Clinical Indicators of Bone Deterioration in Alcoholic Liver Cirrhosis and Chronic Alcohol Abuse: Looking beyond Bone Fracture Occurrence
Abstract
:1. Introduction
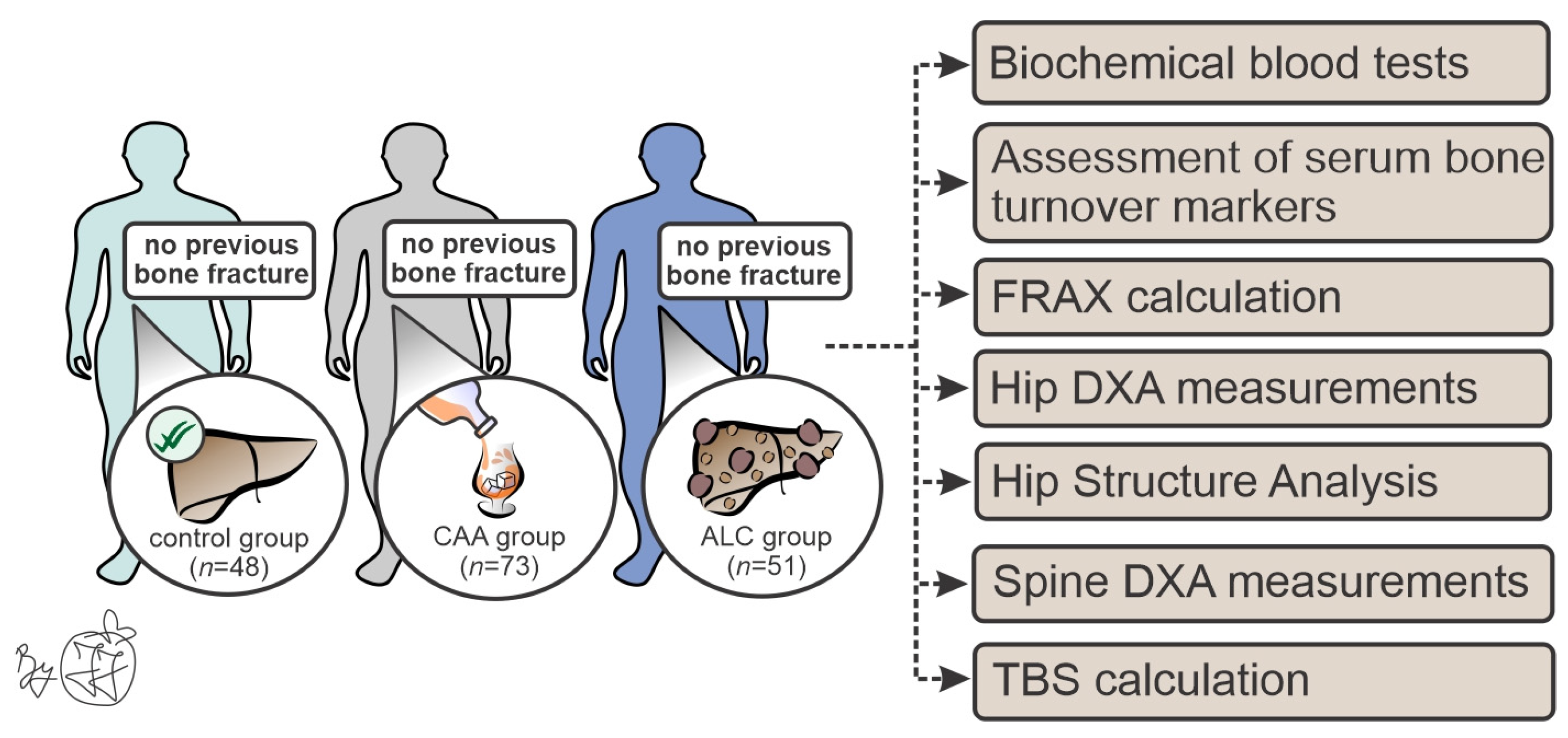
2. Materials and Methods
2.1. Patient Recruitment
2.2. Biochemical Blood Tests and Serum Biomarkers of Bone Metabolism
2.3. Osteodensitometric Measurements and Hip Structural Analysis (HSA)
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Biochemical Analyses
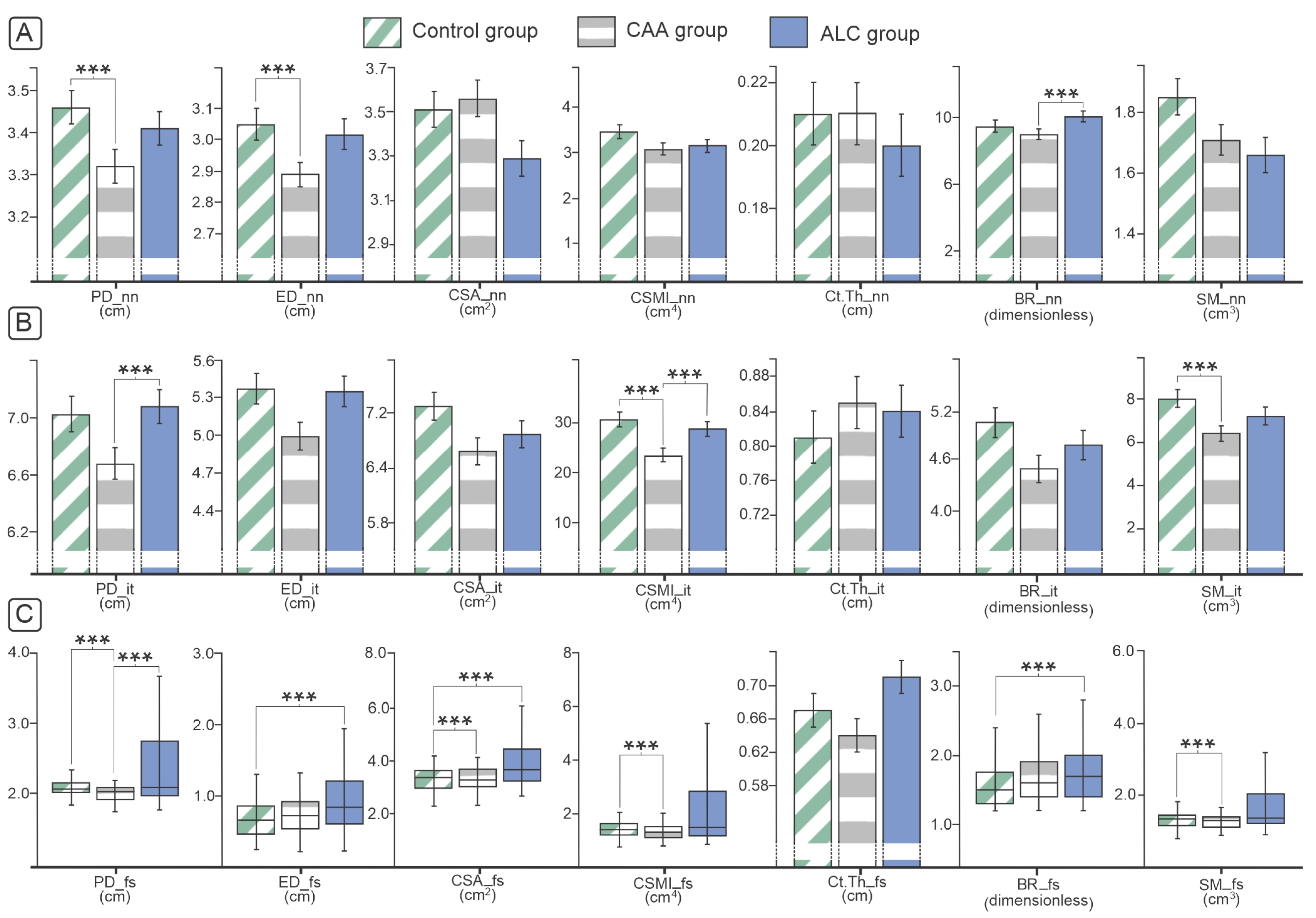
3.2. Osteodensitometry and Geometric Parameters of Proximal Femora and Lumbar Spine
3.3. Bone Turnover Biomarkers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asphaug, L.; Thiele, M.; Krag, A.; Melberg, H.O. Cost-Effectiveness of Noninvasive Screening for Alcohol-Related Liver Fibrosis. Hepatology 2020, 71, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Listabarth, S.; König, D.; Berlakovich, G.; Munda, P.; Ferenci, P.; Kollmann, D.; Gyöeri, G.; Waldhoer, T.; Groemer, M.; van Enckevort, A.; et al. Sex Disparities in Outcome of Patients with Alcohol-Related Liver Cirrhosis within the Eurotransplant Network—A Competing Risk Analysis. J. Clin. Med. 2022, 11, 3646. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Long, J.; Liu, S.; Liu, C.; Li, L.; Yang, L.; Li, Y.; Shu, B. The Burden of Liver Cirrhosis and Underlying Etiologies: Results from the Global Burden of Disease Study 2017. Aging 2021, 13, 279–300. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; De Avila, L.; Afendy, M.; Younossi, I.; Pham, H.; Cable, R.; Younossi, Z.M. Direct and Indirect Economic Burden of Chronic Liver Disease in the United States. Clin. Gastroenterol. Hepatol. 2017, 15, 759–766.e5. [Google Scholar] [CrossRef]
- Jadzic, J.; Djonic, D. Bone Loss in Chronic Liver Diseases: Could Healthy Liver Be a Requirement for Good Bone Health? World J. Gastroenterol. 2023, 29, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Peris, P.; Guañabens, N.; Parés, A.; Pons, F.; del Rio, L.; Monegal, A.; Surís, X.; Caballería, J.; Rodés, J.; Muñoz-Gómez, J. Vertebral Fractures and Osteopenia in Chronic Alcoholic Patients. Calcif. Tissue Int. 1995, 57, 111–114. [Google Scholar] [CrossRef]
- Otete, H.; Deleuran, T.; Fleming, K.M.; Card, T.; Aithal, G.P.; Jepsen, P.; West, J. Hip Fracture Risk in Patients with Alcoholic Cirrhosis: A Population-Based Study Using English and Danish Data. J. Hepatol. 2018, 69, 697–704. [Google Scholar] [CrossRef]
