Optical Coherence Tomography Angiography as a Diagnostic Tool for Diabetic Retinopathy
Abstract
:1. Introduction
2. Methods
3. Principles of Optical Coherence Tomography Angiography
3.1. Optical Coherence Tomography Angiography vs. Fundus Fluorescein Angiography
3.2. Optical Coherence Tomography Angiography Artefacts
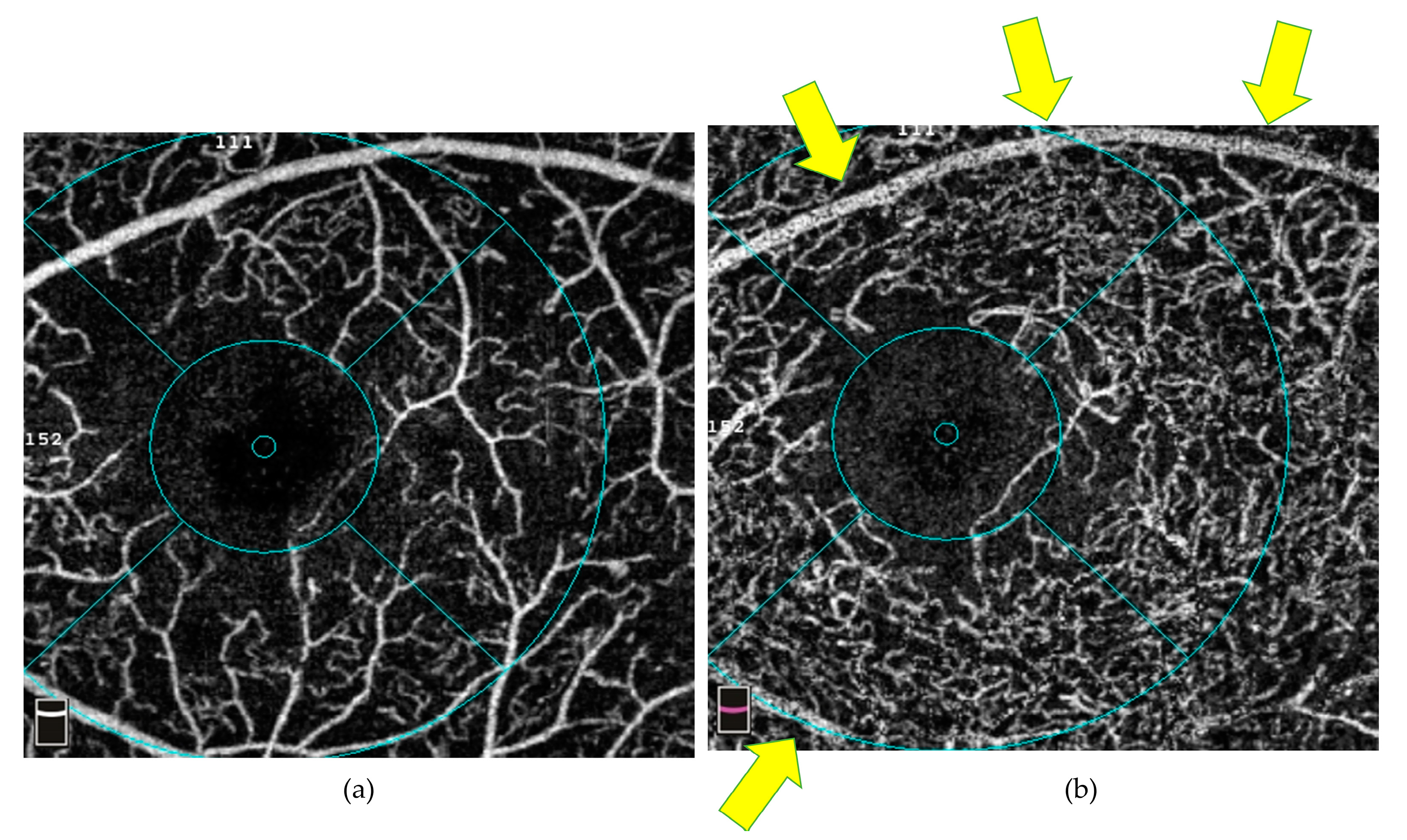
3.2.1. Signal Artefacts
3.2.2. Processing Artefacts
3.3. Spectrum-Domain versus Swept-Source Optical Coherence Tomography Angiography
3.4. Different Metrics on Optical Coherence Tomography Angiography
4. Optical Coherence Tomography Angiography and Diabetic Retinopathy
4.1. Clinical Features of Diabetic Retinopathy
4.2. Diabetes Mellitus without Clinical Signs of Diabetic Retinopathy
4.3. Non-Proliferative Diabetic Retinopathy (NPDR)
4.4. Proliferative Diabetic Retinopathy (PDR)
4.5. Diabetic Macular Oedema (DMO)
4.6. Diabetic Macular Ischaemia (DMI)
4.7. Pre-Diabetes
5. Different Scanning Protocols in Optical Coherence Tomography Angiography
6. Recommended Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas 10th Edition. Available online: www.diabetesatlas.org (accessed on 31 October 2023).
