | 21 (21–21) | 19.5 (9–21) | 0.3679 |
| Locomotion | 13.9 (12–14) | 13.9 (12–14) | 12.8 (6–14) | 0.7165 |
| Communication | 13.9 (12–14) | 13.7 (12–14) | 12.7 (11–14) | 0.0067 * |
| Sphincter Control | 13.9 (12–14) | 14.0 (14–14) | 13.76 (10–14) | 0.2231 |
| Social Awareness | 19.5 (14–21) | 19.5 (15–21) | 19.0 (10–21) | 0.0498 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojan, K.; Adamska, K.; Lewandowska, A.; Procyk, D.; Leporowska, E.; Osztynowicz, K.; Michalak, S. Neural and Onconeural Autoantibodies and Blood–Brain Barrier Disruption Markers in Patients Undergoing Radiotherapy for High-Grade Primary Brain Tumour. Diagnostics 2024, 14, 307. https://doi.org/10.3390/diagnostics14030307
Hojan K, Adamska K, Lewandowska A, Procyk D, Leporowska E, Osztynowicz K, Michalak S. Neural and Onconeural Autoantibodies and Blood–Brain Barrier Disruption Markers in Patients Undergoing Radiotherapy for High-Grade Primary Brain Tumour. Diagnostics. 2024; 14(3):307. https://doi.org/10.3390/diagnostics14030307
Chicago/Turabian StyleHojan, Katarzyna, Krystyna Adamska, Agnieszka Lewandowska, Danuta Procyk, Ewa Leporowska, Krystyna Osztynowicz, and Slawomir Michalak. 2024. "Neural and Onconeural Autoantibodies and Blood–Brain Barrier Disruption Markers in Patients Undergoing Radiotherapy for High-Grade Primary Brain Tumour" Diagnostics 14, no. 3: 307. https://doi.org/10.3390/diagnostics14030307
APA StyleHojan, K., Adamska, K., Lewandowska, A., Procyk, D., Leporowska, E., Osztynowicz, K., & Michalak, S. (2024). Neural and Onconeural Autoantibodies and Blood–Brain Barrier Disruption Markers in Patients Undergoing Radiotherapy for High-Grade Primary Brain Tumour. Diagnostics, 14(3), 307. https://doi.org/10.3390/diagnostics14030307