- López-Larramona, G.; Lucendo, A.J.; González-Delgado, L. Alcoholic Liver Disease and Changes in Bone Mineral Density. Rev. Española Enfermedades Dig. 2013, 105, 609–621. [Google Scholar] [CrossRef]
- Sagnelli, E.; Stroffolini, T.; Sagnelli, C.; Pirisi, M.; Babudieri, S.; Colloredo, G.; Russello, M.; Coppola, N.; Gaeta, G.B.; Cacopardo, B.; et al. Gender Differences in Chronic Liver Diseases in Two Cohorts of 2001 and 2014 in Italy. Infection 2018, 46, 93–101. [Google Scholar] [CrossRef]
- Lupoli, R.; Di Minno, A.; Spadarella, G.; Ambrosino, P.; Panico, A.; Tarantino, L.; Lupoli, G.; Lupoli, G.; Di Minno, M.N.D. The Risk of Osteoporosis in Patients with Liver Cirrhosis: A Meta-Analysis of Literature Studies. Clin. Endocrinol. 2016, 84, 30–38. [Google Scholar] [CrossRef]
- Nakchbandi, I. Osteoporosis and Fractures in Liver Disease: Relevance, Pathogenesis and Therapeutic Implications. World J. Gastroenterol. 2014, 20, 9427–9438. [Google Scholar]
- Handzlik-Orlik, G.; Holecki, M.; Wilczyński, K.; Duława, J. Osteoporosis in Liver Disease: Pathogenesis and Management. Ther. Adv. Endocrinol. Metab. 2016, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Díez-Ruiz, A.; García-Saura, P.L.; García-Ruiz, P.; González-Calvin, J.L.; Gallego-Rojo, F.; Fuchs, D. Bone Mineral Density, Bone Turnover Markers and Cytokines in Alcohol-Induced Cirrhosis. Alcohol Alcohol. 2010, 45, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Gasser, R.W.; Kemmler, G.; Moncayo, R.; Finkenstedt, G.; Kurz, M.; Fleischhacker, W.W. Low Bone Mineral Density and Impaired Bone Metabolism in Young Alcoholic Patients without Liver Cirrhosis: A Cross-Sectional Study. Alcohol. Clin. Exp. Res. 2009, 33, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Sellier, N.; Reboul-Marty, J.; Chalès, G.; Lalatonne, Y.; Bourcier, V.; Grando, V.; Barget, N.; Beaugrand, M.; Trinchet, J.C.; et al. Bone Mineral Density Assessed by Dual-Energy X-Ray Absorptiometry in Patients with Viral or Alcoholic Compensated Cirrhosis. A Prospective Study. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 731–737. [Google Scholar] [CrossRef]
- Laitinen, K.; Lamberg-Allardt, C.; Tunninen, R.; Harkönen, M.; Valimaki, M. Bone Mineral Density and Abstention-Induced Changes in Bone and Mineral Metabolism in Noncirrhotic Male Alcoholics. Am. J. Med. 1992, 93, 642–650. [Google Scholar] [CrossRef]