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Sim, R.; Tan, B.; Wong, D.; Yao, X.; Liu, X.; Ting, D.S.W.; Schmidl, D.; Ang, M.; Garhöfer, G.; et al. Optical coherence tomography angiography in diabetes and diabetic retinopathy. J. Clin. Med. 2020, 9, 1723. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, D.R.; Yi, J.J.; De Koo, L.O.; Ameri, H.; Puliafito, C.A.; Kashani, A.H. Optical coherence tomography angiography of diabetic retinopathy in human subjects. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Fawzi, A.; Teo, K.Y.; Fukuyama, H.; Sen, S.; Tsai, W.S.; Sivaprasad, S. Diabetic macular ischaemia—A new therapeutic target? Prog. Retin. Eye Res. 2022, 89, 101033. [Google Scholar] [CrossRef] [PubMed]
- Palochak, C.M.A.; Lee, H.E.; Song, J.; Geng, A.; Linsenmeier, R.A.; Burns, S.A.; Fawzi, A.A. Retinal blood velocity and flow in early diabetes and diabetic retinopathy using adaptive optics scanning laser ophthalmoscopy. J. Clin. Med. 2019, 8, 1165. [Google Scholar] [CrossRef]
- Fondi, K.; Wozniak, P.A.; Howorka, K.; Bata, A.M.; Aschinger, G.C.; Popa-Cherecheanu, A.; Witkowska, K.J.; Hommer, A.; Schmidl, D.; Werkmeister, R.M.; et al. Retinal oxygen extraction in individuals with type 1 diabetes with no or mild diabetic retinopathy. Diabetologia 2017, 60, 1534–1540. [Google Scholar] [CrossRef]
- Khuu, L.A.; Tayyari, F.; Sivak, J.M.; Flanagan, J.G.; Singer, S.; Brent, M.H.; Huang, D.; Tan, O.; Hudson, C. Aqueous humor endothelin-1 and total retinal blood flow in patients with non-proliferative diabetic retinopathy. Eye 2017, 31, 1443–1450. [Google Scholar] [CrossRef]
- Iwase, T.; Ueno, Y.; Tomita, R.; Terasaki, H. Relationship Between Retinal Microcirculation and Renal Function in Patients with Diabetes and Chronic Kidney Disease by Laser Speckle Flowgraphy. Life 2023, 13, 424. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Mechanisms of Disease Diabetic Retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Hammes, H.P.; Feng, Y.; Pfister, F.; Brownlee, M. Diabetic retinopathy: Targeting vasoregression. Diabetes 2011, 60, 9–16. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, R.K. Optical coherence tomography based angiography [Invited]. Biomed. Opt. Express 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Science 2012, 254, 4710–4725. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, R.K. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt. Express 2008, 16, 11438–11452. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Balaratnasingam, C.; Yu, P.K.; Morgan, W.H.; McAllister, I.L.; Cringle, S.J.; Yu, D.Y. Quantitative morphometry of perifoveal capillary networks in the human retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5502–5514. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, srep42201. [Google Scholar] [CrossRef] [PubMed]
- Linsenmeier, R.A.; Zhang, H.F. Retinal oxygen: From animals to humans. Prog. Retin. Eye Res. 2017, 58, 115–151. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Vingopoulos, F.; Le, R.; Laíns, I.; Wu, D.M.; et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 577–581. [Google Scholar] [CrossRef]
- López-Sáez, M.P.; Ordoqui, E.; Tornero, P.; Baeza, A.; Sainza, T.; Zubeldia, J.M.; Baeza, M.L. Fluorescein-induced allergic reaction. Ann. Allergy Asthma Immunol. 1998, 81, 428–430. [Google Scholar] [CrossRef]
- Amato, A.; Nadin, F.; Borghesan, F.; Cicinelli, M.V.; Chatziralli, I.; Sadiq, S.; Mirza, R.; Bandello, F. Widefield optical coherence tomography angiography in diabetic retinopathy. J. Diabetes Res. 2020, 2020, 855709. [Google Scholar] [CrossRef]
- Tan, A.C.S.; Tan, G.S.; Denniston, A.K.; Keane, P.A.; Ang, M.; Milea, D.; Chakravarthy, U.; Cheung, C.M.G. An overview of the clinical applications of optical coherence tomography angiography. Eye 2018, 32, 262–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, E.S.; Kim, D.G.; Yu, S.Y. Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: Longitudinal study using OCT angiography. Acta Diabetol. 2019, 56, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Nesper, P.L.; Jampol, L.M.; Yu, F.; Fawzi, A.A. Statistical model of optical coherence tomography angiography parameters that correlate with severity of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Camacho, P. The role of Optical Coherence Tomography Angiography to detect early microvascular changes in Diabetic Retinopathy: A systematic review. J. Diabetes Metab. Disord. 2021, 20, 1957–1974. [Google Scholar] [CrossRef] [PubMed]
- Jiaa, Y.; Baileya, S.T.; Hwanga, T.S.; McClintica, S.M.; Gaoa, S.S.; Pennesia, M.E.; Flaxela, C.J.; Lauera, A.K.; Wilsona, D.J.; Horneggerb, J.; et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA 2015, 112, E2395–E2402. [Google Scholar] [CrossRef] [PubMed]
- Sambhav, K.; Abu-Amero, K.K.; Chalam, K.V. Deep capillary macular perfusion indices obtained with oct angiography correlate with degree of nonproliferative diabetic retinopathy. Eur. J. Ophthalmol. 2017, 27, 716–729. [Google Scholar] [CrossRef]
- Garg, I.; Uwakwe, C.; Le, R.; Lu, E.S.; Cui, Y.; Wai, K.M.; Katz, R.; Zhu, Y.; Moon, J.Y.; Li, C.Y.; et al. Nonperfusion Area and Other Vascular Metrics by Wider Field Swept-Source OCT Angiography as Biomarkers of Diabetic Retinopathy Severity. Ophthalmol. Sci. 2022, 2, 100144. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Schwartz, R.; Nicholson, L.; Huemer, J.; El-Bradey, M.H.; Sim, D.A.; Patel, P.J.; Balaskas, K.; Hamilton, R.D.; Keane, P.A.; et al. Widefield optical coherence tomography angiography for early detection and objective evaluation of proliferative diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 118. [Google Scholar] [CrossRef]
