T Cell Immunity in Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma—A Systematic Review
Abstract
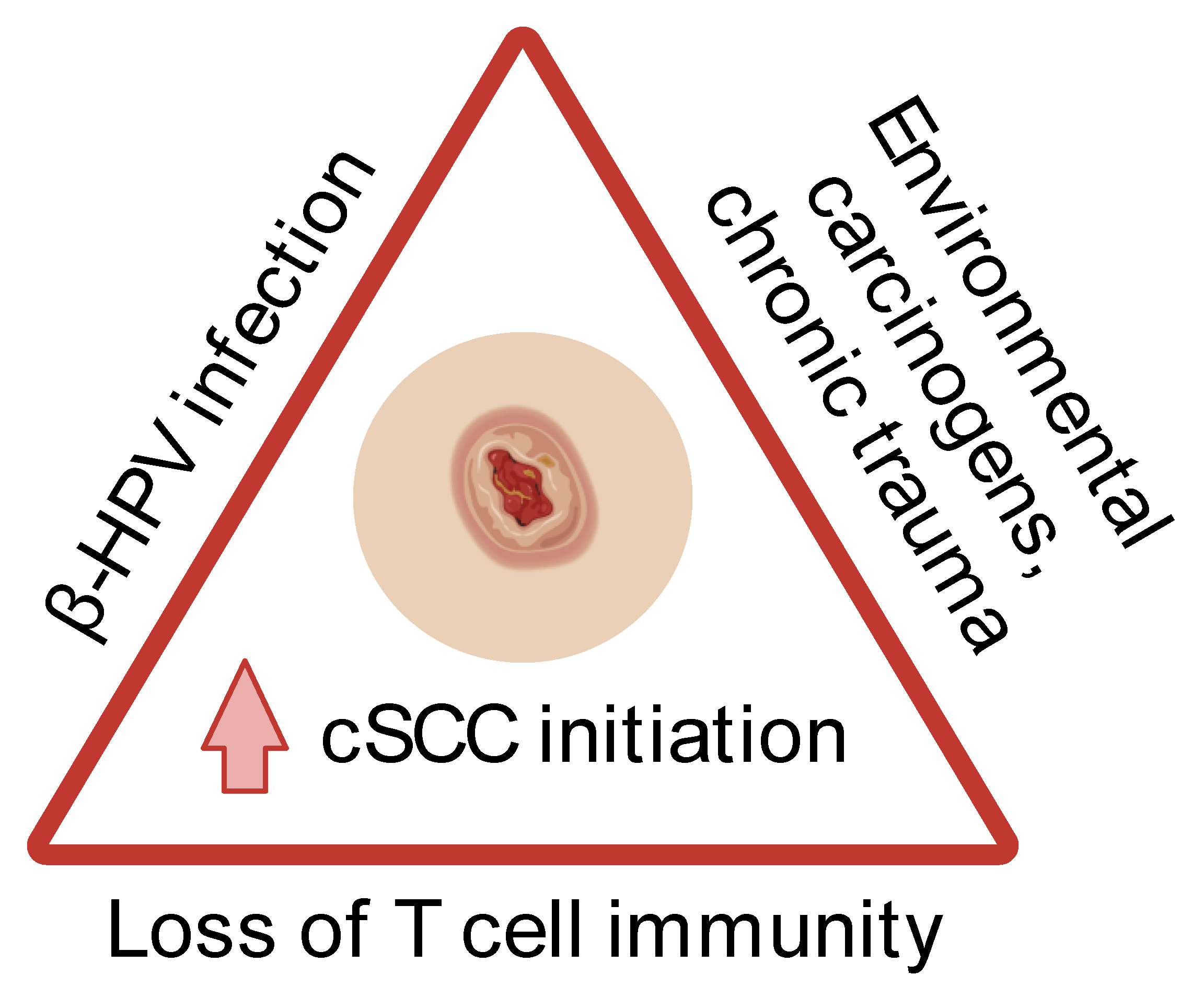
1. Introduction
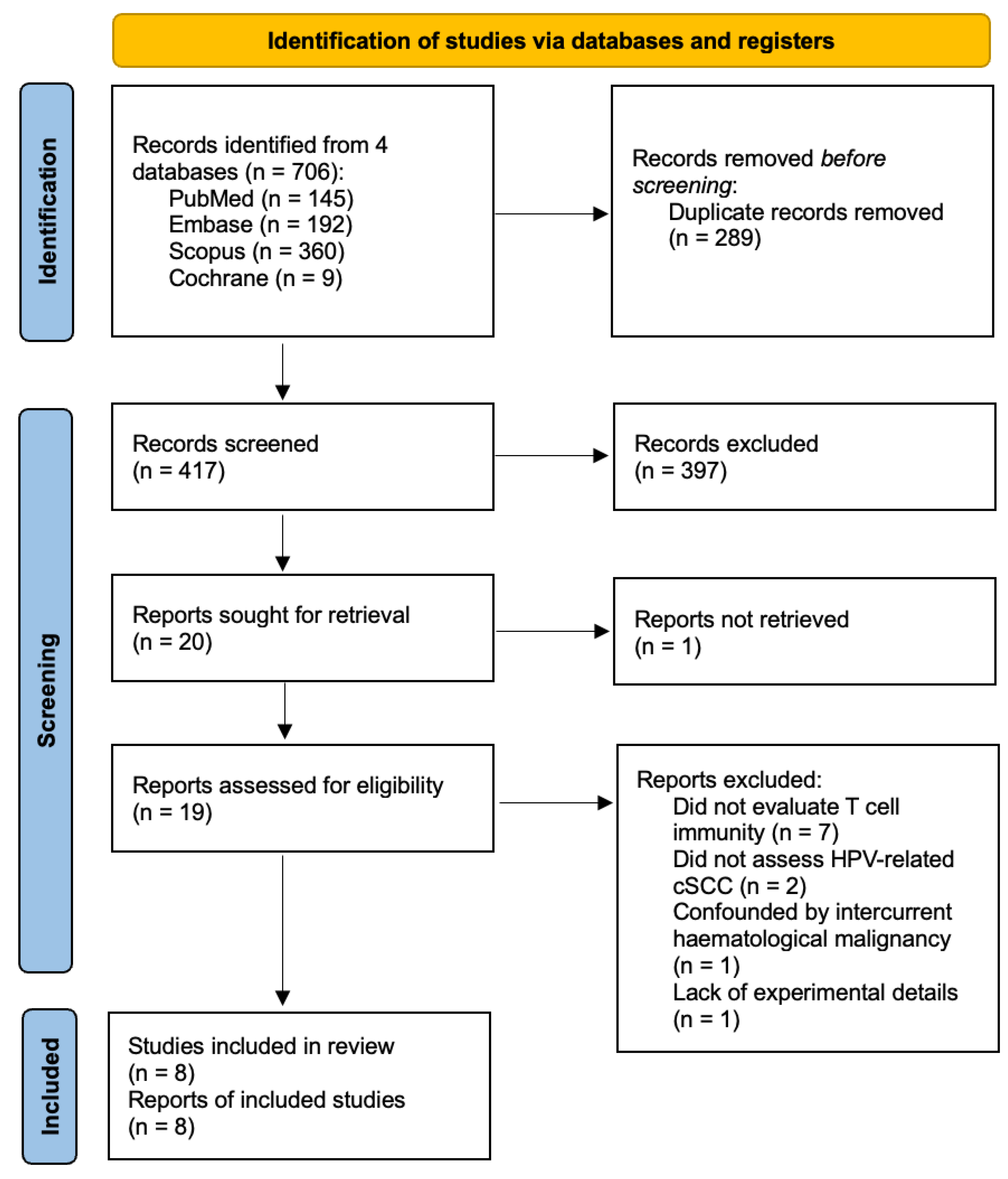
2. Materials and Methods
3. Results
3.1. T Cell Immunity in HPV-Related cSCC Carcinogenesis
3.1.1. T Cell Immunity in α-HPV-Related Epithelial Carcinogenesis
3.1.2. T Cell Immunity in β-HPV-Related Epithelial Carcinogenesis
| Author and Year | Study Population | Key T Cell Perturbations |
|---|---|---|
| Borgogna et al. (2023) [19] | cSCC mouse models
|
|
| Antsiferova et al. (2017) [20] | cSCC mouse models
|
|
3.2. T Cell Immunity in Potential Vaccination Strategies against β-HPV-Related cSCC Carcinogenesis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Gloster, H.M.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–760; quiz 761–764. [Google Scholar] [CrossRef] [PubMed]
- Tokez, S.; Hollestein, L.; Louwman, M.; Nijsten, T.; Wakkee, M. Incidence of Multiple vs First Cutaneous Squamous Cell Carcinoma on a Nationwide Scale and Estimation of Future Incidences of Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2020, 156, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.C.; Jin, A.; Koh, W.P. Trends of cutaneous basal cell carcinoma, squamous cell carcinoma, and melanoma among the Chinese, Malays, and Indians in Singapore from 1968–2016. JAAD Int. 2021, 4, 39–45. [Google Scholar] [CrossRef]
- Chahoud, J.; Semaan, A.; Chen, Y.; Cao, M.; Rieber, A.G.; Rady, P.; Tyring, S.K. Association between β-Genus Human Papillomavirus and Cutaneous Squamous Cell Carcinoma in Immunocompetent Individuals-A Meta-analysis. JAMA Dermatol. 2016, 152, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef] [PubMed]
- Howley, P.M.; Pfister, H.J. Beta genus papillomaviruses and skin cancer. Virology 2015, 479–480, 290–296. [Google Scholar] [CrossRef]
- Tommasino, M. The biology of beta human papillomaviruses. Virus Res. 2017, 231, 128–138. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Garrett, G.L.; Blanc, P.D.; Boscardin, J.; Lloyd, A.A.; Ahmed, R.L.; Anthony, T.; Bibee, K.; Breithaupt, A.; Cannon, J.; Chen, A.; et al. Incidence of and Risk Factors for Skin Cancer in Organ Transplant Recipients in the United States. JAMA Dermatol. 2017, 153, 296–303. [Google Scholar] [CrossRef]