- Culafić, D.; Djonic, D.; Culafic-Vojinovic, V.; Ignjatovic, S.; Soldatovic, I.; Vasic, J.; Beck, T.J.; Djuric, M. Evidence of Degraded BMD and Geometry at the Proximal Femora in Male Patients with Alcoholic Liver Cirrhosis. Osteoporos. Int. 2014, 26, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Danford, C.J.; Trivedi, H.D.; Bonder, A. Bone Health in Patients with Liver Diseases. J. Clin. Densitom. 2019, 23, 212–222. [Google Scholar] [CrossRef]
- Nakchbandi, I.A.; van der Merwe, S.W. Current Understanding of Osteoporosis Associated with Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 660–670. [Google Scholar] [CrossRef]
- López-Larramona, G.; Lucendo, A.J.; González-Castillo, S.; Tenias, J.M. Hepatic Osteodystrophy: An Important Matter for Consideration in Chronic Liver Disease. World J. Hepatol. 2011, 3, 300–307. [Google Scholar] [CrossRef]
- Guañabens, N.; Parés, A. Liver and Bone. Arch. Biochem. Biophys. 2010, 503, 84–94. [Google Scholar] [CrossRef]
- Collier, J. Bone Disorders in Chronic Liver Disease. Hepatology 2007, 46, 1271–1278. [Google Scholar] [CrossRef]
- Casanova-Lara, A.I.; Peniche-Moguel, P.A.; Pérez-Hernández, J.L.; Pérez-Torres, E.; Escobedo González, G.; Córdova-Gallardo, C.J. Osteoporosis and FRAX Risk in Patients with Liver Cirrhosis. Rev. Médica Hosp. Gen. México 2014, 77, 173–178. [Google Scholar] [CrossRef]
- Pasco, J.A.; Anderson, K.B.; Hyde, N.K.; Williams, L.J.; Rufus-Membere, P.; Holloway-Kew, K.L. High Alcohol Intake in Older Men and the Probability of Osteoporotic Fracture According to the FRAX Algorithm. Nutrients 2021, 13, 2955. [Google Scholar] [CrossRef]
- Carey, E.J.; Balan, V.; Kremers, W.K.; Hay, J.E. Osteopenia and Osteoporosis in Patients with End-Stage Liver Disease Caused by Hepatitis C and Alcoholic Liver Disease: Not Just a Cholestatic Problem. Liver Transplant. 2003, 9, 1166–1173. [Google Scholar] [CrossRef]
- Ulhøi, M.P.; Meldgaard, K.; Steiniche, T.; Odgaard, A.; Vesterby, A. Chronic Alcohol Abuse Leads to Low Bone Mass with No General Loss of Bone Structure or Bone Mechanical Strength. J. Forensic Sci. 2017, 62, 131–136. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Harrison, S.L.; Barrett-Connor, E.; Fink, H.A.; Cauley, J.A.; Lewis, C.E.; Orwoll, E.S.; Cummings, S.R. Alcohol Intake and Its Relationship with Bone Mineral Density, Falls, and Fracture Risk in Older Men. J. Am. Geriatr. Soc. 2006, 54, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Balhara, Y.P.S.; Narang, P.; Saha, S.; Kandasamy, D.; Chattopadhyay, N.; Goswami, R. Bone Mineral Density, Bone Microarchitecture and Vertebral Fractures in Male Patients with Alcohol Use Disorders. Alcohol Alcohol. 