- Yoshida, M.; Murakami, T.; Kawai, K.; Nishikawa, K.; Ishihara, K.; Mori, Y.; Tsujikawa, A. Inference of Capillary Nonperfusion Progression on Widefield OCT Angiography in Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2023, 64, 24. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Wu, D.M.; Vavvas, D.G.; Husain, D.; Miller, J.W.; et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Enders, C.; Lang, G.E.; Dreyhaupt, J.; Loidl, M.; Lang, G.K.; Werner, J.U. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS ONE 2019, 14, e0210505. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Hirano, T.; Mizobe, H.; Toba, S. OCT-angiography based artificial intelligence-inferred fluorescein angiography for leakage detection in retina [Invited]. Biomed. Opt. Express 2023, 14, 5851. [Google Scholar] [CrossRef] [PubMed]
- Holmen, I.C.; Konda, S.M.; Pak, J.W.; McDaniel, K.W.; Blodi, B.; Stepien, K.E.; Domalpally, A. Prevalence and Severity of Artifacts in Optical Coherence Tomographic Angiograms. JAMA Ophthalmol. 2020, 138, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in Optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Moult, E.M.; Novais, E.A.; Louzada, R.N.; Cole, E.D.; Lee, B.; Husvogt, L.; Keane, P.A.; Denniston, A.K.; Witkin, A.J.; et al. Visualizing the Choriocapillaris Under Drusen: Comparing 1050-nm Swept-Source Versus 840-nm Spectral-Domain Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT585–OCT590. [Google Scholar] [CrossRef]
- Ferguson, R.D.; Hammer, D.X.; Paunescu, L.A.; Beaton, S.; Schuman, J.S. Tracking optical coherence tomography. Opt. Lett. 2004, 29, 2139–2141. [Google Scholar] [CrossRef]
- Hammer, D.X.; Ferguson, R.D.; Iftimia, N.V.; Ustun, T.; Wollstein, G.; Ishikawa, H.; Gabriele, M.L.; Dilworth, W.D.; Kagemann, L.; Schuman, J.S. Advanced scanning methods with tracking optical coherence tomography. Opt. Express 2005, 13, 7937–7947. [Google Scholar] [CrossRef]
- Vienola, K.V.; Braaf, B.; Sheehy, C.K.; Yang, Q.; Tiruveedhula, P.; Arathorn, D.W.; de Boer, J.F.; Roorda, A. Real-time eye motion compensation for OCT imaging with tracking SLO. Biomed. Opt. Express 2012, 3, 2950–2963. [Google Scholar] [CrossRef]
- Kashani, A.H.; Green, K.M.; Kwon, J.; Chu, Z.; Zhang, Q.; Wang, R.K.; Garrity, S.; Sarraf, D.; Rebhun, C.B.; Waheed, N.K.; et al. Suspended Scattering Particles in Motion: A Novel Feature of OCT Angiography in Exudative Maculopathies. Ophthalmol. Retin. 2018, 2, 694–702. [Google Scholar] [CrossRef]
- Merkle, C.W.; Zhu, J.; Bernucci, M.T.; Srinivasan, V.J. Dynamic Contrast Optical Coherence Tomography reveals laminar microvascular hemodynamics in the mouse neocortex in vivo. Neuroimage 2019, 202, 116067. [Google Scholar] [CrossRef]
- Fedosov, D.A.; Caswell, B.; Popel, A.S.; Karniadakis, G.E.M. Blood Flow and Cell-Free Layer in Microvessels. Microcirculation 2010, 17, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ledesma-Gil, G. Novel method for image averaging of optical coherence tomography angiography images. Retina 2020, 40, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Uji, A.; Balasubramanian, S.; Lei, J.; Baghdasaryan, E.; Al-Sheikh, M.; Borrelli, E.; Sadda, S.R. Multiple enface image averaging for enhanced optical coherence tomography angiography imaging. Acta Ophthalmol. 2018, 96, e820–e827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Wang, R.K. Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomed. Opt. Express 2015, 6, 4130–4143. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.; Boehringer, D.; Rothaus, K.; Cakir, B.; Bucher, F.; Daniel, M.; Lang, S.J.; Lagrèze, W.A.; Agostini, H.; Lange, C. Swept-source optical coherence tomography angiography alleviates shadowing artifacts caused by subretinal fluid. Int. Ophthalmol. 2020, 40, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Lentzsch, A.; Schöllhorn, L.; Schnorr, C.; Siggel, R.; Liakopoulos, S. Comparison of swept-source versus spectral-domain optical coherence tomography angiography for detection of macular neovascularization. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Parravano, M.; Costanzo, E.; Borrelli, E.; Sacconi, R.; Virgili, G.; Sadda, S.V.R.; Scarinci, F.; Varano, M.; Bandello, F.; Querques, G. Appearance of cysts and capillary non perfusion areas in diabetic macular edema using two different OCTA devices. Sci. Rep. 2020, 10, 800. [Google Scholar] [CrossRef]
- Xu, F.; Li, Z.; Yang, X.; Gao, Y.; Li, Z.; Li, G.; Wang, S.; Ning, X.; Li, J. Assessment of choroidal structural changes in patients with pre- and early-stage clinical diabetic retinopathy using wide-field SS-OCTA. Front. Endocrinol. 2023, 13, 1036625. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, W.; Yang, S.; Chen, Y.; Zhu, Z.; Huang, W. Choriocapillaris flow deficit and the risk of referable diabetic retinopathy: A longitudinal SS-OCTA study. Br. J. Ophthalmol. 2023, 107, 1319–1323. [Google Scholar] [CrossRef]
- Faatz, H.; Rothaus, K.; Ziegler, M.; Book, M.; Lommatzsch, C.; Spital, G.; Gutfleisch, M.; Pauleikhoff, D.; Lommatzsch, A. Quantitative comparison of the vascular structure of macular neovascularizations between swept-source and spectral-domain optical coherence tomography angiography. Clin. Ophthalmol. 2020, 14, 3179–3186. [Google Scholar] [CrossRef]
