Parenchymal Cavitations in Pulmonary Tuberculosis: Comparison between Lung Ultrasound, Chest X-ray and Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Patient Selection
2.2. TB Diagnosis and Imaging
2.3. Statistical Analysis
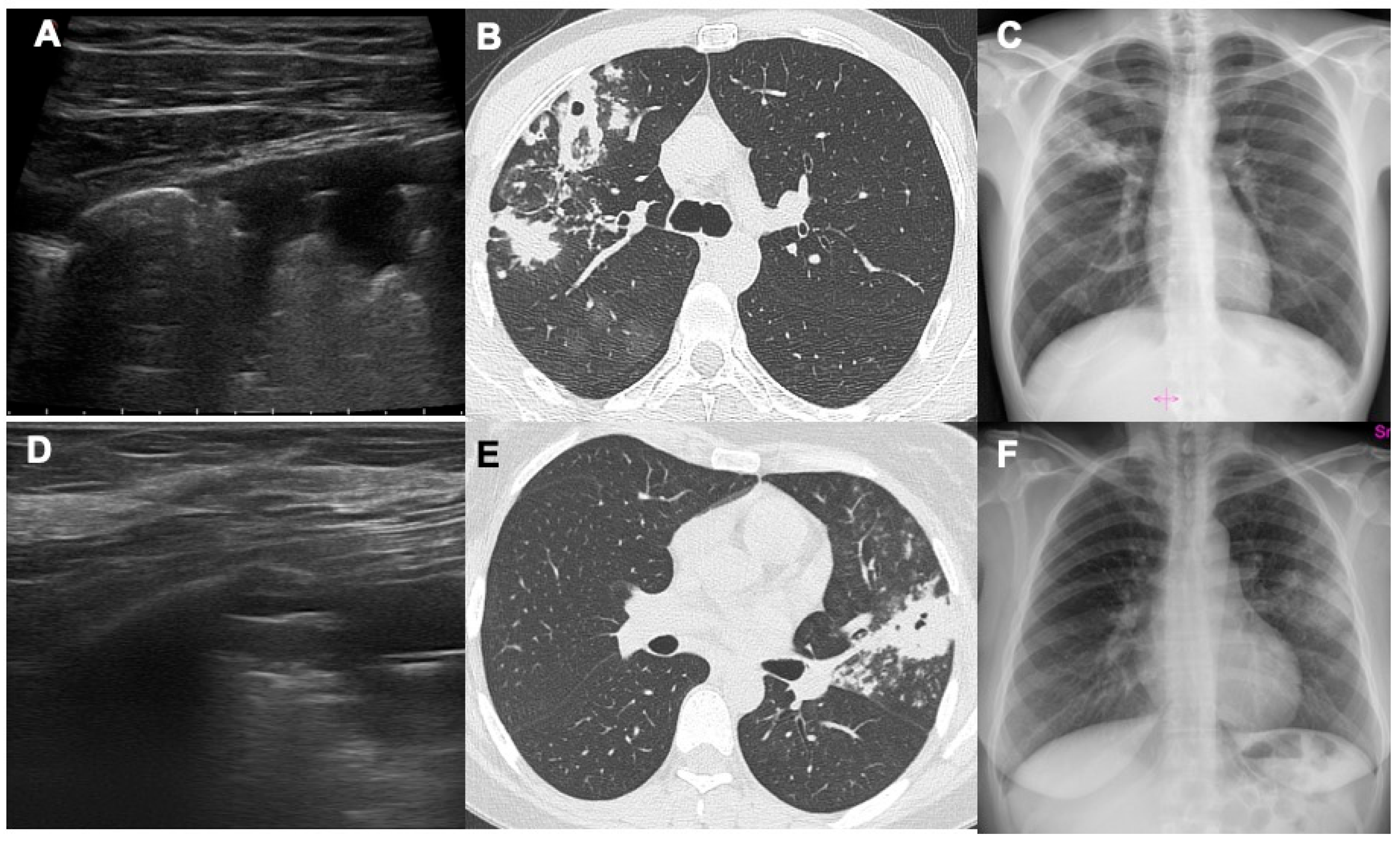
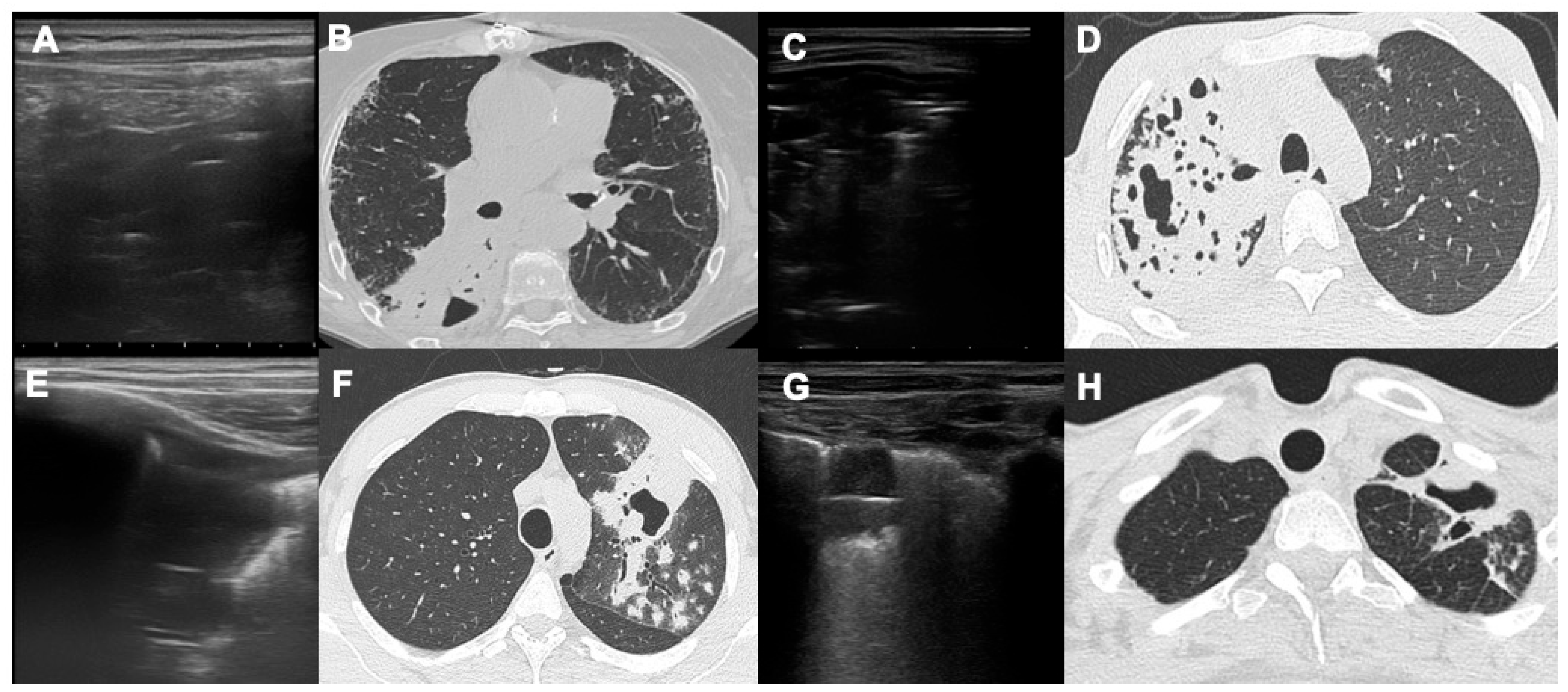
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240083851 (accessed on 2 January 2024).
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- Available online: https://www.gov.uk/government/publications/tuberculosis-tb-by-country-rates-per-100000-people/who-estimates-of-tuberculosis-incidence-by-country-and-territory-2020-accessible-text-version (accessed on 2 January 2024).
