Abstract
Lung cancer is often triggered by genetic alterations that result in the expression of oncogenic tyrosine kinases. Specifically, ALK, RET, and ROS1 chimeric receptor tyrosine kinases are observed in approximately 5–7%, 1–2%, and 1–2% of NSCLC patients, respectively. The presence of these fusion genes determines the response to tyrosine kinase inhibitors. Thus, accurate detection of these gene fusions is essential in cancer research and precision oncology. To address this need, we have developed a multiplexed RT-qPCR assay using xeno nucleic acid (XNA) molecular clamping technology to detect lung cancer fusions. This assay can quantitatively detect thirteen ALK, seven ROS1, and seven RET gene fusions in FFPE samples. The sensitivity of the assay was established at a limit of detection of 50 copies of the synthetic template. Our assay has successfully identified all fusion transcripts using 50 ng of RNA from both reference FFPE samples and cell lines. After validation, a total of 77 lung cancer patient FFPE samples were tested, demonstrating the effectiveness of the XNA-based fusion gene assay with clinical samples. Importantly, this assay is adaptable to highly degraded RNA samples with low input amounts. Future steps involve expanding the testing to include a broader range of clinical samples as well as cell-free RNAs to further validate its applicability and reliability.
1. Introduction
Lung cancer is one of the most common types of cancer and stands as the leading cause of tumor-related fatalities, accounting for nearly a quarter (approximately 25%) of such cases [1]. According to estimates, there were 117,560 new lung cancer cases in men and 120,790 in women in 2023. The estimated deaths due to lung cancer were 67,160 in men and 59,910 in women in 2023 [2]. Over 80% of lung tumors are classified histologically as either adenocarcinomas, squamous cell carcinomas, or large cell carcinomas, collectively categorized as non-small-cell lung cancers (NSCLCs). Various genetic alterations have been identified as drivers of oncogenic processes in NSCLC. These alterations encompass point mutations, deletions, insertions, and gene fusions. In Western nations, approximately 53% of NSCLC cases exhibit mutations in three genes: the Kirsten rat sarcoma virus (KRAS), epidermal growth factor receptor (EGFR), or B-Raf Proto-Oncogene, Serine/Threonine Kinase (BRAF). In addition, driver gene fusions and splicing variants are found in 10–15% of patients [3,4]. Among the prevalent gene fusions observed in NSCLC, three genes encode membrane receptors, namely, anaplastic lymphoma receptor tyrosine kinase (ALK), the ret proto-oncogene (RET), and the ROS proto-oncogene 1, receptor tyrosine kinase (ROS1). ALK fusion has a frequency of 5–7%, while RET and ROS1 fusions have a similar frequency of 1–2% [5]. The fusion of the echinoderm microtubule-associated protein-like 4 (EML4) and the kinesin family member 5B (KIF5B) are significant fusion partners of the ALK gene. These fusions are important biomarkers for predicting responses to tyrosine kinase inhibitors (TKIs). As per the National Comprehensive Cancer Network (NCCN) guidelines, patients with ALK-fusion-positive tumors have shown positive responses to ALK TKIs. Alectinib has demonstrated superior efficacy with respect to crizotinib as an initial treatment option for these patients [6]. RET rearrangement was first identified in NSCLC in 2012 using a next-generation sequencing assay [7]. The kinase domain of the RET gene combines with the N-terminal region of various gene partners to form fusions. This fusion leads to the ligand-independent and continuous activation of RET, which stimulates cell proliferation and enhances cell survival [8]. KIF5B is the most prevalent partner in 70–80% of cases, followed by CCDC6 and NCOA4 [9]. The FDA has approved two TKIs that target RET specifically, namely, selpercatinib and pralsetinib, for the treatment of advanced RET-positive NSCLC [10]. The ROS1 gene was first discovered in the 1980s, and its role as a proto-oncogene was identified in brain tumors in 2003 [11]. In lung cancer, two ROS1 fusion transcripts, namely, SLC34A2-ROS1 and CD74-ROS1, were initially identified as proto-oncogenes [12]. The rearrangement of ROS1 primarily occurs in exons 32, 34, 35, or 36, as well as in introns 31 or 33 [13,14]. Among the fusion partners, CD74 is the most prevalent (accounting for 38–54% of cases), followed by EZR (13–24%), SDC4 (9–13%), SLC34A2 (5–10%), and GOPC (2–3%) [14,15,16]. The test for ROS1 rearrangement is currently recommended for all cases of metastatic lung carcinomas. The FDA has approved two tyrosine kinase inhibitors, crizotinib and entrectinib, as initial treatment options.
Numerous rare fusions involving receptor tyrosine kinase (RTK) have been detected in lung cancer, including fibroblast growth factor receptor (FGFR), neuregulin 1 (NRG1), and MET Proto-Oncogene, Receptor Tyrosine Kinase (MET). The FGFR family comprises four members: FGFR1, FGFR2, FGFR3, and FGFR4 [17]. Although FGFR fusions are infrequent, they have been observed in lung cancer, with the most prevalent being FGFR3-TACC3 [18]. Drugs targeting FGFRs are currently being developed, such as erdafitinib and ARQ-087, which have shown limited responses with manageable toxicity [19,20]. NRG1, a ligand of the Human Epidermal Growth Factor Receptor (HER) family, stimulates the activation of RTKs. NRG1 fusion is very rare, and drug development is supported by small-cohort studies and case reports [21,22]. MET fusion (TPR-MET) was initially discovered in an osteogenic sarcoma cell line, with an incidence of approximately 0.5% in lung cancer. Despite the identification of numerous fusion partners, the biological and therapeutic implications remain to be evaluated [23].
Various diagnostic techniques can be used to identify gene fusions, including immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), reverse transcription quantitative polymerase chain reaction (RT-qPCR), and next-generation sequencing (NGS). FISH has traditionally been considered the gold standard for detecting RTK fusions induced by chromosomal translocations and intrachromosomal rearrangements [24]. RTK gene fusions often lead to elevated mRNA and protein levels, making RT-qPCR and IHC effective alternatives [25]. A positive ALK IHC result has been utilized to prescribe ALK inhibitors [24]. FISH requires no sophisticated equipment and is valuable as a validation tool after positive IHC or NGS results [26]. Despite being considered as a benchmark, FISH is associated with higher costs, technical challenges related to limited tumor cell availability, and high operator variability [27,28]. Additionally, it examines only one alteration per test, necessitating more material for comprehensive analyses, and has limitations in detecting small intrachromosomal rearrangements due to its analytical resolution of 100–200 kb, limiting sensitivity [29]. IHC, on the other hand, is more cost-effective than FISH and is useful for preselecting tumors for confirmatory FISH testing, offering excellent sensitivity with specific antibodies. However, it does not identify the fusion partner and relies on antibody specificity [30]. With the growing demand for comprehensive genomic assessment, NGS-based fusion detection is becoming a preferred approach. NGS provides extensive genetic information, including fusion breakpoints at single-nucleotide resolution and the detection of unknown fusion partners. Despite its numerous advantages, NGS also has many limitations, such as restricted access to panels, dependency on sample quality and quantity, the need for bioinformatic support increasing overall costs, and the requirement for complex and expensive equipment [31]. RT-qPCR-based methods offer high specificity, providing robust and detailed information. Using ROS1 fusion-specific primers, RT-qPCR demonstrates outstanding performance with 100% sensitivity and 85.1% specificity for ROS1 fusion detection [32]. However, its results are contingent on RNA quality, and primers must be designed based on known fusion partners, as it is unable to detect unknown partners.
Initially, identifying and characterizing gene fusions in clinical biopsies focused on single alterations using the polymerase chain reaction (PCR). However, the continuously expanding array of targetable fusions prompted the advancement of multiplex techniques. These methods enable the investigation of multiple alterations in a single assay.