2022, 57, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Von Skrbensky, G.; Holzer, L.A.; Pichl, W. Hip Fractures and the Contribution of Cortical versus Trabecular Bone to Femoral Neck Strength. J. Bone Miner. Res. 2009, 24, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Jadzic, J.; Milovanovic, P.; Cvetkovic, D.; Ivovic, M.; Tomanovic, N.; Bracanovic, M.; Zivkovic, V.; Nikolic, S.; Djuric, M.; Djonic, D. Mechano-Structural Alteration in Proximal Femora of Individuals with Alcoholic Liver Disease: Implications for Increased Bone Fragility. Bone 2021, 150, 116020. [Google Scholar] [CrossRef] [PubMed]
- Seabra, O.; Pereira, V.G.; Espindula, A.P.; Cardoso, F.A.G.; Volpon, J.B.; Pereira, S.A.L.; Rosa, R.C. Even without Changing the Bone Mineral Density, Alcohol Consumption Decreases the Percentage of Collagen, the Thickness of Bone Trabeculae, and Increases Bone Fragility. An. Acad. Bras. Cienc. 2022, 94, e20210661. [Google Scholar] [CrossRef]
- Zietz, B.; Lock, G.; Plach, B.; Drobnik, W.; Grossmann, J.; Schölmerich, J.; Straub, R.H. Dysfunction of the Hypothalamic-Pituitary-Glandular Axes and Relation to Child-Pugh Classification in Male Patients with Alcoholic and Virus-Related Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Mowat, N.A.G.; Edwards, C.R.W.; Fisher, R.; McNeilly, A.S.; Green, J.R.; Dawson, A.M. Hypothalamic Pituitary Gonadal Function in Men with Cirrhosis of the Liver. Gut 1976, 17, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Monegal, A.; Navasa, M.; Guañabens, N.; Peris, P.; Pons, F.; Martinez De Osaba, M.J.; Rimola, A.; Rodés, J.; Muñoz-Gómez, J. Osteoporosis and Bone Mineral Metabolism Disorders in Cirrhotic Patients Referred for Orthotopic Liver Transplantation. Calcif. Tissue Int. 1997, 60, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Uehara, S.; Udagawa, N.; Takahashi, N. Regulation of Bone Metabolism by Wnt Signals. J. Biochem. 2016, 159, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Menéndez, L.; Sádaba, M.C.; Puche, J.E.; Lavandera, J.L.; de Castro, L.F.; de Gortázar, A.R.; Castilla-Cortázar, I. IGF-I Increases Markers of Osteoblastic Activity and Reduces Bone Resorption via Osteoprotegerin and RANK-Ligand. J. Transl. Med. 2013, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Adamek, A.; Kasprzak, A.; Seraszek, A.; Mikoś, H.; Bura, A.; Mozer-Lisewska, I. Alterations of Insulin-like Growth Factor I (IGF-I) and Estradiol Serum Levels in Chronic Hepatitis C. Wspolczesna Onkol. 2012, 16, 234–239. [Google Scholar] [CrossRef]