- Lanz, J.; Bouwes Bavinck, J.N.; Westhuis, M.; Quint, K.D.; Harwood, C.A.; Nasir, S.; Van-de-Velde, V.; Proby, C.M.; Ferrándiz, C.; Genders, R.E.; et al. Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol. 2019, 155, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Urso, B.; Kelsey, A.; Bordelon, J.; Sheiner, P.; Finch, J.; Cohen, J.L. Risk factors and prevention strategies for cutaneous squamous cell carcinoma in transplant recipients. Int. J. Dermatol. 2022, 61, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Zavdy, O.; Coreanu, T.; Bar-On, D.Y.; Ritter, A.; Bachar, G.; Shpitzer, T.; Kurman, N.; Mansour, M.; Ad-El, D.; Rozovski, U.; et al. Cutaneous Squamous Cell Carcinoma in Immunocompromised Patients-A Comparison between Different Immunomodulating Conditions. Cancers 2023, 15, 1764. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Korets, L.V.; Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005, 7, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Arbeit, J.M.; Münger, K.; Howley, P.M.; Hanahan, D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J. Virol. 1994, 68, 4358–4368. [Google Scholar] [CrossRef] [PubMed]
- Mombaerts, P.; Iacomini, J.; Johnson, R.S.; Herrup, K.; Tonegawa, S.; Papaioannou, V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992, 68, 869–877. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Rollison, D.E.; Amorrortu, R.P.; Zhao, Y.; Messina, J.L.; Schell, M.J.; Fenske, N.A.; Cherpelis, B.S.; Giuliano, A.R.; Sondak, V.K.; Pawlita, M.; et al. Cutaneous Human Papillomaviruses and the Risk of Keratinocyte Carcinomas. Cancer Res. 2021, 81, 4628–4638. [Google Scholar] [CrossRef]
- Borgogna, C.; Martuscelli, L.; Olivero, C.; Lo Cigno, I.; De Andrea, M.; Caneparo, V.; Boldorini, R.; Patel, G.; Gariglio, M. Enhanced Spontaneous Skin Tumorigenesis and Aberrant Inflammatory Response to UVB Exposure in Immunosuppressed Human Papillomavirus Type 8-Transgenic Mice. J. Investig. Dermatol. 2023, 143, 740–750.e744. [Google Scholar] [CrossRef]
- Antsiferova, M.; Piwko-Czuchra, A.; Cangkrama, M.; Wietecha, M.; Sahin, D.; Birkner, K.; Amann, V.C.; Levesque, M.; Hohl, D.; Dummer, R.; et al. Activin promotes skin carcinogenesis by attraction and reprogramming of macrophages. EMBO Mol. Med. 2017, 9, 27–45. [Google Scholar] [CrossRef]