Xeno nucleic acid (XNA) is a synthetic DNA analog, originally developed to store genetic information and evolve in response to external stimuli [33]. XNAs have proven to be highly effective in binding to specific regular DNA sequences, making them useful as molecular clamps in quantitative real-time PCR or as precise molecular probes for identifying specific nucleic acid sequences [34]. The attachment of XNA to its designated sequence blocks the extension of the DNA strand by DNA polymerase. In cases where there is a mismatch in the target site sequence, the XNA–DNA duplex lacks stability, allowing the extension of the strand by DNA polymerase [35,36].
In this study, we present the development and verification of a novel multiplex RT-qPCR assay that uses XNA. The assay is designed to identify gene fusions that are frequently observed in lung cancer cases in a simultaneous and qualitative manner. The fusion biomarker assay can detect fusion events in ALK, RET, and ROS1 target genes.
2. Materials and Methods
2.1. Clinical Samples and Cell Lines
Twenty preserved samples in formalin-fixed paraffin-embedded (FFPE) format were provided by the lung External Quality Assessment (EQA) program conducted by the European Society of Pathology (ESP). Details about the EQA scheme can be found at http://lung.eqascheme.org/info/public/alk/index.xhtml (accessed on 19 February 2024). The ESP schemes followed the required guidelines for EQA programs in the field of molecular pathology [37]. The clinical information was not provided by the organization. Fifty-seven de-identified FFPE samples from lung cancer patients were obtained from Amsbio (Cambridge, MA, USA). Approval for ethical considerations was granted by the institutional review board (IRB) at WIRB-Copernicus Group, Inc., and sample collection was conducted with written informed consent. Specimen details are listed in Supplementary Table S1. Fusion-positive and -negative FFPE reference materials were obtained from Horizon Discovery (Cambridge, UK). RNAs were extracted from FFPE sections using the RNeasy FFPE kit (QAIGEN, Hilden, Germany) according to the manufacturer’s instructions. Two lung cancer cell lines, A549 (a fusion-negative cell line) and H2228 (an EML4-ALK V3a/b-positive cell line) were purchased from the American Type Culture Collection (ATCC). A CCDC6-RET-positive cell line, LC-2/ad, was purchased from Millipore Sigma (Burlington, MA, USA). An SLC34A2-ROS1-positive cell line, HCC78, was purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ). Total RNA was isolated from 1 million cells using a Direct-zol RNA miniprep kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. Total lung tissue RNA was purchased from Biochain (Newark, CA, USA). RNAs were quantified using the Qubit RNA BR assay kit (Invitrogen, Waltham, MA, USA).
2.2. Detection of ALK, RET, or ROS1 Fusion Using RT-qPCR
ALK, RET, or ROS1 fusion was detected using the QfusionTM ALK fusion detection kit, the QfusionTM RET fusion detection kit, and the QfusionTM ROS1 fusion detection kit (DiaCarta Inc., Pleasanton, CA, USA), which can simultaneously detect thirteen ALK fusions, seven RET fusions, and seven ROS1 fusions, respectively (Supplementary Figure S1). Briefly, 50 ng of RNAs was subjected to RT-qPCR using Bio-Rad CFX384 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The thermal cycling condition was 10 min at 50 °C for reverse transcription (RT) and 2 min at 95 °C for RT inactivation, followed by 45 cycles of 5 s at 95 °C for denaturation, 40 s at 70 °C for XNA binding, 30 s at 64 °C for primer binding, and elongation with a slow ramp rate (3 °C per second). The experimental workflow is shown in Supplementary Figure S2.
2.3. Determination of Analytical Sensitivity
The analytical sensitivity was assessed using synthetic double-stranded DNAs (Supplementary Table S2), which were purchased from Integrated DNA Technologies (Coralville, IA, USA) as gBlocks gene fragments. Three distinct copy numbers (500, 100, and 50 copies) of gBlocks were used as inputs, and the RT-qPCR assay was conducted with 18 replicates.
To determine the limit of detection of RNA fusion targets in cell lines, 50 ng of total RNA was used as input. The total RNA from each cell line underwent serial dilution with normal lung tissue RNA (Biochain) and then was checked using the QfusionTM ALK fusion detection kit, the QfusionTM RET fusion detection kit, or the QfusionTM ROS1 fusion detection kit (DiaCarta Inc.). Similarly, for RNA fusion targets in the FFPE RNA reference standard (Horizon Discovery), RNAs were diluted with normal lung tissue FFPE RNA (Horizon Discovery) and tested using the QfusionTM assays.
2.4. Detection of ALK, RET, or ROS1 Fusion Using Next-Generation Sequencing (NGS)
Lung cancer-specific fusions were analyzed using an OptiSeqTM lung cancer fusion NGS panel (DiaCarta Inc., Pleasanton, CA, USA). Fifty nanograms of RNAs was used as an input for library preparation. The libraries were sequenced on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) with read lengths of 150 bases, generating approximately 0.1 million paired end reads per library. The obtained results were analyzed using the QIAGEN CLC Genomics Workbench version 20.0.4 (QIAGEN, Aarhus, Denmark) for fusion detection. A fusion event was deemed confirmed if a minimum of 10 reads were identified as crossing the fusion junction.
2.5. Preparation of Fusion and Wild-Type Transcripts by In Vitro Transcription
The EML4-ALK V1 and wild-type ALK transcripts were synthesized using the Megascript T7 transcription kit (Thermo Fisher Scientific, Carlsbad, CA, USA) following the manufacturer’s guidelines. The RNAs were then purified using the RNA Clean & Concentrator-5 kit (Zymo Research) and quantified using the Qubit RNA BR assay kit (Invitrogen), following the manufacturer’s guidelines.
3. Results
3.1. Analytical Sensitivity, Limit of Detection (LoD)
We conducted 18 replicate reactions to check the analytical sensitivity of each QfusionTM assay designed for detecting ALK, RET, or ROS1 fusions. We used 500, 100, and 50 copies of synthetic double-stranded fusion templates for the test (Supplementary Table S2). The analytical sensitivity was expressed as the number of copies detected per reaction. The results demonstrated a 100% detection rate when testing with 500, 100, and 50 copies per reaction for most templates. However, there was a slightly lower detection rate of 89% for the EML4-ALK V6 template and 83% for the GOPC-ROS1 G8;R35 template (Table 1). Therefore, the limit of detection for all three detection assays was established at 50 copies of the target per reaction.

Table 1.
(a) Analytical sensitivity of the QfusionTM ALK fusion detection assay. (b) Analytical sensitivity of the QfusionTM RET fusion detection assay. (c) Analytical sensitivity of the QfusionTM ROS1 fusion detection assay.
3.2. Enhancement of QfusionTM Assay Specificity and Sensitivity with XNA
Off-target amplification is a common issue in multiplex PCR-based assays, especially when there is a significant background of wild-type sequences [38]. This often leads to false positives when detecting mutations or fusions due to the promiscuous binding of primers and probes to sequences that closely resemble the target [35].
To address the issue of non-specific amplification in the presence of abundant wild-type backgrounds, we utilized XNA clamp probes designed to hinder the amplification of wild-type transcripts. XNA forms stable complexes with its targets, while mismatches in fusion partners result in weak complexes, allowing the amplification of fusion targets (Supplementary Figure S3). Given the characteristics of XNA, we hypothesized that its application could enhance the specificity and sensitivity of the QfusionTM assays.