- Mendes, L.; Marques, I.P.; Cunha-Vaz, J. Comparison of Different Metrics for the Identification of Vascular Changes in Diabetic Retinopathy Using OCTA. Front. Neurosci. 2021, 15, 755730. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Durbin, M.K.; Shi, Y.; Uji, A.; Balasubramanian, S.; Baghdasaryan, E.; Al-Sheikh, M.; Sadda, S.R. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA Ophthalmol. 2017, 135, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Lauermann, P.; van Oterendorp, C.; Storch, M.W.; Khattab, M.H.; Feltgen, N.; Hoerauf, H.; Bemme, S. Distance-thresholded intercapillary area analysis versus vessel-based approaches to quantify retinal ischemia in OCTA. Transl. Vis. Sci. Technol. 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Dolz-Marco, R.; Freund, K.B.; Balaratnasingam, C.; Dansingani, K.; Gilani, F.; Mehta, N.; Young, E.; Klifto, M.R.; Chae, B.; et al. Fractal dimensional analysis of optical coherence tomography angiography in eyes with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4940–4947. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, E.S.; Yu, S.Y. Longitudinal changes in retinal microvasculature after panretinal photocoagulation in diabetic retinopathy using swept-source OCT angiography. Sci. Rep. 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Tang, F.; Wong, R.; Lok, J.; Szeto, S.K.H.; Chan, J.C.K.; Chan, C.K.M.; Tham, C.C.; Ng, D.S.; Cheung, C.Y. OCT Angiography Metrics Predict Progression of Diabetic Retinopathy and Development of Diabetic Macular Edema: A Prospective Study. Ophthalmology 2019, 126, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.A.; Durrani, A.K.; Patel, S. Optical coherence tomography angiography characteristics in diabetic patients without clinical diabetic retinopathy. Eye 2019, 33, 648–652. [Google Scholar] [CrossRef]
- Zhang, K.; Ferreyra, H.A.; Grob, S.; Bedell, M.; Zhang, J.J. Diabetic Retinopathy: Genetics and Etiologic Mechanisms. In Retina, 15th ed.; Elsevier: Amsterdam, The Netherlands, 2013; 925p. [Google Scholar]
- Early Treatment Diabetic Retinopathy Study. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS reports number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, G.; Chihara, E.; Takahashi, H.; Amano, H.; Okazaki, K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 190–196. [Google Scholar] [CrossRef]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Tomasso, L.; Querques, L.; Zerbini, G.; Scorcia, V.; Bandello, F.; Querques, G. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017, 54, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, H.; Chu, Z.; Zhang, Q.; Chao, J.R.; Rezaei, K.A.; Wang, R.K. Microvascular changes in the choriocapillaris of diabetic patients without retinopathy investigated by swept-source OCT angiography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 50. [Google Scholar] [CrossRef] [PubMed]
- Nesper, P.L.; Roberts, P.K.; Onishi, A.C.; Chai, H.; Liu, L.; Jampol, L.M.; Fawzi, A.A. quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO307–BIO315. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, T.E.; Chin, A.T.; Bonini Filho, M.A.; Adhi, M.; Branchini, L.; Salz, D.A.; Baumal, C.R.; Crawford, C.; Reichel, E.; Witkin, A.J.; et al. Detection of microvascular changes in eyes with diabetes but not clinical diabetic retinopathy using Optical Coherence Tomography Angiography. Retina 2015, 35, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Takase, N.; Nozaki, M.; Kato, A.; Ozeki, H.; Yoshida, M.; Ogura, Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face Optical Coherence Tomography Angiography. Retina 2015, 35, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Soetikno, B.T.; Fawzi, A.A. Characterization of the Middle Capillary Plexus using Optical Coherence Tomography Angiography in Healthy and Diabetic Eyes. Retina 2016, 36, 2039. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical coherence tomography angiography in diabetic retinopathy: A prospective pilot study. Am. J. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef]
- Couturier, A.; Mané, V.; Bonnin, S.; Erginay, A.; Massin, P.; Gaudric, A.; Tadayoni, R. Capillary Plexus Anomalies in Diabetic Retinopathy on Optical Coherence Tomography Angiography. 2015. Available online: http://journals.lww.com/retinajournal (accessed on 31 October 2023).
- Parravano, M.; De Geronimo, D.; Scarinci, F.; Querques, L.; Virgili, G.; Simonett, J.M.; Varano, M.; Bandello, F.; Querques, G. Diabetic Microaneurysms Internal Reflectivity on Spectral-Domain Optical Coherence Tomography and Optical Coherence Tomography Angiography Detection. Am. J. Ophthalmol. 2017, 179, 90–96. [Google Scholar] [CrossRef]
- Menean, M.; Sacconi, R.; Tombolini, B.; Fantaguzzi, F.; Bandello, F.; Querques, G. Combined wide-field imaging in grading diabetic retinopathy. Eye 2024, 38, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Sorour, O.; Chaudhri, J.; Alibhai, Y.; Waheed, N.K.; Duker, J.S.; Baumal, C.R. Distinguishing Intraretinal Microvascular Abnormalities from Retinal Neovascularization using Optical Coherence Tomography Angiography. Retina 2020, 40, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.P.; Saraf, S.S.; Zhang, Q.; Wang, R.K.; Rezaei, K.A. Ultra-Widefield Protocol Enhances Automated Classification of Diabetic Retinopathy Severity with OCT Angiography. Ophthalmol. Retin. 2020, 4, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Hagag, A.M.; Wang, J.; Zhang, M.; Smith, A.; Wilson, D.J.; Huang, D.; Jia, Y. Automated quantification of nonperfusion areas in 3 vascular plexuses with optical coherence tomography angiography in eyes of patients with diabetes. JAMA Ophthalmol. 2018, 136, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rosen, R. Optical Coherence Tomography Angiography in Diabetes. Curr. Diabetes Rep. 2016, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Reste-Ferreira, D.; Marques, I.P.; Santos, T.; Ribeiro, M.L.; Mendes, L.; Santos, A.R.; Lobo, C.; Cunha-Vaz, J. Retinal neurodegeneration in eyes with NPDR risk phenotypes: A two-year longitudinal study. Acta Ophthalmol. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Americal Academy of Ophthalmology. Summary Benchmarks for Preferred Practice Pattern® Guidelines. 2022. Available online: https://www.aao.org/education/summary-benchmark-detail/summary-benchmarks-full-set-2020#:~:text=Preferred%20Practice%20Patterns%20provide%20guidance,the%20needs%20of%20all%20patients (accessed on 31 October 2023).
- Cheung, N.; Mitchell, P.; Wong, T.Y. Seminar Diabetic Retinopathy. 2010. p. 376. Available online: www.thelancet.com (accessed on 31 October 2023).
- Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Takahashi, A.; Omae, T.; Song, Y.S.; Takahashi, T.; Yoshida, A. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6247–6255. [Google Scholar] [CrossRef]
- Elbendary, A.M.; Abouelkheir, H.Y. Bimodal imaging of proliferative diabetic retinopathy vascular features using swept source optical coherence tomography angiography. Int. J. Ophthalmol. 2018, 11, 1528–1533. [Google Scholar]
- Papayannis, A.; Tsamis, E.; Stringa, F.; Iacono, P.; Battaglia Parodi, M.; Stanga, P.E. Swept-source optical coherence tomography angiography vitreo-retinal segmentation in proliferative diabetic retinopathy. Eur. J. Ophthalmol. 2021, 31, 1925–1932. [Google Scholar] [CrossRef]
- Pan, J.; Chen, D.; Yang, X.; Zou, R.; Zhao, K.; Cheng, D.; Huang, S.; Zhou, T.; Yang, Y.; Chen, F. Characteristics of Neovascularization in Early Stages of Proliferative Diabetic Retinopathy by Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 192, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Dela Cruz, A.J.; Ledesma, M.G.; Van Hemert, J.; Radwan, A.; Cavallerano, J.D.; Aiello, L.M.; Sun, J.K.; Aiello, L.P. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.I.; Nam, K.Y.; Lee, S.E.; Lee, M.W.; Lim, H.B.; Jo, Y.J.; Kim, J.Y. Peripapillary microvasculature in patients with diabetes mellitus: An optical coherence tomography angiography study. Sci. Rep. 2019, 9, 15814. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, D.; Tang, Z.; Ng, D.S.; Cheung, C.Y. Optical coherence tomography angiography in diabetic retinopathy: An updated review. Eye 2020, 35, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lan, H.; Hu, Y.; Chen, Z.; Ouyang, P.; Luo, J. Application of Improved U-Net Convolutional Neural Network for Automatic Quantification of the Foveal Avascular Zone in Diabetic Macular Ischemia. J. Diabetes Res. 2022, 2022, 4612554. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, E.H.; Liu, G.; Shi, Y.; Zheng, F.; Flynn, H.W.; Gregori, G.; Rosenfeld, P.J. Widefield swept-source optical coherence tomography angiography of proliferative diabetic retinopathy. Ophthalmic Surg. Lasers Imaging Retina 2019, 50, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.F.; Shi, Y.; Hinkle, J.W.; Scott, N.L.; Fan, K.C.; Lyu, C.; Gregori, G.; Rosenfeld, P.J. Longitudinal Wide-Field Swept-Source OCT Angiography of Neovascularization in Proliferative Diabetic Retinopathy after Panretinal Photocoagulation. Ophthalmol. Retin. 2019, 3, 350–361. [Google Scholar] [CrossRef]
- Acar, O.P.A.; Onur, I.U. Effect of panretinal photocoagulation on retina and choroid in diabetic retinopathy: An optical coherence tomography angiography study. Photodiagnosis Photodyn. Ther. 2022, 40, 103166. [Google Scholar] [CrossRef]
- Faghihi, H.; Riazi-Esfahani, H.; Khodabande, A.; Khalili Pour, E.; Mirshahi, A.; Ghassemi, F.; Mirshahi, R.; Khojasteh, H.; Bazvand, F.; Hashemi, A.; et al. Effect of panretinal photocoagulation on macular vasculature using optical coherence tomography angiography. Eur. J. Ophthalmol. 2020, 31, 1877–1884. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Y.; Liu, D.; Stewart, J.M. Optical Coherence Tomography Angiography Assessment of Macular Choriocapillaris and Choroid Following Panretinal Photocoagulation in a Diverse Population with Advanced Diabetic Retinopathy. Asia-Pac. J. Ophthalmol. 2021, 10, 203–207. [Google Scholar] [CrossRef]
- Fawzi, A.A.; Fayed, A.E.; Linsenmeier, R.A.; Gao, J.; Yu, F. Improved Macular Capillary Flow on Optical Coherence Tomography Angiography After Panretinal Photocoagulation for Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 2019, 206, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Mirshahi, A.; Ghassemi, F.; Fadakar, K.; Mirshahi, R.; Bazvand, F.; Riazi-Esfahani, H. Effects of panretinal photocoagulation on retinal vasculature and foveal avascular zone in diabetic retinopathy using optical coherence tomography angiography: A pilot study. J. Curr. Ophthalmol. 2019, 31, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, A.S.; Stanca, H.T.; Tăbăcaru, B.; Danielescu, C.; Munteanu, M.; Stanca, S. Quantitative Parameters Relevant for Diabetic Macular Edema Evaluation by Optical Coherence Tomography Angiography. Medicina 2023, 59, 1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Van Dijk, E.H.C.; Borrelli, E.; Fragiotta, S.; Breazzano, M.P. OCT and OCT Angiography Update: Clinical Application to Age-Related Macular Degeneration, Central Serous Chorioretinopathy, Macular Telangiectasia, and Diabetic Retinopathy. Diagnostics 2023, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Kim, Y.H.; Ha, S.J.; Byeon, H.E.; Cho, C.H.; Kim, J.H.; Lee, K. Review Article Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography. J. Diabetes Res. 2019, 2019, 8164250. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, W.J.; Qin, Y.Z.; Qiu, X.Y.; Qin, L.; Li, J.M. Aqueous angiopoietin-like levels correlate with optical coherence tomography angiography metrics in diabetic macular edema. Int. J. Ophthalmol. 2021, 14, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, Y.; Bulloch, G.; Xiong, K.; Chen, Y.; Li, Y.; Liao, H.; Huang, W.; Zhu, Z.; Wang, W. Parapapillary Choroidal Microvasculature Predicts Diabetic Retinopathy Progression and Diabetic Macular Edema Development: A Three-Year Prospective Study. Am. J. Ophthalmol. 2023, 245, 164–173. [Google Scholar] [CrossRef]