- Schaper, I.D.; Marcuzzi, G.P.; Weissenborn, S.J.; Kasper, H.U.; Dries, V.; Smyth, N.; Fuchs, P.; Pfister, H. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005, 65, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Namwanje, M.; Brown, C.W. Activins and Inhibins: Roles in Development, Physiology, and Disease. Cold Spring Harb. Perspect. Biol. 2016, 8, a021881. [Google Scholar] [CrossRef] [PubMed]
- Antsiferova, M.; Huber, M.; Meyer, M.; Piwko-Czuchra, A.; Ramadan, T.; MacLeod, A.S.; Havran, W.L.; Dummer, R.; Hohl, D.; Werner, S. Activin enhances skin tumourigenesis and malignant progression by inducing a pro-tumourigenic immune cell response. Nat. Commun. 2011, 2, 576. [Google Scholar] [CrossRef] [PubMed]
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Li, T.; Hasegawa, T.; Ha, D.T.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 575, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.H.; Son, H.G.; Ha, D.T.; Strickley, J.D.; Joh, J.; Demehri, S. Compromised T Cell Immunity Links Increased Cutaneous Papillomavirus Activity to Squamous Cell Carcinoma Risk. JID Innov. 2022, 3, 100163. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, S.; Strasser, K.; Schröckenfuchs, G.; Bonelli, M.; Bauer, W.; Kittler, H.; Cataisson, C.; Fischer, M.B.; Lichtenberger, B.M.; Handisurya, A. Mus musculus papillomavirus 1 is a key driver of skin cancer development upon immunosuppression. Am. J. Transplant. 2020, 21, 525–539. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. Mus musculus Papillomavirus 1: A New Frontier in Animal Models of Papillomavirus Pathogenesis. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Marcuzzi, G.P.; Awerkiew, S.; Hufbauer, M.; Schädlich, L.; Gissmann, L.; Eming, S.; Pfister, H. Tumor prevention in HPV8 transgenic mice by HPV8-E6 DNA vaccination. Med. Microbiol. Immunol. 2014, 203, 155–163. [Google Scholar] [CrossRef]
- Hufbauer, M.; Rattay, S.; Hagen, C.; Quaas, A.; Pfister, H.; Hartmann, G.; Coch, C.; Akgül, B. Poly(I:C) Treatment Prevents Skin Tumor Formation in the Preclinical HPV8 Transgenic Mouse Model. J. Investig. Dermatol. 2022, 143, 1197–1207.e1193. [Google Scholar] [CrossRef]