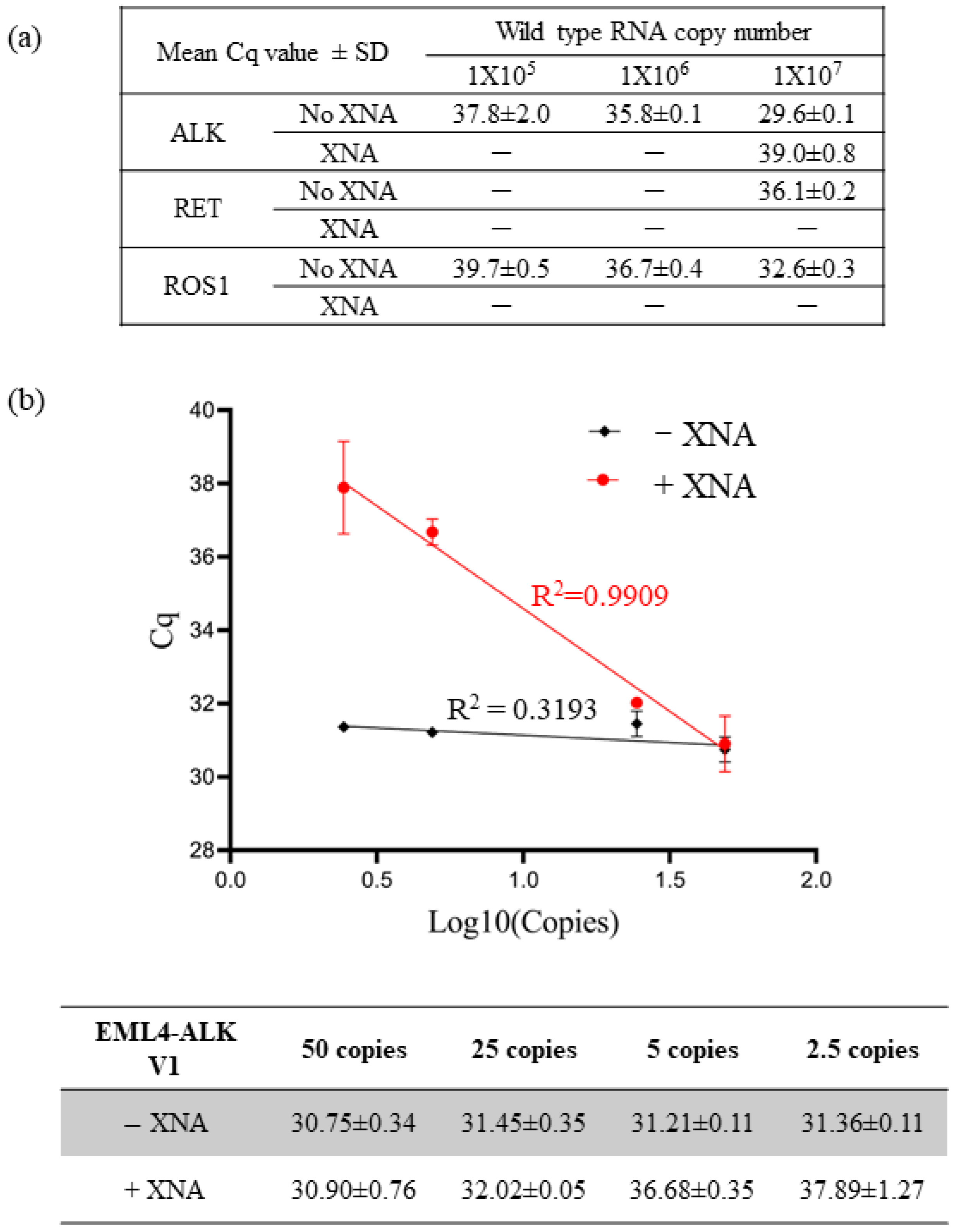
First, to evaluate the impact of XNA on specificity, we tested the detection efficiency of each QfusionTM assay by using 10-fold serial dilutions of ALK, RET, or ROS1 wild-type RNA transcripts in the presence or absence of XNA. As shown in Figure 1a, each assay exhibited non-specific detection of wild-type RNA transcripts. However, the presence of XNA efficiently reduced the detection of wild-type RNAs. This suggests that XNA enhances the assay’s specificity, reducing the occurrence of false positives.
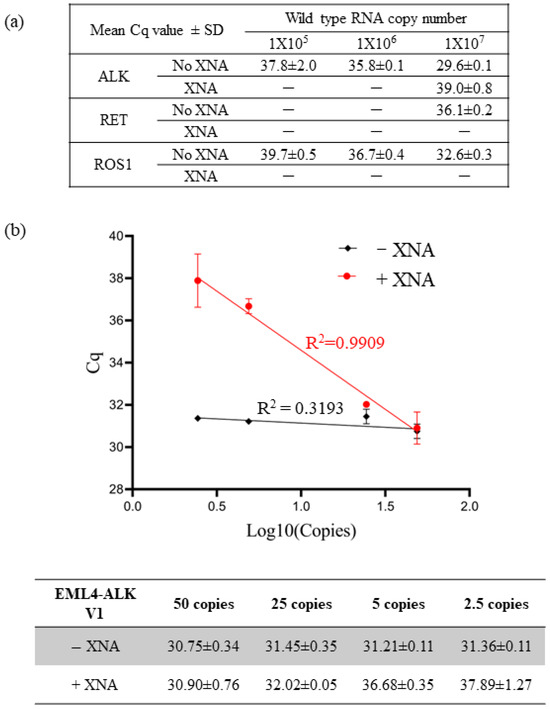
Figure 1.
Enhancing assay specificity and sensitivity with XNA. (a) XNA enhanced specificity by preventing non-specific amplification of abundant wild-type sequences. (b) XNA improved assay sensitivity by inhibiting the wild-type backgrounds.
We conducted multiple measurements using varying quantities of EML4-ALK V1 synthetic DNA fragments to evaluate the impact of XNA on assay sensitivity. We performed the tests in the presence and absence of 10,000,000 copies of wild-type ALK DNA fragments. Since the presence of abundant wild types could lead to non-specific amplification in the reaction (Figure 1a), we hypothesized that XNA would enhance the detection limit of the fusion target by preventing non-specific amplification of wild types. Furthermore, we postulated that XNA would not affect the detection of fusion transcripts in the absence of wild-type ALK.
As anticipated, our results affirmed that XNA had no significant impact on the detection of EML4-ALK V1 fusion (Supplementary Table S3). In the absence of XNA, the quantification cycle (Cq) value remained unchanged for different copy numbers of fusion targets due to preferential amplification of abundant wild types, masking the amplification of fusion targets (Figure 1b). Therefore, the assay was unable to distinguish between 50 copies and 2.5 copies, resulting in a 50-copy LoD. However, XNA effectively prevented the amplification of wild types, enabling the detection of low copy numbers of fusion targets in a linear, dose-dependent manner ranging from 2.5 to 50 copies. This implies that the LoD was enhanced to the 2.5-copy level, demonstrating increased sensitivity.
In summary, XNA inhibited the amplification of wild types, increasing the specificity and sensitivity of the QfusionTM assay.
