Regression of Cardiac Rhabdomyomas Producing a Severe Aortic Stenosis: Case Report and Discussion of the Literature
Abstract
1. Introduction
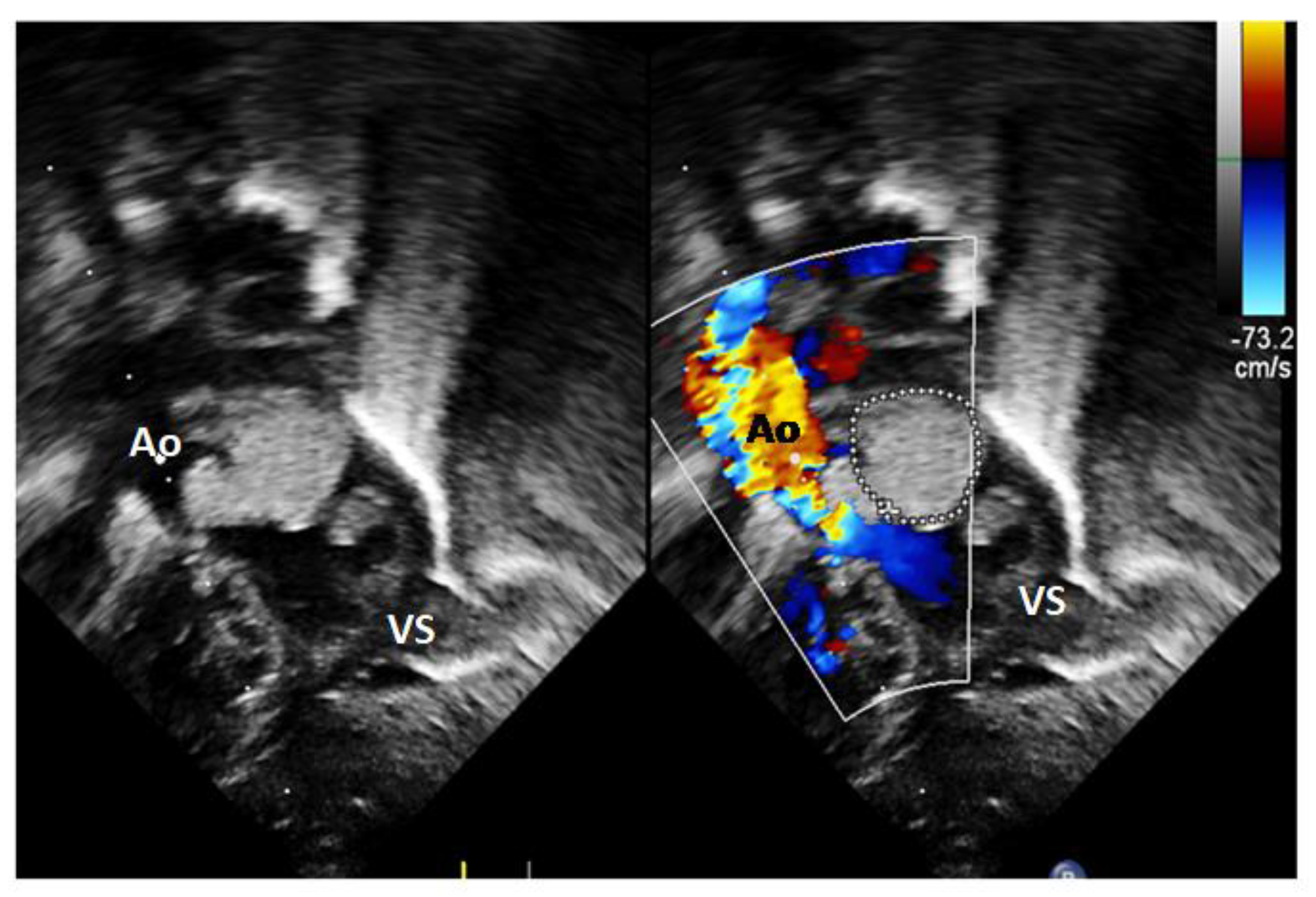
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holley, D.G.; Martin, G.R.; Brenner, J.I.; Fyfe, D.A.; Huhta, J.C.; Kleinman, C.S. Diagnosis and management of fetal cardiac tumors: A multicenter experience and review of published reports. J. Am. Coll. Cardiol. 1995, 26, 516–520. [Google Scholar] [CrossRef]
- Fesslova, V.; Villa, L.; Rizzuti, T.; Mastrangelo, M.; Mosca, F. Natural history and long-term outcome of cardiac rhabdomyomas detected prenatally. Prenat. Diagn. 2004, 24, 241–248. [Google Scholar] [CrossRef]
- Smythe, J.F.; Dyck, J.D.S.; Smallhorn, J.E.; Freedom, R.M. Natural history of cardiac rhabdomyoma in infancy and childhood. Am. J. Cardiol. 1990, 66, 1247–1249. [Google Scholar] [CrossRef]
- Black, M.D.; Kadletz, M.; Smallhorn, J.F.; Freedom, R.M. Cardiac rhabdomyomas and obstructive left heart disease: Histologically but not functionally benign. Ann. Thorac. Surg. 1998, 65, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Guereta, L.G.; Burgueros, M.; Elorza, M.D.; Alix, A.G.; Benito, F.; Gamallo, C. Cardiac rhabdomyoma presenting as fetal hydrops. Pediatr. Cardiol. 1986, 7, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.O.; Pagon, R.A. Incidence of tuberous sclerosis in patients with cardiac rhabdomyoma. Am. J. Med. Genet. 1990, 37, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, P.; Giacchi, V.; Mattia, C.; Greco, F.; Smilari, P.; Betta, P.; Distefano, G. Rhabdomyomas and tuberous sclerosis complex: Our experience in 33 cases. BMC Cardiovasc. Disord. 2014, 9, 14–66. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.B.; Prakash, A.; Romp, R.L.; Krueger, D.A.; Knilans, T.K. Cardiovascular manifestations of tuberous sclerosis complex and summary of the revised diagnostic criteria and surveillance and management recommendations from the international tuberous sclerosis consensus group. J. Am. Heart Assoc. 2014, 3, e001493. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Oliver, K.; Mueller, R.; Sampson, J. A cross sectional study of renal involvement in tuberous sclerosis. J. Med. Genet. 1996, 33, 480–484. [Google Scholar] [CrossRef]
- De Waele, L.; Lagae, L.; Mekahli, D. Tuberous sclerosis complex: The past and the future. Pediatr. Nephrol. 2015, 30, 1771–1780. [Google Scholar] [CrossRef]
- Nir, A.; Ekstein, S.; Nadjari, M.; Rass-Rotschild, A.; Rein, A.J.J.T. Rhabdomyoma in the fetus: Illustration of tumor growth during the second half of gestation. Pediatr. Cardiol. 2001, 22, 515–518. [Google Scholar] [CrossRef]