- De Carlo, T.E.; Chin, A.T.; Joseph, T.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Waheed, N.K. Distinguishing diabetic macular edema from capillary nonperfusion using optical coherence tomography angiography. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 108–114. [Google Scholar] [CrossRef]
- Mané, V.; Dupas, B.; Gaudric, A.; Bonnin, S.; Pedinielli, A.; Erginay, A.; Tadayoni, R.; Couturier, A. Correlation between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by Optical Coherence Tomography Angiography. Retina 2016, 36, S102–S110. [Google Scholar] [CrossRef]
- Hasegawa, N.; Nozaki, M.; Takase, N.; Yoshida, M.; Ogura, Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT348–OCT355. [Google Scholar] [CrossRef]
- Lee, J.; Moon, B.G.; Cho, A.R.; Yoon, Y.H. Optical Coherence Tomography Angiography of DME and Its Association with Anti-VEGF Treatment Response. Ophthalmology 2016, 123, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Nanegrungsunk, O.; Patikulsila, D.; Sadda, S.R. Ophthalmic imaging in diabetic retinopathy: A review. Clin. Exp. Ophthalmol. 2022, 50, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- AttaAllah, H.R.; Mohamed, A.A.M.; Ali, M.A. Macular vessels density in diabetic retinopathy: Quantitative assessment using optical coherence tomography angiography. Int. Ophthalmol. 2019, 39, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Chan, E.O.; Sun, Z.; Wong, R.; Lok, J.; Szeto, S.; Chan, J.C.; Lam, A.; Tham, C.C.; Ng, D.S.; et al. Clinically relevant factors associated with quantitative optical coherence tomography angiography metrics in deep capillary plexus in patients with diabetes. Eye Vis. 2020, 7, 7. [Google Scholar] [CrossRef]
- Suciu, C.I.; Suciu, V.I.; Nicoara, S.D. Optical Coherence Tomography (Angiography) Biomarkers in the Assessment and Monitoring of Diabetic Macular Edema. J. Diabetes Res. 2020, 2020, 6655021. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, S.J.; Kang, S.W.; Son, K.Y.; Hwang, S. Local ocular factors associated with the development of diabetic macular edema: An inter-eye study. Sci. Rep. 2023, 13, 14868. [Google Scholar] [CrossRef] [PubMed]
- Kwan, C.C.; Fawzi, A.A. Imaging and Biomarkers in Diabetic Macular Edema and Diabetic Retinopathy. Curr. Diabetes Rep. 2019, 19, 95. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Z.; Shi, J.; Ran, A.; Tang, F.; Tang, Z.; Lok, J.; Chb, M.B.; Szeto, S.; Chan, J.; et al. A Multitask Deep-Learning System for assessment of Diabetic Macular Ischemia on Optical Coherence Tomography Angiography Images. Retina 2022, 42, 184–194. [Google Scholar] [CrossRef]
- Sim, D.A.; Keane, P.A.; Zarranz-Ventura, J.; Fung, S.; Powner, M.B.; Platteau, E.; Bunce, C.V.; Fruttiger, M.; Patel, P.J.; Tufail, A.; et al. The effects of macular ischemia on visual acuity in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2353–2360. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Classification of Diabetic Retinopathy from Fluorescein Angiograms: ETDRS Report Number 11. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Bradley, P.D.; Sim, D.A.; Keane, P.A.; Cardoso, J.; Agrawal, R.; Tufail, A.; Egan, C.A. The evaluation of diabetic macular ischemia using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.S.; Thottarath, S.; Gurudas, S.; Pearce, E.; Giani, A.; Chong, V.; Gemmy Cheung, C.M.; Sivaprasad, S. Characterization of the Structural and Functional Alteration in Eyes with Diabetic Macular Ischemia. Ophthalmol. Retina 2023, 7, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.C.; Ashraf, M.; Soetikno, B.T.; Fawzi, A.A. Multilevel Ischemia in Disorganization of the retinal inner layers on projection resolved Optical Coherence Tomography Angiography. Retina 2019, 39, 1588. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Lin, M.M.; Lammer, J.; Prager, S.; Sarangi, R.; Silva, P.S.; Aiello, L.P. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014, 132, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- DaCosta, J.; Bhatia, D.; Talks, J. The use of optical coherence tomography angiography and optical coherence tomography to predict visual acuity in diabetic retinopathy. Eye 2020, 34, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.W.; Tang, Z.Q.; Tang, F.Y.; Szeto, S.K.H.; Chan, J.; Yip, F.; Wong, C.Y.K.; Ran, A.R.; Lai, T.Y.Y.; Cheung, C.Y. Clinically relevant factors associated with a binary outcome of diabetic macular ischaemia: An OCTA study. Br. J. Ophthalmol. 2023, 107, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Lima, T.T.; Louzada, R.N.; Rassi, A.T.; Isaac, D.L.C.; Avila, M. Diabetic macular ischemia diagnosis: Comparison between optical coherence tomography angiography and fluorescein angiography. J. Ophthalmol. 2016, 2016, 3989310. [Google Scholar] [CrossRef] [PubMed]
- Scarinci, F.; Varano, M.; Parravano, M. Retinal Sensitivity Loss Correlates with Deep Capillary Plexus Impairment in Diabetic Macular Ischemia. J. Ophthalmol. 2019, 2019, 7589841. [Google Scholar] [CrossRef]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. International Diabetes Federation Atlas (9th Edition). 2019. Available online: https://www.diabetesatlas.org/ (accessed on 31 October 2023).