3.3. Evaluation of the QfusionTM Assays’ Performance with Cell Lines and FFPE RNA Reference Standards
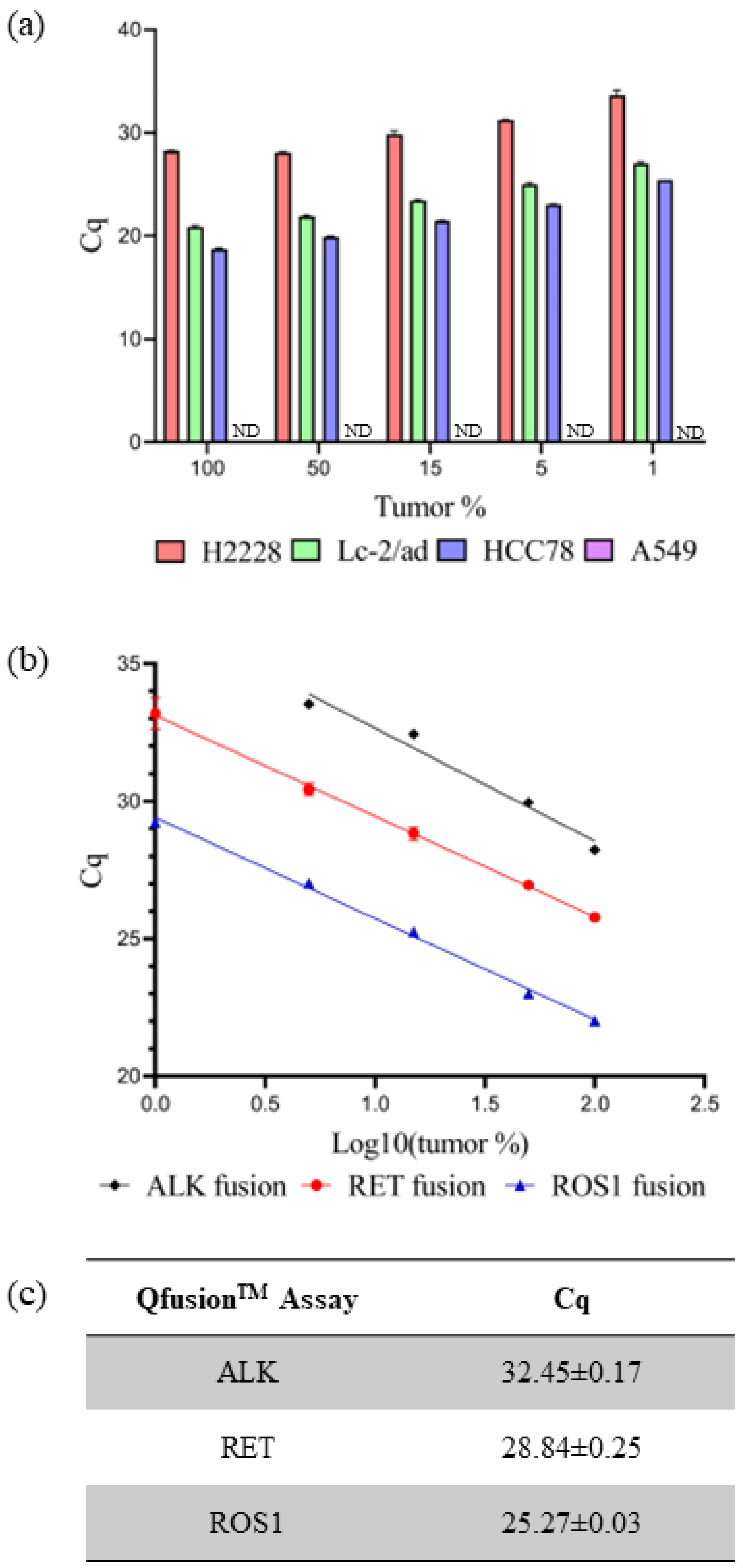
The sensitivity and specificity of individual QfusionTM assays were subsequently assessed using fusion-negative and -positive cancer cell lines. RNA extraction was performed on four cell lines: A549 (fusion-negative), H2228 (EML4-ALK V3/b-positive), LC-2/ad (CCDC6-RET C1; R12-positive), and HCC78 (SLC34A2-ROS1 S4; R32-positive). RNA dilution was conducted using normal lung tissue RNA along with RNAs from cancer cell lines, ranging from 100% to 0% representation of tumor portions. Through the systematic dilution of cancer cell line RNAs, the sensitivity and specificity of the QfusionTM assay were assessed. The individual XNA-mediated ALK, RET, or ROS1 QfusionTM assays successfully identified all fusion targets with 1% minimum dilution. The corresponding Cq values for ALK, RET, and ROS1 fusions were 33.62 ± 0.50, 27.03 ± 0.13, and 25.42 ± 0.10, respectively. No fusion signals were detected in the A549 fusion-negative cell line (Figure 2a).
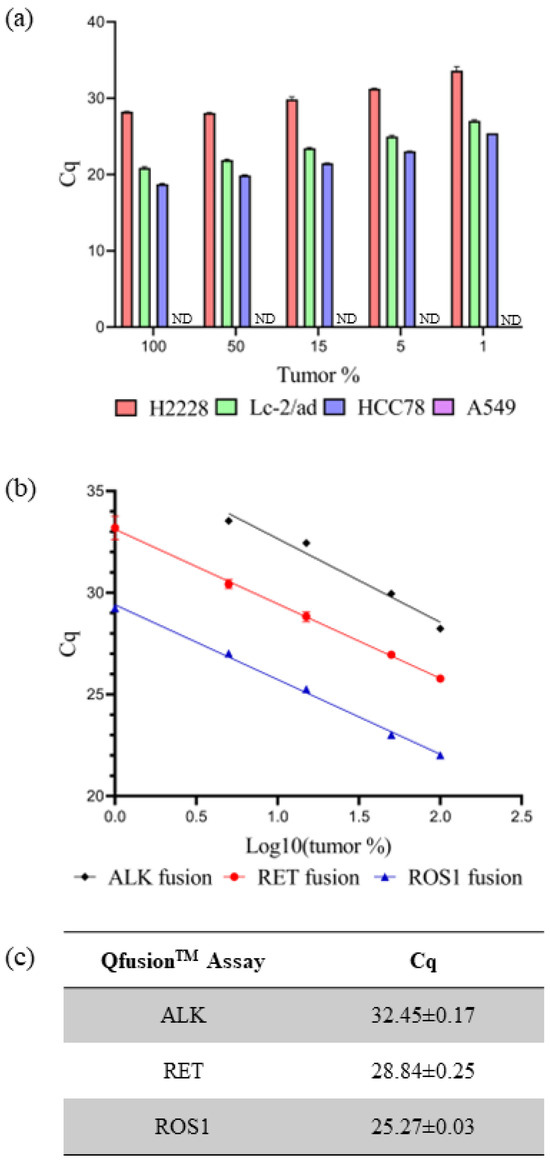
Figure 2.
Evaluating QfusionTM assay performance. (a) Each QfusionTM assay was tested with the corresponding cell lines: A549, H2228 (EML4-ALK), LC-2/ad (CCDC6-RET), and HCC78 (SLC45A-ROS1). The A549 was a fusion-negative cell line and Cq was not determined (ND). (b,c) FFPE RNA reference standard was diluted with normal FFPE RNA and analyzed with each QfusionTM assay. The x-axis represents the logarithm base 10 value of tumor percentage. Cq values were determined for 15% of tumor samples.
The current gold standard diagnostic method for detecting ALK, RET, or ROS1 fusion is FISH. A positive fusion result is typically determined if at least 15% of tumor cells exhibit rearrangement [39]. The QfusionTM assay is specifically designed to detect fusions in both archived and fresh tissue samples. The Cq values for each QfusionTM assay at the 15% tumor cell threshold were determined. To ascertain these Cq values, RNAs were prepared from commercially available FFPE reference standards and normal lung FFPE samples. The QfusionTM assay was then used to analyze various concentrations of reference-standard FFPE RNAs that were diluted with normal lung FFPE RNAs. The resulting Cq values for the QfusionTM ALK, RET, or ROS1 fusion assays at a 15% tumor fraction were determined as 32.45 ± 0.17, 28.84 ± 0.25, and 25.27 ± 0.03, respectively (Figure 2b,c).