- Alkalay, A.L.; Ferry, D.A.; Lin, B.; Fink, B.W.; Pomerance, J.J. 1987.Spontaneous regression of rhabdomyoma in tuberous sclerosis. Clin. Pediatr. 1987, 25, 532–535. [Google Scholar] [CrossRef]
- Brand, J.M.; Frieberg, D.Z. Spontaneous regression of a primary cardiac tumor presenting as fetal tachyarrhythmias. J. Perinatol. 1992, 12, 48–50. [Google Scholar]
- Goyer, I.; Dahdah, N.; Major, P. Use of mTOR inhibitor everolimus in three neonates for treatment of tumors associated with tuberous sclerosis complex. Pediatr. Neurol. 2015, 52, 450–453. [Google Scholar] [CrossRef]
- Weiland, M.D.; Bonello, K.; Hill, K.D. Rapid regression of large cardiac rhabdomyomas in Neonates after sirolimus therapy. Cardiol. Young 2018, 28, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Abraham, S.; Ferdman, D. Rapid Regression of Prenatally Identified Intrapericardial Giant Rhabdomyomas with Sirolimus. CASE (Phila) 2018, 2, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Will, J.C.; Siedentopf, N.; Schmid, O.; Gruber, T.M.; Henrich, W.; Hertzberg, C.; Weschke, B. Successful prenatal treatment of cardiac rhabdomyoma in a Fetus with Tuberous Sclerosis. Pediatr. Rep. 2023, 15, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Vachon-Marceau, C.; Guerra, V.; Jaeggi, E.; Chau, V.; Ryan, G.; Van Mieghem, T. In-utero treatment of large symptomatic rhabdomyoma with sirolimus. Ultrasound Obstet. Gynecol. 2019, 53, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.S.; Chao, A.; Wang, T.H.; Chang, Y.C.; Chang, Y.L.; Hsieh, C.C. Outcome of antenatally diagnosed cardiac rhabdomyoma: Case series and a meta-analysis. Ultrasound Obstet. Gynecol. 2008, 31, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Yinon, Y.; Chitayat, D.; Blaser, S.; Seed, M.; Amsalem, H.; Yoo, S.J.; Jaeggi, E.T. Fetal cardiac tumors: A single-center experience of 40 cases. Prenat. Diagn. 2010, 30, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Sun, H.; Gu, X.; Hao, X.; Fu, Y.; Zhang, Y.; Liu, X.; Zhang, H.; Han, L.; et al. Fetal cardiac tumor: Echocardiography, clinical outcome and genetic analysis in 53 cases. Ultrasound Obstet. Gynecol. 2019, 54, 103–109. [Google Scholar] [CrossRef]
- Degueldre, S.C.; Chockalingam, P.; Mivelaz, Y.; Di Bernardo, S.; Pfammatter, J.P.; Barrea, C. Considerations for prenatal counselling of patients with cardiac rhabdomyomas based on their cardiac and neurologic outcomes. Cardiol. Young 2010, 20, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Kwiatkowski, D.J.; Roach, E.S.; Dobyns, W.B.; Lewis, R.A.; Herman, G.E.; Rodriguez, E., Jr.; Daiger, S.P.; Blanton, S.H. Evidence for genetic heterogenecity in tuberous sclerosis: One locus on chromosome 9 and at least one locus elsewhere. Am. J. Hum. Genet. 1992, 51, 709–720. [Google Scholar]