- Ronsoni, M.F.; Lazzarotto, C.; Fayad, L.; Silva, M.C.; Nogueira, C.L.; Bazzo, M.L.; Narciso-Schiavon, J.L.; Dantas-Corrêa, E.B.; de Lucca Schiavon, L. IGF-I and IGFBP-3 Serum Levels in Patients Hospitalized for Complications of Liver Cirrhosis. Ann. Hepatol. 2013, 12, 456–463. [Google Scholar] [CrossRef]
- Baim, S.; Miller, P.D. Assessing the Clinical Utility of Serum CTX in Postmenopausal Osteoporosis and Its Use in Predicting Risk of Osteonecrosis of the Jaw. J. Bone Miner. Res. 2009, 24, 561–574. [Google Scholar] [CrossRef]
- Marrone, J.A.; Maddalozzo, G.F.; Branscum, A.J.; Hardin, K.; Cialdella-Kam, L.; Philbrick, K.A.; Breggia, A.C.; Rosen, C.J.; Turner, R.T.; Iwaniec, U.T. Moderate Alcohol Intake Lowers Biochemical Markers of Bone Turnover in Postmenopausal Women. Menopause 2012, 19, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Sripanyakorn, S.; Jugdaohsingh, R.; Mander, A.; Davidson, S.L.; Thompson, R.P.H.; Powell, J.J. Moderate Ingestion of Alcohol Is Associated with Acute Ethanol-Induced Suppression of Circulating CTX in a PTH-Independent Fashion. J. Bone Miner. Res. 2009, 24, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- García-Valdecasas-Campelo, E.; González-Reimers, E.; Santolaria-Fernández, F.; De la Vega-Prieto, M.J.; Milena-Abril, A.; Sánchez-Pérez, M.J.; Martínez-Riera, A.; De Los Ángeles Gómez-Rodríguez, M. Serum Osteoprotegerin and Rankl Levels in Chronic Alcoholic Liver Disease. Alcohol Alcohol. 2006, 41, 261–266. [Google Scholar] [CrossRef]
- Fábrega, E.; Orive, A.; Garciá-Suarez, C.; Gaciá-Unzueta, M.; Amado, J.A.; Pons-Romero, F. Osteoprotegerin and RANKL in Alcoholic Liver Cirrhosis. Liver Int. 2005, 25, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Prystupa, A.; Dąbrowska, A.; Sak, J.J.; Tarach, J.; Toruń-Jurkowska, A.; Lachowska-Kotowska, P.; Dzida, G. Concentrations of Fetuin-A, Osteoprotegerin and α-Klotho in Patients with Alcoholic Liver Cirrhosis. Exp. Ther. Med. 2016, 12, 3464–3470. [Google Scholar] [CrossRef]