- Kirthi, V.; Nderitu, P.; Alam, U.; Evans, J.R.; Nevitt, S.; Malik, R.A.; Hopkins, D.; Jackson, T.L. The prevalence of retinopathy in prediabetes: A systematic review. Surv. Ophthalmol. 2022, 67, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.D.; Arango, F.J.; Parra, M.M.; Sánchez-Ávila, R.M.; Parra-Serrano, G.A.; Hoyos, A.T.; Granados, S.J.; Viteri, E.J.; Gaibor-Santos, I.; Perez, Y. Early microvascular changes in patients with prediabetes evaluated by optical coherence tomography angiography. Ther. Adv. Ophthalmol. 2021, 13, 25158414211047020. [Google Scholar] [CrossRef] [PubMed]
- Kirthi, V.; Reed, K.I.; Alattar, K.; Zuckerman, B.P.; Bunce, C.; Nderitu, P.; Alam, U.; Clarke, B.; Hau, S.; Al-Shibani, F.; et al. Multimodal testing reveals subclinical neurovascular dysfunction in prediabetes, challenging the diagnostic threshold of diabetes. Diabet. Med. 2023, 40, e14952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Toyama, T.; Hashimoto, Y.; Kawai, H.; Azuma, K.; Shiraya, T.; Kato, S.; Watanabe, Y.; Hirano, H.; Fujiwara, Y.; et al. Association of Prediabetes with Retinal Microvasculature on Swept-Source Optical Coherence Tomography Angiography in the Elderly: OTASSHA Study. Retina 2022, 42, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, X.; Wang, Y.; Chu, Z.; Wang, R.K.; Lu, L.; Zou, H. Early Retinal Microvasculopathy in Prediabetic Patients and Correlated Factors. Ophthalmic Res. 2023, 66, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li Rudvan, A.; Can, M.; Efe, F.; Keskin, M.; Beyan, E. Evaluation of retinal microvascular changes in patients with prediabetes. Niger. J. Clin. Pract. 2021, 24, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Ratra, D.; Dalan, D.; Prakash, N.; Kaviarasan, K.; Thanikachalam, S.; Das, U.N.; Angayarkanni, N. Quantitative analysis of retinal microvascular changes in prediabetic and diabetic patients. Indian. J. Ophthalmol. 2021, 69, 3226–3234. [Google Scholar] [CrossRef]
- Zagst, A.J.; Smith, J.D.; Wang, R.; Harrison, W.W. Foveal avascular zone size and mfERG metrics in diabetes and prediabetes: A pilot study of the relationship between structure and function. Doc. Ophthalmol. 2023, 147, 99–107. [Google Scholar] [CrossRef]
- El Sawy, S.; Bekhit, M.; Abdelhamid, A.; Esmat, S.; Ashraf, H.; Naguib, M. Assessment of early macular microangiopathy in subjects with prediabetes using optical coherence tomography angiography and fundus photography. Acta Diabetol. 2023, 61, 69–77. [Google Scholar] [CrossRef]
- Ho, J.; Dans, K.; You, Q.; Nudleman, E.D.; Freeman, W.R. Comparison of 3 MM × 3 MM versus 6 MM × 6 MM Optical Coherence Tomography Angiography Scan Sizes in the Evaluation of Non-Proliferative Diabetic Retinopathy. Retina 2019, 39, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Kitahara, J.; Toriyama, Y.; Kasamatsu, H.; Murata, T.; Sadda, S. Quantifying vascular density and morphology using different swept-source optical coherence tomography angiographic scan patterns in diabetic retinopathy. Br. J. Ophthalmol. 2019, 103, 216. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Warren, L.H.; Santos, A.R.; Marques, I.P.; Kubach, S.; Mendes, L.G.; de Sisternes, L.; Madeira, M.H.; Durbin, M.; Cunha-Vaz, J.G. Swept-source OCTA quantification of capillary closure predicts ETDRS severity staging of NPDR. Br. J. Ophthalmol. 2022, 106, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.R.; Kashani, A.H.; Tadayoni, R.; Korobelnik, J.F.; Wolf, S.; Pichi, F.; Tian, M. Standardization of OCT Angiography Nomenclature in Retinal Vascular Diseases: First Survey Results. Ophthalmol. Retin. 2021, 5, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.R.; Kashani, A.H.; Tadayoni, R.; Korobelnik, J.F.; Wolf, S.; Pichi, F.; Koh, A.; Ishibazawa, A.; Gaudric, A.; Loewenstein, A.; et al. Recommendations for OCT angiography reporting in retinal vascular disease: A Delphi approach by international experts. Ophthalmol. Retin. 2022, 6, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Waheed, N.K.; Rosen, R.B.; Jia, Y.; Munk, M.R.; Huang, D.; Fawzi, A.; Chong, V.; Nguyen, Q.D.; Sepah, Y.; Pearce, E. Optical coherence tomography angiography in diabetic retinopathy. Prog. Retin. Eye Res. 2023, 97, 101206. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jiang, H.; Shi, Y.; Qu, D.; Gregori, G.; Zheng, F.; Rundek, T.; Wang, J. Age-related alterations in the retinal microvasculature, microcirculation, and microstructure. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3804–3817. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, H.; Hwang, S.J.; Jeon, B.; Seong, M.; Yeom, H.; Kang, M.H.; Lim, H.W.; Shin, Y.U. Changes in the retinal microvasculature measured using optical coherence tomography angiography according to age. J. Clin. Med. 2020, 9, 883. [Google Scholar] [CrossRef]
- Tan, C.S.; Lim, L.W.; Chow, V.S.; Chay, I.W.; Tan, S.; Cheong, K.X.; Tan, G.T.; Sadda, S.V.R. Optical coherence tomography angiography evaluation of the parafoveal vasculature and its relationship with ocular factors. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT224–OCT234. [Google Scholar] [CrossRef]
- Borrelli, E.; Lonngi, M.; Balasubramanian, S.; Tepelus, T.C.; Baghdasaryan, E.; Iafe, N.A.; Pineles, S.L.; Velez, F.G.; Sarraf, D.; Sadda, S.R.; et al. Macular Microvascular Networks in Healthy Pediatric Subjects. Retina 2019, 39, 1216–1224. [Google Scholar] [CrossRef]
- Bhanushali, D.; Anegondi, N.; Gadde, S.G.K.; Srinivasan, P.; Chidambara, L.; Yadav, N.K.; Roy, A.S. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 519–525. [Google Scholar] [CrossRef]