- Marcuzzi, G.P.; Hufbauer, M.; Kasper, H.U.; Weißenborn, S.J.; Smola, S.; Pfister, H. Spontaneous tumour development in human papillomavirus type 8 E6 transgenic mice and rapid induction by UV-light exposure and wounding. J. Gen. Virol. 2009, 90, 2855–2864. [Google Scholar] [CrossRef]
- Pfefferle, R.; Marcuzzi, G.P.; Akgül, B.; Kasper, H.U.; Schulze, F.; Haase, I.; Wickenhauser, C.; Pfister, H. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J. Investig. Dermatol. 2008, 128, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Xu, F. Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol. Ther. 2010, 10, 1219–1223. [Google Scholar] [CrossRef]
- Landini, M.M.; Borgogna, C.; Peretti, A.; Colombo, E.; Zavattaro, E.; Boldorini, R.; Miglio, U.; Doorbar, J.; Ravanini, P.; Kumar, R.; et al. α- and β-Papillomavirus infection in a young patient with an unclassified primary T-cell immunodeficiency and multiple mucosal and cutaneous lesions. J. Am. Acad. Dermatol. 2014, 71, 108–115.e101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saluzzo, S.; Pandey, R.V.; Gail, L.M.; Dingelmaier-Hovorka, R.; Kleissl, L.; Shaw, L.; Reininger, B.; Atzmüller, D.; Strobl, J.; Touzeau-Römer, V.; et al. Delayed antiretroviral therapy in HIV-infected individuals leads to irreversible depletion of skin- and mucosa-resident memory T cells. Immunity 2021, 54, 2842–2858.e2845. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Saeidian, A.H.; Youssefian, L.; Saffarian, Z.; Casanova, J.L.; Béziat, V.; Jouanguy, E.; Vahidnezhad, H. Recalcitrant Warts, Epidermodysplasia Verruciformis, and the Tree-Man Syndrome: Phenotypic Spectrum of Cutaneous Human Papillomavirus Infections at the Intersection of Genetic Variability of Viral and Human Genomes. J. Investig. Dermatol. 2022, 142, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. Model systems of human papillomavirus-associated disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Thomson, J.; Harwood, C.; Leigh, I. The Role of the Immune System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2009. [Google Scholar] [CrossRef]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Nelemans, P.J.; Kouloubis, N.; Arits, A.H.M.M.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Essers, B.A.B.; Steijlen, P.M.; Kelleners-Smeets, N.W.J.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef]