3.4. Evaluation of the QfusionTM Assays’ Performance Using Clinical Samples
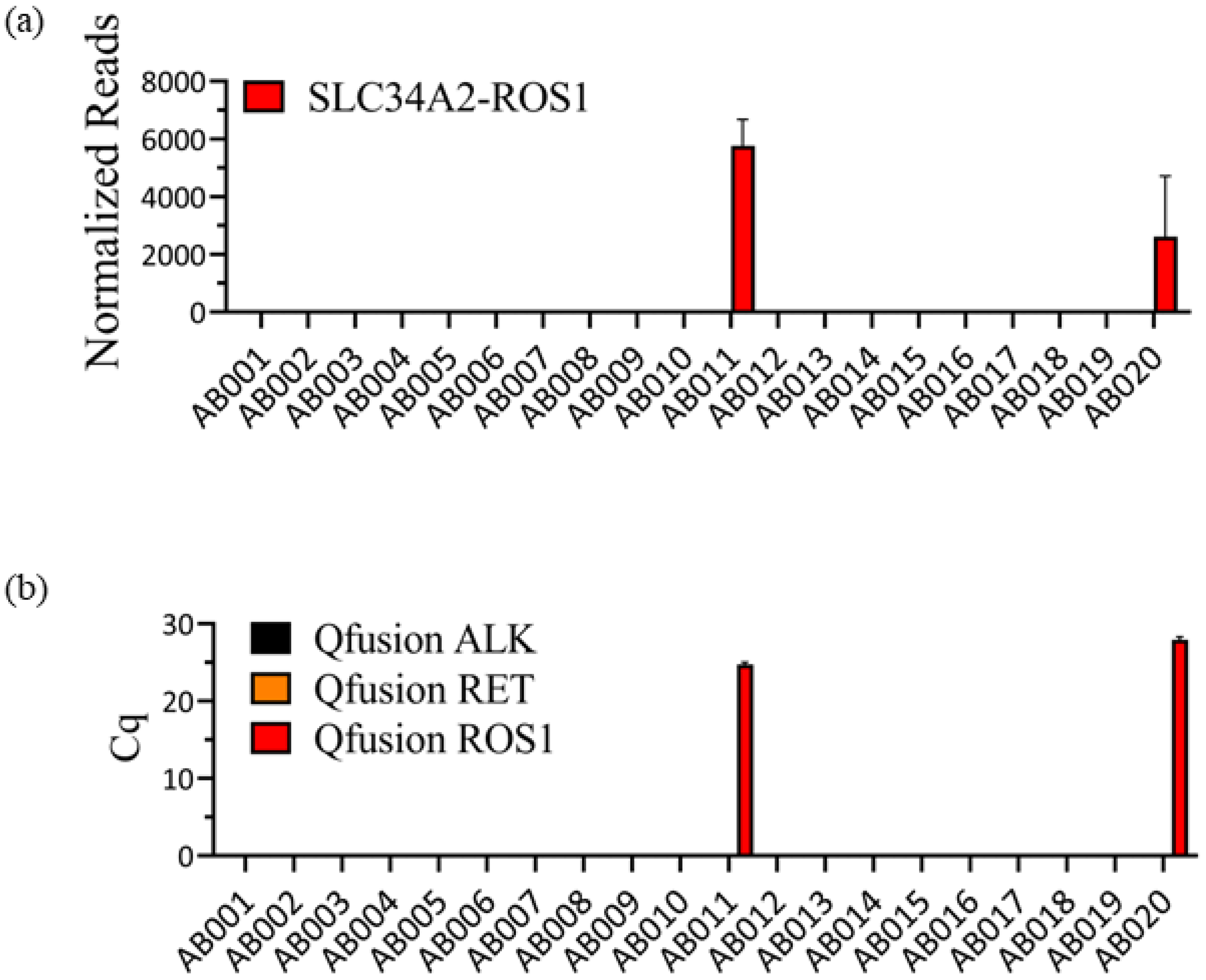
In our previous study, we analyzed twenty FFPE patient samples acquired from the EQA program of the ESP using the OptiSeqTM lung cancer fusion NGS panel [40]. This panel, which was specifically designed for the detection of 63 known lung cancer-specific fusion genes, including ALK, RET, and ROS1 fusion genes, successfully identified SLC45A-ROS1 fusions in two patient samples (Figure 3a). However, there were no instances of ALK or RET fusions. Subsequently, the same twenty samples were assessed using the QfusionTM ALK, RET, or ROS1 fusion detection assay. As shown in Figure 3b, the QfusionTM ROS1 fusion detection assay detected the same patients as ROS1 fusion positive. Conversely, both the QfusionTM ALK and RET fusion detection assays showed that all tested samples were negative for ALK or RET fusion.

Figure 3.
Consistency between OptiSeqTM lung cancer fusion NGS panel and QfusionTM ALK, RET, or ROS1 fusion detection assay results. (a) Analysis of twenty FFPE samples using the OptiSeqTM lung cancer fusion NGS panel revealed the presence of SLC34A2-ROS1 fusion in two patient samples. The Y-axis represents normalized reads, and the error bars indicate the standard deviations of three replicates. (b) The same twenty FFPE samples were subjected to analysis using QfusionTM assays. The results confirmed ROS1 fusion positivity in two patients with SLC34A2-ROS1 fusion. QfusionTM ALK and RET fusion detection assays showed no ALK and RET fusions. The error bars depict the standard deviations of three replicates.
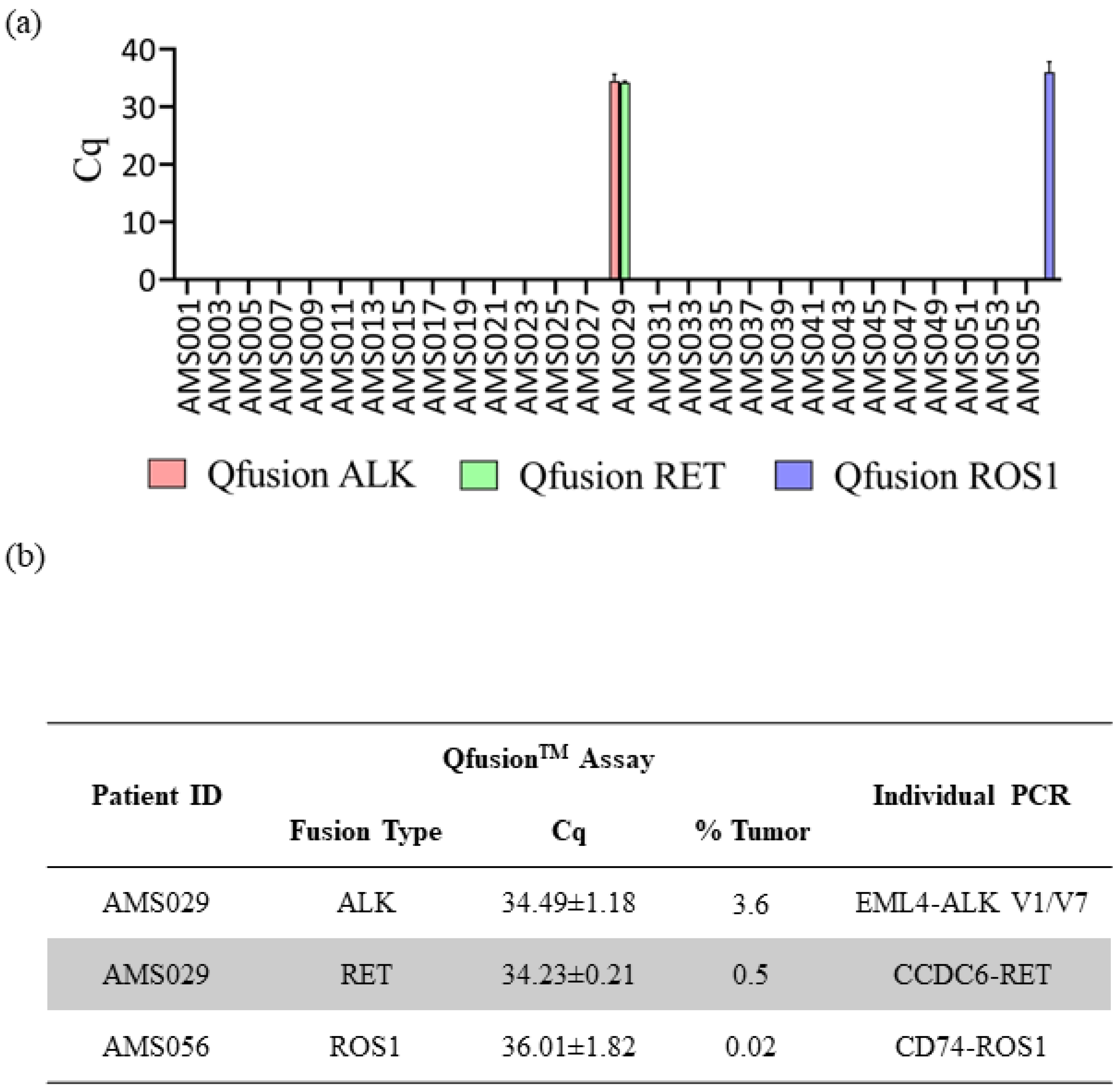
The consistent findings observed between the QfusionTM assays and the NGS assay prompted us to extend our investigation to a larger set of patient samples. The QfusionTM ALK, RET, and ROS1 fusion detection assays were employed to assess 57 distinct clinical FFPE samples obtained from Amsbio (Cambridge, MA, USA). Out of the 57 samples, one patient was identified as ALK fusion-positive, one as RET fusion-positive, and one as ROS1 fusion-positive (Figure 4a). Interestingly, one patient sample, AMS029, exhibited concurrent ALK and RET fusions. To validate the outcomes derived from the QfusionTM assay and determine the specific fusion genes implicated, all fusion-positive samples underwent a reanalysis employing individual PCR amplicons. The QfusionTM assay product (amplicon) served as the template for the second individual PCR, wherein a single primer pair and probe were employed to discern the specific fusion variant.