- Yoder, M.A.; Lamichane, G.; Bishai, W.R. Cavitary pulmonary tuberculosis: The holy grail of disease transmission. Curr. Sci. 2004, 86, 74–81. [Google Scholar]
- Suttels, V.; Du Toit, J.D.; Fiogbé, A.A.; Wachinou, A.P.; Guendehou, B.; Alovokpinhou, F.; Toukoui, P.; Hada, A.R.; Sefou, F.; Vinasse, P.; et al. Point-of-care ultrasound for tuberculosis management in Sub-Saharan Africa: A balanced SWOT analysis. Int. J. Infect. Dis. 2022, 123, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Urbanowski, M.E.; Ordonez, A.A.; Ruiz-Bedoya, C.A.; Jain, S.K.; Bishai, W.R. Cavitary tuberculosis: The gateway of disease transmission. Lancet Infect. Dis. 2020, 20, e117–e128. [Google Scholar] [CrossRef] [PubMed]
- Jamal, F.; Hammer, M.M. Nontuberculous Mycobacterial infections. Radiol. Clin. N. Am. 2022, 60, 399–408. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yu, X.; Smith, B.; Liu, X.; Gao, J.; Diacon, A.H.; Dawson, R.; Tameris, M.; Zhu, H.; Qu, Y.; et al. Radiological and functional evidence of the bronchial spread of tuberculosis: An observational analysis. Lancet Microbe 2021, 2, e518–e526. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G. Point-of-care lung ultrasound. Praxis 2014, 103, 711–716. [Google Scholar] [CrossRef]
- Nazerian, P.; Volpicelli, G.; Vanni, S.; Gigli, C.; Betti, L.; Bartolucci, M.; Zanobetti, M.; Ermini, F.R.; Iannello, C.; Grifoni, S. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am. J. Emerg. Med. 2015, 33, 620–625. [Google Scholar] [CrossRef]
- Giannelli, F.; Cozzi, D.; Cavigli, E.; Campolmi, I.; Rinaldi, F.; Giachè, S.; Rogasi, P.G.; Miele, V.; Bartolucci, M. Lung ultrasound (LUS) in pulmonary tuberculosis: Correlation with chest CT and X-ray findings. J. Ultrasound 2022, 25, 625–634. [Google Scholar] [CrossRef]
- Giordani, M.T.; Heller, T. Role of ultrasound in the diagnosis of tuberculosis. Eur. J. Intern. Med. 2019, 66, 27–28. [Google Scholar] [CrossRef]
- Montuori, M.; Casella, F.; Casazza, G.; Franzetti, F.; Pini, P.; Invernizzi, C.; Torzillo, D.; Rizzardini, G.; Galli, M.; Cogliati, C. Lung ultrasonography in pulmonary tuberculosis: A pilot study on diagnostic accuracy in a high-risk population. Eur. J. Intern. Med. 2019, 66, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Busi Rizzi, E.; Schininà, V.; Palmieri, F.; Girardi, E.; Bibbolino, C. Cavitary pulmonary tuberculosis HIV-related. Eur. J. Radiol. 2004, 52, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Alshoabi, S.A.; Almas, K.M.; Aldofri, S.A.; Hamid, A.M.; Alhazmi, F.H.; Alsharif, W.M.; Abdulaal, O.M.; Qurashi, A.A.; Aloufi, K.M.; Alsultan, K.D.; et al. The diagnostic decriver: Radiological pictorial review of tuberculosis. Diagnostics 2022, 12, 306. [Google Scholar] [CrossRef]
- Cardinale, L.; Parlatano, D.; Boccuzzi, F.; Onoscuri, M.; Volpicelli, G.; Veltri, A. The imaging spectrum of pulmonary tuberculosis. Acta Radiol. 2015, 56, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, D.; Cavigli, E.; Moroni, C.; Smorchkova, O.; Zantonelli, G.; Pradella, S.; Miele, V. Ground-glass opacity (GGO): A review of the differential diagnosis in the era of COVID-19. Jpn. J. Radiol. 2021, 39, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, D.; Moroni, C.; Addeo, G.; Danti, G.; Lanzetta, M.M.; Cavigli, E.; Falchini, M.; Marra, F.; Piccolo, C.L.; Brunese, L.; et al. Radiological patterns of lung involvement in inflammatory bowel disease. Gastroenterol. Res. Pract. 2018, 2018, 5697846. [Google Scholar] [CrossRef] [PubMed]
- Holt, M.R.; Chan, E.D. Chronic cavitary infections other than tuberculosis: Clinical aspects. J. Thorac. Imaging 2018, 33, 322–333. [Google Scholar] [CrossRef]