- Borella, F.; Gallio, N.; Mangherini, L.; Cassoni, P.; Bertero, L.; Benedetto, C.; Preti, M. Recent advances in treating female genital human papillomavirus related neoplasms with topical imiquimod. J. Med. Virol. 2023, 95, e29238. [Google Scholar] [CrossRef]
- Lewis, S.M.; Asselin-Labat, M.L.; Nguyen, Q.; Berthelet, J.; Tan, X.; Wimmer, V.C.; Merino, D.; Rogers, K.L.; Naik, S.H. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 2021, 18, 997–1012. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Budiarto, B.R.; Wang, Y.F.; Lin, C.Y.; Gwo, M.C.; So, D.K.; Tzeng, Y.S.; Chen, S.Y. Spatial multi-omics analyses of the tumor immune microenvironment. J. Biomed. Sci. 2022, 29, 96. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Study Population | Key T Cell Perturbations |
|---|---|---|
| De Visser et al. (2005) [14] | cSCC mouse models
|
|
| Author and Year | Study Population | Key T Cell Perturbations |
|---|---|---|
| Strickley et al. (2019) [24] | cSCC mouse models
|
|
| Johnson et al. (2022) [25] | cSCC mouse model
|
|
| Dorfer et al. (2020) [26] | cSCC mouse models
|
|
| Author and Year | Study Population | Vaccination Strategy | Key T Cell Perturbations |
|---|---|---|---|
| Marcuzzi et al. (2014) [28] | cSCC mouse model
| HPV8 E6 DNA tattooing onto skin |
|
| Hufbauer et al. (2022) [29] | cSCC mouse model
| Poly(I:C) tattooing onto skin |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, S.H.; Oh, C.C. T Cell Immunity in Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma—A Systematic Review. Diagnostics 2024, 14, 473. https://doi.org/10.3390/diagnostics14050473
Tay SH, Oh CC. T Cell Immunity in Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma—A Systematic Review. Diagnostics. 2024; 14(5):473. https://doi.org/10.3390/diagnostics14050473
Chicago/Turabian StyleTay, Shi Huan, and Choon Chiat Oh. 2024. "T Cell Immunity in Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma—A Systematic Review" Diagnostics 14, no. 5: 473. https://doi.org/10.3390/diagnostics14050473
APA StyleTay, S. H., & Oh, C. C. (2024). T Cell Immunity in Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma—A Systematic Review. Diagnostics, 14(5), 473. https://doi.org/10.3390/diagnostics14050473