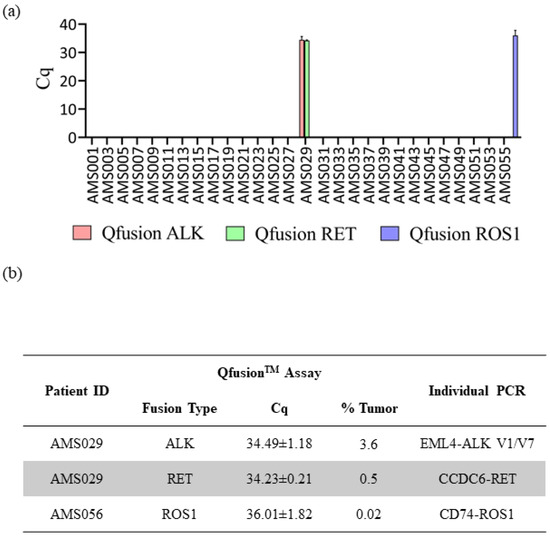
Figure 4.
Assessment of QfusionTM assays’ performance utilizing clinical samples. (a) Results from the analysis of 57 FFPE lung cancer patient samples using the QfusionTM ALK, RET, or ROS1 fusion detection assay. The data reveals the identification of one patient with ALK and RET fusions and one patient with ROS1 fusion. (b) Validation of distinct fusion events through singleplex RT-qPCR. The identified ALK fusions included EML4-ALK V1/V7, while the RET fusion involved CCDC6-RET. Additionally, a single ROS1 fusion event, CD74-ROS1, was confirmed.
The results of the singleplex individual PCR test showed that the AMS029 patient harbored two types of EML4-ALK fusions, V1 and V7 variants, and one CCDC6-RET fusion. In the case of the ROS1 fusion-positive AMS056 patient, the singleplex individual PCR identified the CD74-ROS1 fusion variant (Figure 4b). Subsequently, each individual PCR product was analyzed through sanger sequencing, which confirmed the presence of the corresponding fusion variants (Supplementary Figure S4).
4. Discussion
Chromosomal translocations resulting in the formation of fusion genes represent a significant causative factor in cancer. Accurate diagnosis of these events is crucial for effective treatment. The commonly used diagnostic method, FISH, relies on pre-existing annotations, exhibiting low throughput and limited resolution. In contrast to FISH, RNA NGS sequencing (RNAseq) provides a high-resolution approach to detect fusion genes capable of identifying both established and novel fusions. While the high-throughput nature of RNAseq expands diagnostic possibilities, it also poses a challenge in terms of increased false-positive rates. This indicates potential inaccuracies in fusion gene calls and the identification of non-driver fusion events, such as recurrent chimeric fusion RNAs in non-cancerous tissues and cells [41].
Incorporating RNAseq into routine clinical practice for primary fusion detection is impractical due to its elevated cost, long turnaround time, and complex procedural and data analysis requirements. Therefore, our focus has shifted towards developing a cost-effective, high-throughput fusion detection assay utilizing a multiplex-based reverse RT-qPCR approach.
Numerous RT-qPCR assays have been published for the detection of ALK, RET, and ROS1 fusion genes. Kuang et al. conducted an analysis of RT-qPCR and NGS for EML4-ALK detection, reporting a concordance rate of 95.16%. In approximately 5% of discordant samples, RT-qPCR further identified additional ALK fusions among those with negative NGS results [42]. Lu et al. reported the incidence of EML4-ALK detected by IHC (9.51%), RT-qPCR (11.62%), and NGS (5.84%) [43], suggesting that RT-qPCR has the potential advantage in clinical scenarios for ALK fusion detection. Regarding RET fusions, Kim et al. investigated the frequency of the KIF5B-RET fusion gene and its association with other oncogenic drivers. They analyzed expression in archival tumor tissues using RT-qPCR, detecting the fusion gene in 5.8% of cases, which was confirmed by FISH [44]. In another study, Tsai et al. aimed to detect two types of RET fusions, KIF5B-RET and CCDC6-RET, simultaneously, using a multiplex RT-qPCR platform. They identified RET rearrangements in 2.5% of patients, suggesting the feasibility of screening for RET fusions in a large population [45]. Another large-scale study demonstrated the ease of ROS1 fusion detection by RT-qPCR using a commercial ROS1 fusion detection RT-qPCR kit. Zhang et al. analyzed 2358 cytological specimens and identified ROS1 fusions in 41 patients (1.95%). Furthermore, they monitored 14 patients treated with crizotinib but found no significant differences in the objective response rate, highlighting the prognostic implications of RT-qPCR based fusion detection [46].
Digital PCR (dPCR) is emerging as a promising alternative to NGS for cancer biomarker testing due to its straightforward workflow, minimal sample requirements, rapid turnaround time, and cost-effectiveness [47]. However, conventional dPCR’s clinical utility is hindered by its intrinsic limitation in multiplexing, which restricts the simultaneous assessment of all actionable biomarkers in a single assay with a limited sample quantity. In an attempt to address this limitation, Cabrera et al. developed a multiplex dPCR panel for fusion detection. Their study evaluated the analytical performance and concordance of this multiplex dPCR panel with NGS, achieving a concordance rate exceeding 97%. This demonstrates that multiplex dPCR significantly reduces turnaround time and maintains analytical sensitivity and reactivity comparable to currently accepted amplicon-based NGS methodologies. The simplified workflow and analysis of dPCR offer a promising solution for enhancing accessibility to biomarker testing [48].
To design assays for the identification of gene fusion transcripts, we used our existing XNA technology, which was previously used for detecting single-nucleotide polymorphism (SNP) in genomic DNA samples [35]. Our panels targeted 28 well-known fusion transcripts, encompassing over 90% of ALK, RET, or ROS1 fusion transcripts [49]. We systematically tested the assays on in vitro transcribed RNAs, cell lines, and FFPE samples and successfully identified both known fusion transcripts and fusions in patient FFPE samples with previously unknown fusion status.
The use of a multiplex PCR method can result in decreased specificity and sensitivity compared to singleplex PCR [50,51]. As illustrated in Figure 1, non-specific amplification of wild-type ALK transcripts was observed despite using fusion-specific primer and probe sets. However, the introduction of XNA effectively mitigated this non-specific amplification, thereby increasing assay specificity and sensitivity. Our utilization of XNA technology, originally employed for SNP detection on DNA, has been extended to include fusion detection on RNA.
All three QfusionTM assays were able to detect synthetic targets even at levels as low as 50 copies per reaction, with 18 replicate measurements. In addition, these assays were able to identify corresponding targets in cell lines and FFPE RNA references even when the tumor fraction was as low as 1%. However, it is noteworthy that the criteria for fusion positivity was established based on FISH standards, where a tumor cell population of 15% exhibiting fusion is considered a positive case.
When applying this criterion to 57 FFPE samples from patients with unknown fusion, we found two fusion-positive cases, each exhibiting a frequency of 1.8% for ALK fusion, RET fusion, and ROS1 fusion. Interestingly, one patient displayed two distinct fusions, ALK and RET fusions, which was confirmed by singleplex PCR. This is a rare co-occurrence of such dual fusions in a single patient and could be due to the nature of intratumor heterogeneity [52]. The low frequency of fusion-positive cases among lung cancer patients observed in this study indicates the challenges posed by the relative rarity of these events in the broader patient population. This underscores the importance of using sensitive and comprehensive diagnostic approaches to detect such infrequent but clinically significant occurrences, which can contribute to our understanding of the prevalence of fusion-positive cases in lung cancer.