- Chun, L.Y.; Silas, M.R.; Dimitroyannis, R.C.; Ho, K.; Skondra, D. Differences in macular capillary parameters between healthy black and white subjects with Optical Coherence Tomography Angiography (OCTA). PLoS ONE 2019, 14, e0223142. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Filho, M.B.; Rebhun, C.B.; Moult, E.M.; Lee, B.; Alibhai, Y.; Witkin, A.J.; Baumal, C.R.; Duker, J.S.; Fujimoto, J.G.; et al. Analyzing Relative Flow Speeds in Diabetic Retinopathy Using Variable Interscan Time Analysis OCT Angiography. Ophthalmol. Retin. 2021, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, D.; Mao, M.; Li, M.; Liu, S.; Li, F.; Chen, L.; Liu, M.; Leng, H.; Wang, Y.; et al. Ultra-widefield color fundus photography combined with high-speed ultra-widefield swept-source optical coherence tomography angiography for non-invasive detection of lesions in diabetic retinopathy. Front. Public Health 2022, 10, 1047608. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Tang, F.; Ting, D.S.W.; Tan, G.S.W.; Wong, T.Y. Artificial Intelligence in Diabetic Eye Disease Screening. Asia-Pac. J. Ophthalmol. 2019, 8, 158–164. [Google Scholar]
- Sandhu, H.S.; Eladawi, N.; Elmogy, M.; Keynton, R.; Helmy, O.; Schaal, S.; El-Baz, A. Automated diabetic retinopathy detection using optical coherence tomography angiography: A pilot study. Br. J. Ophthalmol. 2018, 102, 1564. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Zhang, Y.; Lim, J.I.; Chan, R.V.P.; Yang, M.; Yao, X. Quantitative Optical Coherence Tomography Angiography features for objective classification and staging of Diabetic Retinopathy. Retina 2020, 40, 322–332. [Google Scholar] [CrossRef]
- Alam, M.; Le, D.; Lim, J.I.; Chan, R.V.P.; Yao, X. Supervised machine learning based multi-task artificial intelligence classification of retinopathies. J. Clin. Med. 2019, 8, 872. [Google Scholar] [CrossRef]
- Malone, J.D.; El-Haddad, M.T.; Yerramreddy, S.S.; Oguz, I.; Tao, Y.K.K. Handheld spectrally encoded coherence tomography and reflectometry for motion-corrected ophthalmic optical coherence tomography and optical coherence tomography angiography. Neurophotonics 2019, 6, 1. [Google Scholar] [CrossRef]
- Rank, E.A.; Sentosa, R.; Harper, D.J.; Salas, M.; Gaugutz, A.; Seyringer, D.; Nevlacsil, S.; Maese-Novo, A.; Eggeling, M.; Muellner, P.; et al. Toward optical coherence tomography on a chip: In vivo three-dimensional human retinal imaging using photonic integrated circuit-based arrayed waveguide gratings. Light. Sci. Appl. 2021, 10, 6. [Google Scholar] [CrossRef]
- Lee, W.D.; Devarajan, K.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Optical coherence tomography angiography for the anterior segment. Eye Vis. 2019, 6, 4. [Google Scholar] [CrossRef]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef]
- Brunner, M.; Romano, V.; Steger, B.; Vinciguerra, R.; Lawman, S.; Williams, B.; Hicks, N.; Czanner, G.; Zheng, Y.; Willoughby, C.E.; et al. Imaging of corneal neovascularization: Optical coherence tomography angiography and fluorescence angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1263–1269. [Google Scholar] [CrossRef]






| OCTA | FFA |
|---|---|
| Non-invasive | Invasive—dye required, risk of anaphylaxis |
| Rapid | Time-consuming |
| Distinguishes retinal layers | Unable to distinguish retinal layers |
| 3D image | 2D image |
| Superior high image resolution | Lower image resolution |
| Quantitative data | Qualitative data |
| Artefacts may cause interpretation errors | Artefacts less common |
| Smaller field of view | Wider field of view |
| Able to detect flow but not leakage | Able to detect flow and leakage |
| No validated technique | Validated technique |
| Easier to conduct on patients with poor venous access | Difficult to conduct on patients with poor venous access |
| More suitable for imaging in children | Less suitable for imaging in children |
| OCTA Metric | Description |
|---|---|
| Foveal Avascular Zone (FAZ) area | Measurement of the FAZ size in mm2 |
| Foveal Avascular Zone (FAZ) circularity | Describes how circular FAZ appears at the fovea |
| Fractal Dimensions (FD) | Irregularity of blood vessel pattern |
| Intercapillary Spaces (IS) | Space between adjacent capillaries |
| Non Perfusion Area (NPA) | Area of absent blood flow |
| Perfusion Density (PD) | A similar concept of VD used by Zeiss |
| Vessel Density (VD) | Proportion of vessel area with blood flow |
| Vessel Skeleton Density (VSD) | Density of binarised vessel network |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijesingha, N.; Tsai, W.-S.; Keskin, A.M.; Holmes, C.; Kazantzis, D.; Chandak, S.; Kubravi, H.; Sivaprasad, S. Optical Coherence Tomography Angiography as a Diagnostic Tool for Diabetic Retinopathy. Diagnostics 2024, 14, 326. https://doi.org/10.3390/diagnostics14030326
Wijesingha N, Tsai W-S, Keskin AM, Holmes C, Kazantzis D, Chandak S, Kubravi H, Sivaprasad S. Optical Coherence Tomography Angiography as a Diagnostic Tool for Diabetic Retinopathy. Diagnostics. 2024; 14(3):326. https://doi.org/10.3390/diagnostics14030326
Chicago/Turabian StyleWijesingha, Naomi, Wei-Shan Tsai, Ayse Merve Keskin, Christopher Holmes, Dimitrios Kazantzis, Swati Chandak, Heena Kubravi, and Sobha Sivaprasad. 2024. "Optical Coherence Tomography Angiography as a Diagnostic Tool for Diabetic Retinopathy" Diagnostics 14, no. 3: 326. https://doi.org/10.3390/diagnostics14030326
APA StyleWijesingha, N., Tsai, W.-S., Keskin, A. M., Holmes, C., Kazantzis, D., Chandak, S., Kubravi, H., & Sivaprasad, S. (2024). Optical Coherence Tomography Angiography as a Diagnostic Tool for Diabetic Retinopathy. Diagnostics, 14(3), 326. https://doi.org/10.3390/diagnostics14030326