- Hofbauer, L.C. CLINICIAN’ S CORNER Clinical Implications of the Osteoprotegerin/RANKL/RANK System for Bone. Endocrinology 2011, 292, 490–495. [Google Scholar]
- Guarino, M.; Loperto, I.; Camera, S.; Cossiga, V.; Di Somma, C.; Colao, A.; Caporaso, N.; Morisco, F. Osteoporosis across Chronic Liver Disease. Osteoporos. Int. 2016, 27, 1967–1977. [Google Scholar] [CrossRef]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-α Induces Osteoclastogenesis by Direct Stimulation of Macrophages Exposed to Permissive Levels of RANK Ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef]

| ALC Group (Mean ± SE) | CAA Group (Mean ± SE) | Control Group (Mean ± SE) | p Value Overall | p Value ALC vs. CAA | p Value ALC vs. Control | p Value CAA vs. Control | |
|---|---|---|---|---|---|---|---|
| PT (s) | 16.99 ± 0.61 | 11.89 ± 0.12 | 11.50 ± 0.09 | p < 0.001 | p < 0.001 | p < 0.001 | 1.00 |
| Fibrinogen (g/L) | 2.79 ± 0.14 | 3.98 ± 0.11 | 3.38 ± 0.12 | p < 0.001 | p < 0.001 | 0.015 | 0.007 |
| Albumin (g/L) | 36.47 ± 0.86 | 43.96 ± 0.39 | 46.56 ± 0.51 | p < 0.001 | p < 0.001 | p < 0.001 | 0.015 |
| Total bilirubin (μmol/L) | 47.84 ± 7.65 | 8.72 ± 0.78 | 14.11 ± 1.17 | p < 0.001 | p < 0.001 | p < 0.001 | 1.000 |
| Direct bilirubin (μmol/L) | 25.44 ± 5.19 | 2.68 ± 0.41 | 4.06 ± 0.33 | p < 0.001 | p < 0.001 | p < 0.001 | 1.000 |
| AST (U/L) | 47.74 ± 4.78 | 31.18 ± 3.00 | 24.23 ± 1.76 | p < 0.001 | 0.002 | 0.000 | 0.629 |
| ALT (U/L) | 32.61 ± 2.74 | 35.33 ± 3.92 | 32.37 ± 3.13 | p > 0.05 | / | / | / |
| ALP (U/L) | 112.63 ± 7.48 | 70.89± 2.09 | 69.23 ± 2.47 | p < 0.001 | p < 0.001 | p < 0.001 | 1.000 |
| GGT (U/L) | 95.04 ± 14.69 | 71.20 ± 8.28 | 30.44 ± 3.52 | p = 0.001 | 0.279 | 0.001 | 0.036 |
| Ca (mmol/L) | 2.34 ± 0.02 | 2.40 ± 0.01 | 2.43 ± 0.02 | p = 0.001 | 0.010 | 0.002 | 0.882 |
| Ca2+ (mmol/L) | 1.27 ± 0.01 | 1.30 ± 0.01 | 1.29 ± 0.01 | p = 0.025 | 0.024 | 0.195 | 1.000 |
| P (mmol/L) | 1.07 ± 0.04 | 1.02 ± 0.03 | 0.89 ± 0.03 | p = 0.004 | 0.720 | 0.003 | 0.034 |
| Vitamin D (nmol/L) | 31.48 ± 3.24 | 29.99 ± 2.58 | 45.93 ± 37.59 | p = 0.002 | 1.000 | 0.009 | 0.003 |
| PTH (pg/mL) | 37.41 ± 3.55 | 36.82 ± 2.89 | 71.12 ± 5.26 | p < 0.001 | 1.000 | p < 0.001 | p < 0.001 |
| Osteocalcin (μg/L) | 17.54 ± 1.71 | 17.27 ± 1.24 | 19.91 ± 1.25 | p > 0.05 | / | / | / |
| Testosteron total (nmol/L) | 16.19 ± 1.27 | 19.13 ± 1.30 | 20.04 ± 1.48 | p > 0.05 | / | / | / |
| Testosteron free (nmol/L) | 4.68 ± 0.84 | 7.83 ± 1.37 | 9.98 ± 0.96 | p = 0.002 | 0.156 | 0.001 | 0.469 |