Patients harboring RTK fusions have exhibited favorable responses to FDA-approved TKIs. Specifically, alectinib and crizotinib have demonstrated efficacy for ALK fusions [6], while selpercatinib and pralsetinib have shown effectiveness for RET fusions [10]. Additionally, crizotinib and entrectinib have been effective for ROS1 fusions [46]. For instance, a cohort of fifty-three patients received crizotinib and were followed for an average duration of 62.6 months. The objective response rate (ORR) was 72%, comprising 6 complete responses and 32 partial responses. This investigation underscores the efficacy of crizotinib in treating ROS1 fusion-positive patients [46]. In line with our assay development objectives, we aim to not only facilitate disease diagnosis but also monitor treatment response. Our goal is to extend the applicability of our assays to longitudinal studies, enabling the evaluation of treatment response over time.
The timely and accurate identification of biomarkers that can be acted upon is a significant step forward in making testing more accessible. To achieve this, we are introducing RT-qPCR panels that comprehensively cover actionable targets and show a high level of agreement with NGS results. These panels have a streamlined workflow and simple analysis, making them a promising solution to improve the accessibility of biomarker testing, thereby contributing to the advancement of personalized medicine guidance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14050488/s1, Table S1. Amsbio FFPE sample information; Table S2. Sequence information of synthetic fusion and wild type templates; Table S3. The effect of XNA on fusion detection; Figure S1. Schematic of QfusionTM ALK, RET, or ROS1 fusion detection assay targets; Figure S2. Schematic of RT-qPCR workflow for QfusionTM ALK, RET, or ROS1 fusion detection assay; Figure S3. XNA blocker principal and PCR cycle; Figure S4. Confirmation of fusion genes by Sanger sequencing.
Author Contributions
Conceptualization, B.L. and M.Y.S.; Methodology, B.L., A.C. and A.Y.F.; Formal analysis, B.L.; Writing—original draft, B.L.; Supervision, A.Z. and M.Y.S.; Project administration, M.Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of WIRB-Copernicus Group, Inc (BST-ARCH-001-VZ, 12 April 2023; BST-FPB-17, 7 April 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
B.L., A.C., A.Y.F., A.Z. and M.Y.S. are employee of DiaCarta Inc.
References
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Chevallier, M.; Borgeaud, M.; Addeo, A.; Friedlaender, A. Oncogenic driver mutations in non-small cell lung cancer: Past, present and future. World J. Clin. Oncol. 2021, 12, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Fontanini, G. Next Generation Sequencing for Gene Fusion Analysis in Lung Cancer: A Literature Review. Diagnostics 2020, 10, 521. [Google Scholar] [CrossRef]
- Suda, K.; Mitsudomi, T. Emerging oncogenic fusions other than ALK, ROS1, RET, and NTRK in NSCLC and the role of fusions as resistance mechanisms to targeted therapy. Transl. Lung Cancer Res. 2020, 9, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.; Capelletti, M.; Yelensky, R.; Otto, G.; Parker, A.; Jarosz, M.; Curran, J.A.; Balasubramanian, S.; Bloom, T.; Brennan, K.W.; et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat. Med. 2012, 18, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Tsuta, K.; Kohno, T.; Yoshida, A.; Shimada, Y.; Asamura, H.; Furuta, K.; Kushima, R. RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br. J. Cancer 2014, 110, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Auger, N.; Auclin, E.; Besse, B. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 27–45. [Google Scholar] [CrossRef]
- Subbiah, V.; Velcheti, V.; Tuch, B.B.; Ebata, K.; Busaidy, N.L.; Cabanillas, M.E.; Wirth, L.J.; Stock, S.; Smith, S.; Lauriault, V.; et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 2018, 29, 1869–1876. [Google Scholar] [CrossRef]
- Charest, A.; Lane, K.; McMahon, K.; Park, J.; Preisinger, E.; Conroy, H.; Housman, D. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21). Genes Chromosomes Cancer 2003, 37, 58–71. [Google Scholar] [CrossRef]
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Doebele, R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Han, Y.; Li, P.; Zhang, J.; Ou, Q.; Tong, X.; Zhao, R.; Dong, N.; Wu, X.; Li, W.; et al. Molecular and clinicopathological characteristics of ROS1-rearranged non-small-cell lung cancers identified by next-generation sequencing. Mol. Oncol. 2020, 14, 2787–2795. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Haberberger, J.; Sokol, E.; Schrock, A.B.; Danziger, N.; Madison, R.; Trabucco, S.; Jin, D.; Pavlick, D.; Ramanan, V.; et al. Clinicopathologic, genomic and protein expression characterization of 356 ROS1 fusion driven solid tumors cases. Int. J. Cancer 2021, 148, 1778–1788. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Gottberg-Williams, A.; Vang, P.; Yang, S.; Britt, N.; Kaur, J.; Haberberger, J.; Danziger, N.; Owens, C.; Beckloff, S.E.; et al. Correlating ROS1 Protein Expression with ROS1 Fusions, Amplifications, and Mutations. JTO Clin. Res. Rep. 2021, 2, 100100. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Su, F.; Kalyana-Sundaram, S.; Khazanov, N.; Ateeq, B.; Cao, X.; Lonigro, R.J.; Vats, P.; Wang, R.; Lin, S.F.; et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013, 3, 636–647. [Google Scholar] [CrossRef]
- Qin, A.; Johnson, A.; Ross, J.S.; Miller, V.A.; Ali, S.M.; Schrock, A.B.; Gadgeel, S.M. Detection of Known and Novel FGFR Fusions in Non-Small Cell Lung Cancer by Comprehensive Genomic Profiling. J. Thorac. Oncol. 2019, 14, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Bahleda, R.; Dienstmann, R.; Infante, J.R.; Mita, A.; Italiano, A.; Calvo, E.; Moreno, V.; Adamo, B.; Gazzah, A.; et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2015, 33, 3401–3408. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; El-Rayes, B.F.; Tolcher, A.W.; Patnaik, A.; Rasco, D.W.; Harvey, R.D.; LoRusso, P.M.; Sachdev, J.C.; Abbadessa, G.; Savage, R.E.; et al. A Phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br. J. Cancer 2017, 117, 1592–1599. [Google Scholar] [CrossRef]
- Gay, N.D.; Wang, Y.; Beadling, C.; Warrick, A.; Neff, T.; Corless, C.L.; Tolba, K. Durable Response to Afatinib in Lung Adenocarcinoma Harboring NRG1 Gene Fusions. J. Thorac. Oncol. 2017, 12, e107–e110. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Somwar, R.; Mangatt, B.P.; Edgren, H.; Desmeules, P.; Ruusulehto, A.; Smith, R.S.; Delasos, L.; Vojnic, M.; Plodkowski, A.J.; et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov. 2018, 8, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wu, W.; Wang, L.; Qu, J.; Han, Q.; Wang, H.; Song, S.; Liu, N.; Wang, Y.; Hou, H. Identification of MET fusions as novel therapeutic targets sensitive to MET inhibitors in lung cancer. J. Transl. Med. 2023, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Tuononen, K.; Sarhadi, V.K.; Wirtanen, A.; Ronty, M.; Salmenkivi, K.; Knuuttila, A.; Remes, S.; Telaranta-Keerie, A.I.; Bloor, S.; Ellonen, P.; et al. Targeted resequencing reveals ALK fusions in non-small cell lung carcinomas detected by FISH, immunohistochemistry, and real-time RT-PCR: A comparison of four methods. BioMed Res. Int. 2013, 2013, 757490. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Suarez-Gauthier, A.; Benito, A.; Garrido, P.; Garcia-Campelo, R.; Biscuola, M.; Paz-Ares, L.; Hardisson, D.; de Castro, J.; Camacho, M.C.; et al. Accurate identification of ALK positive lung carcinoma patients: Novel FDA-cleared automated fluorescence in situ hybridization scanning system and ultrasensitive immunohistochemistry. PLoS ONE 2014, 9, e107200. [Google Scholar] [CrossRef]