- Mas, C.; Penny, D.J.; Menahem, S. Pre-excitation syndrome secondary to cardiac rhabdomyomas in tuberous sclerosis. J. Pediatr. Child. Health 2000, 36, 84–86. [Google Scholar] [CrossRef]
- Satge, D.; De Geeter, B. Cardiac rhabdomyoma and apoptosis: Are regression controlled by the body? Arch. Mal. Coeur Vaiss. 1992, 85, 603–608. [Google Scholar]
- Wu, S.S.; Collins, M.H.; De Chadarévian, J.P. Study of the regression process in cardiac rhabdomyomas. Pediatr. Dev. Pathol. 2002, 5, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, Y.; Ishikura, H.; Takada, A.; Yokoyama, S.; Imamura, M.; Yoshiki, T.; Sato, H. Massive apoptosis in infantile myofibromatosis. A putative mechanism of tumor regression. Am. J. Pathol. 1994, 144, 480–485. [Google Scholar] [PubMed]
- Dorywalski, A.; Kraszewski, W.; Walczak, F. Natural history of congenital aortic valve stenosis. Kardiol. Pol. 1972, 15, 271–278. [Google Scholar]
- Kanwar, A.; Thaden, J.J.; Nkomo, V.T. Management of Patients with Aortic Valve Stenosis. Mayo Clin. Proc. 2018, 93, 488–508. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fesslova, V.M.E.; Evangelista, M.; Piazza, L.; Saracino, A.; Andronache, A.; Chiarello, C.; Varrica, A.; Giamberti, A.; Frigiola, A. Regression of Cardiac Rhabdomyomas Producing a Severe Aortic Stenosis: Case Report and Discussion of the Literature. Diagnostics 2024, 14, 470. https://doi.org/10.3390/diagnostics14050470
Fesslova VME, Evangelista M, Piazza L, Saracino A, Andronache A, Chiarello C, Varrica A, Giamberti A, Frigiola A. Regression of Cardiac Rhabdomyomas Producing a Severe Aortic Stenosis: Case Report and Discussion of the Literature. Diagnostics. 2024; 14(5):470. https://doi.org/10.3390/diagnostics14050470
Chicago/Turabian StyleFesslova, Vlasta M. E., Martina Evangelista, Luciane Piazza, Antonio Saracino, Andreea Andronache, Carmelina Chiarello, Alessandro Varrica, Alessandro Giamberti, and Alessandro Frigiola. 2024. "Regression of Cardiac Rhabdomyomas Producing a Severe Aortic Stenosis: Case Report and Discussion of the Literature" Diagnostics 14, no. 5: 470. https://doi.org/10.3390/diagnostics14050470
APA StyleFesslova, V. M. E., Evangelista, M., Piazza, L., Saracino, A., Andronache, A., Chiarello, C., Varrica, A., Giamberti, A., & Frigiola, A. (2024). Regression of Cardiac Rhabdomyomas Producing a Severe Aortic Stenosis: Case Report and Discussion of the Literature. Diagnostics, 14(5), 470. https://doi.org/10.3390/diagnostics14050470