| Estradiol (pmol/L) | 164.05 ± 16.02 | 140.03 ± 9.09 | 131.22 ± 5.54 | p > 0.05 | / | / | / |
| LH (mIU/mL) | 6.87 ± 5.13 | 6.67 ± 4.71 | 3.09 ± 2.59 | p = 0.001 | 1.000 | 0.002 | 0.007 |
| FSH (mIU/mL) | 9.07 ± 1.40 | 8.92 ± 2.11 | 5.49 ± 0.64 | p > 0.05 | / | / | / |
| DHEAS (μmol/L) | 4.46 ± 0.99 | 7.09 ± 1.03 | 4.28 ± 0.76 | p > 0.05 | / | / | / |
| SHBG (nmol/L) | 69.82 ± 3.40 | 59.34 ± 3.77 | 44.54 ± 3.24 | p < 0.001 | 0.111 | p < 0.001 | 0.015 |
| ALC Group | CAA Group | Control Group | p Value Overall | p Value ALC vs. CAA | p Value ALC vs. Control | p Value CAA vs. Control | |
|---|---|---|---|---|---|---|---|
| β-CTX (pg/mL) | 6202.10 ± 437.96 | 2819.23 ± 390.46 | 4224.12 ± 497.75 | p < 0.001 | p < 0.001 | 0.011 | 0.087 |
| OPG (pg/mL) | 391.88 ± 19.48 | 309.58 ± 20.54 | 252.59 ± 21.73 | p < 0.001 | 0.014 | p < 0.001 | 0.182 |
| RANKL (pg/mL) | 2584.00 [1171–5657] | 2737 [1408–4980] | 2701.79 [1408–7900] | p > 0.05 | / | / | / |
| RANKL/OPG ratio | 7.65 [1.43–21.02] | 7.80 [3.27–23.50] | 11.28 [5.11–27.49] | p = 0.003 | 0.502 | 0.003 | 0.004 |
| IGF-1 (ng/mL) | 38.60 [23.97–122.90] | 35.31 [24.21–136.30] | 52.72 [31.30–233.50] | p < 0.001 | 0.660 | 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stulic, M.; Jadzic, J.; Dostanic, N.; Zivkovic, M.; Stojkovic, T.; Aleksic, J.; Stojkovic, S.; Stojkovic Lalosevic, M.; Vojnovic, M.; Vlaisavljevic, Z.; et al. Clinical Indicators of Bone Deterioration in Alcoholic Liver Cirrhosis and Chronic Alcohol Abuse: Looking beyond Bone Fracture Occurrence. Diagnostics 2024, 14, 510. https://doi.org/10.3390/diagnostics14050510
Stulic M, Jadzic J, Dostanic N, Zivkovic M, Stojkovic T, Aleksic J, Stojkovic S, Stojkovic Lalosevic M, Vojnovic M, Vlaisavljevic Z, et al. Clinical Indicators of Bone Deterioration in Alcoholic Liver Cirrhosis and Chronic Alcohol Abuse: Looking beyond Bone Fracture Occurrence. Diagnostics. 2024; 14(5):510. https://doi.org/10.3390/diagnostics14050510
Chicago/Turabian StyleStulic, Milos, Jelena Jadzic, Natasa Dostanic, Milica Zivkovic, Tihomir Stojkovic, Jelena Aleksic, Stefan Stojkovic, Milica Stojkovic Lalosevic, Marko Vojnovic, Zeljko Vlaisavljevic, and et al. 2024. "Clinical Indicators of Bone Deterioration in Alcoholic Liver Cirrhosis and Chronic Alcohol Abuse: Looking beyond Bone Fracture Occurrence" Diagnostics 14, no. 5: 510. https://doi.org/10.3390/diagnostics14050510