- Zito Marino, F.; Rossi, G.; Cozzolino, I.; Montella, M.; Micheli, M.; Bogina, G.; Munari, E.; Brunelli, M.; Franco, R. Multiplex fluorescence in situ hybridisation to detect anaplastic lymphoma kinase and ROS proto-oncogene 1 receptor tyrosine kinase rearrangements in lung cancer cytological samples. J. Clin. Pathol. 2020, 73, 96–101. [Google Scholar] [CrossRef]
- Ginestet, F.; Lambros, L.; Le Flahec, G.; Marcorelles, P.; Uguen, A. Evaluation of a Dual ALK/ROS1 Fluorescent In Situ Hybridization Test in Non-Small-cell Lung Cancer. Clin. Lung Cancer 2018, 19, e647–e653. [Google Scholar] [CrossRef]
- Cui, C.; Shu, W.; Li, P. Fluorescence In situ Hybridization: Cell-Based Genetic Diagnostic and Research Applications. Front. Cell Dev. Biol. 2016, 4, 89. [Google Scholar] [CrossRef]
- Sholl, L.M.; Sun, H.; Butaney, M.; Zhang, C.; Lee, C.; Janne, P.A.; Rodig, S.J. ROS1 immunohistochemistry for detection of ROS1-rearranged lung adenocarcinomas. Am. J. Surg. Pathol. 2013, 37, 1441–1449. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Lian, F.; Guo, L.; Qiu, T.; Ling, Y.; Ying, J.; Lin, D. Detection of ROS1 gene rearrangement in lung adenocarcinoma: Comparison of IHC, FISH and real-time RT-PCR. PLoS ONE 2015, 10, e0120422. [Google Scholar] [CrossRef] [PubMed]
- Anosova, I.; Kowal, E.A.; Dunn, M.R.; Chaput, J.C.; Van Horn, W.D.; Egli, M. The structural diversity of artificial genetic polymers. Nucleic Acids Res. 2016, 44, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Giuffrida, M.C.; Spoto, G. Peptide Nucleic Acid-Based Biosensors for Cancer Diagnosis. Molecules 2017, 22, 1951. [Google Scholar] [CrossRef]
- Sun, Q.; Pastor, L.; Du, J.; Powell, M.J.; Zhang, A.; Bodmer, W.; Wu, J.; Zheng, S.; Sha, M.Y. A novel xenonucleic acid-mediated molecular clamping technology for early colorectal cancer screening. PLoS ONE 2021, 16, e0244332. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Shen, S.; Fu, Y.A.; Cao, A.; Sun, Q.; Cataldi, D.; Li, S.; Lu, M.C.; Zhang, A.; et al. A Sub-Hour Point-of-Care Testing of SARS-CoV-2 Variants Identified by XNA-Based RT-qPCR. Fortune J. 2023, 7, 487–497. [Google Scholar] [CrossRef]
- van Krieken, J.H.; Normanno, N.; Blackhall, F.; Boone, E.; Botti, G.; Carneiro, F.; Celik, I.; Ciardiello, F.; Cree, I.A.; Deans, Z.C.; et al. Guideline on the requirements of external quality assessment programs in molecular pathology. Virchows Arch. 2013, 462, 27–37. [Google Scholar] [CrossRef]
- McCall, C.M.; Mosier, S.; Thiess, M.; Debeljak, M.; Pallavajjala, A.; Beierl, K.; Deak, K.L.; Datto, M.B.; Gocke, C.D.; Lin, M.T.; et al. False positives in multiplex PCR-based next-generation sequencing have unique signatures. J. Mol. Diagn. 2014, 16, 541–549. [Google Scholar] [CrossRef]
- Kwak, E.L.; Bang, Y.J.; Camidge, D.R.; Shaw, A.T.; Solomon, B.; Maki, R.G.; Ou, S.H.; Dezube, B.J.; Janne, P.A.; Costa, D.B.; et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 1693–1703. [Google Scholar] [CrossRef]
- Lee, B.; Babu, A.; Zhang, A.; Sha, M.Y. Abstract 2191: Detection of actionable lung cancer fusion genes with known and novel partners from highly degraded FFPE material. Cancer Res. 2023, 83, 2191. [Google Scholar] [CrossRef]
- Babiceanu, M.; Qin, F.; Xie, Z.; Jia, Y.; Lopez, K.; Janus, N.; Facemire, L.; Kumar, S.; Pang, Y.; Qi, Y.; et al. Recurrent chimeric fusion RNAs in non-cancer tissues and cells. Nucleic Acids Res. 2016, 44, 2859–2872. [Google Scholar] [CrossRef]
- Kuang, Y.; Xu, P.; Wang, J.; Zheng, Y.; Sun, X.; Li, Z.; Gan, R.; Li, H.; Guo, Y.; Yao, F.; et al. Detecting ALK Rearrangement with RT-PCR: A Reliable Approach Compared with Next-Generation Sequencing in Patients with NSCLC. Mol. Diagn. Ther. 2021, 25, 487–494. [Google Scholar] [CrossRef]
- Lu, S.; Lu, C.; Xiao, Y.; Zhu, W.; He, Q.; Xie, B.; Zhou, J.; Tao, Y.; Liu, S.; Xiao, D. Comparison of EML4-ALK fusion gene positive rate in different detection methods and samples of non-small cell lung cancer. J. Cancer 2020, 11, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Lee, J.; Shin, J.Y.; Oh, J.E.; Jung, C.K.; Park, J.K.; Sung, S.W.; Bae, S.J.; Min, H.J.; Kim, D.; et al. KIF5B-RET Fusion gene may coincide oncogenic mutations of EGFR or KRAS gene in lung adenocarcinomas. Diagn. Pathol. 2015, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Wu, S.G.; Hsieh, M.S.; Yu, C.J.; Yang, J.C.; Shih, J.Y. Clinical and prognostic implications of RET rearrangements in metastatic lung adenocarcinoma patients with malignant pleural effusion. Lung Cancer 2015, 88, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Riely, G.J.; Bang, Y.J.; Kim, D.W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Michaelidou, K.; Koutoulaki, C.; Mavridis, K.; Vorrias, E.; Papadaki, M.A.; Koutsopoulos, A.V.; Mavroudis, D.; Agelaki, S. Detection of KRAS G12/G13 Mutations in Cell Free-DNA by Droplet Digital PCR, Offers Prognostic Information for Patients with Advanced Non-Small Cell Lung Cancer. Cells 2020, 9, 2514. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, K.; Gole, J.; Leatham, B.; Springer, M.J.; Smith, M.; Herdt, L.; Jacky, L.; Brown, B.A. Analytical Performance and Concordance with Next-Generation Sequencing of a Rapid, Multiplexed dPCR Panel for the Detection of DNA and RNA Biomarkers in Non-Small-Cell Lung Cancer. Diagnostics 2023, 13, 3299. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Bhamra, G.; Bamford, S.; Dawson, E.; Kok, C.; Clements, J.; Menzies, A.; Teague, J.W.; Futreal, P.A.; Stratton, M.R. The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr. Protoc. Hum. Genet. 2008, 57, 10.11.1–10.11.26. [Google Scholar] [CrossRef] [PubMed]
- Markoulatos, P.; Siafakas, N.; Moncany, M. Multiplex polymerase chain reaction: A practical approach. J. Clin. Lab. Anal. 2002, 16, 47–51. [Google Scholar] [CrossRef]
- Sint, D.; Raso, L.; Traugott, M. Advances in multiplex PCR: Balancing primer efficiencies and improving detection success. Methods Ecol. Evol. 2012, 3, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, S.M.; Dunagin, M.C.; Torborg, S.R.; Torre, E.A.; Emert, B.; Krepler, C.; Beqiri, M.; Sproesser, K.; Brafford, P.A.; Xiao, M.; et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 2017, 546, 431–435. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).