- Ketai, L.; Currie, B.J.; Holt, M.R.; Chan, E.D. Radiology of chronic cavitary infections. J. Thorac. Imaging 2018, 33, 334–343. [Google Scholar] [CrossRef]
- Cozzi, D.; Bargagli, E.; Calabrò, A.G.; Torricelli, E.; Giannelli, F.; Cavigli, E.; Miele, V. Atypical HRCT manifestations of pulmonary sarcoidosis. Radiol. Med. 2018, 123, 174–184. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Demi, L. The role of ultrasound lung artifacts in the diagnosis of respiratory disease. Expert Rev. Respir. Med. 2019, 13, 163–172. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Lascols, N.; Mezière, G.; Gepner, A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004, 30, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Amatya, Y.; Rupp, J.; Russell, F.M.; Saunders, J.; Bales, B.; House, D.R. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int. J. Emerg. Med. 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Esayag, Y.; Nikitin, I.; Bar-Ziv, J.; Cytter, R.; Hadas-Halpern, I.; Zalut, T.; Yinnon, A.M. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am. J. Med. 2010, 123, 88.e1–88.e5. [Google Scholar] [CrossRef] [PubMed]
- Hagaman, J.T.; Panos, R.J.; Rouan, G.W.; Shipley, R.T. Admission chest radiograph lacks sensitivity in the diagnosis of community-acquired pneumonia. Am. J. Med. Sci. 2009, 337, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Hayden, G.E.; Wrenn, K. Chest radiograph vs. computed tomography scan in the evaluation of pneumonia. J. Emerg. Med. 2008, 36, 266–270. [Google Scholar] [CrossRef]
- Kovalchik, S.A.; Tammemagi, M.; Berg, C.D.; Caporaso, N.E.; Riley, T.L.; Korch, M.; Silvestri, G.A.; Chaturvedi, A.K.; Katki, H.A. Targeting of Low-Dose CT screening according to the risk of lung-cancer death. N. Engl. J. Med. 2013, 369, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Morello, R.; De Rose, C.; Ferrari, V.; Valentini, P.; Musolino, A.M.; Biasucci, D.G.; Vetrugno, L.; Buonsenso, D. Utility and limits of lung ultrasound in childhood pulmonary tuberculosis: Lessons from a case series and literature review. J. Clin. Med. 2022, 11, 5714. [Google Scholar] [CrossRef]
- Giacomelli, I.L.; Barros, M.; Pacini, G.S.; Altmayer, S.; Zanon, M.; Dias, A.B.; Nin, C.S.; Rodrigues, R.P.; Marchiori, E.; Watte, G.; et al. Multiple cavitary lung lesions on CT: Imaging findings to differentiate between malignant and benign etiologies. J. Bras. Pneumol. 2019, 46, e20190024. [Google Scholar] [CrossRef]
- Heller, T.; Mtemang’ombe, E.A.; Huson, M.A.; Heuvelings, C.C.; Belard, S.; Janssen, S.; Phiri, S.; Grobusch, M.P. Ultrasound for patients in a high HIV/tuberculosis prevalence setting: A needs assessment and review of focused applications for Sub-Saharan Africa. Int. J. Infect. Dis. 2017, 56, 229–236. [Google Scholar] [CrossRef]
- WHO. Chest Radiography in Tuberculosis Detection; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Di Gennaro, F.; Pisani, L.; Veronese, N.; Pizzol, D.; Lippolis, V.; Saracino, A.; Monno, L.; Huson, M.A.; Copetti, R.; Putoto, G.; et al. Potential Diagnostic properties of chest ultrasound in thoracic tuberculosis: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 2235. [Google Scholar] [CrossRef]
- Cocco, G.; Boccatonda, A.; Rossi, I.; D’Ardes, D.; Corvino, A.; Delli Pizzi, A.; Ucciferri, C.; Katia, F.; Jacopo, V. Early detection of pleuro-pulmonary tuberculosis by bedside lung ultrasound: A case report and review of literature. Clin. Case Rep. 2022, 10, e05739. [Google Scholar] [CrossRef]
- Rea, G.; Sperandeo, M.; Lieto, R.; Bocchino, M.; Quarato, C.M.I.; Feragalli, B.; Valente, T.; Scioscia, G.; Giuffreda, E.; Foschino Barbaro, M.P.; et al. Chest imaging in the diagnosis and management of pulmonary tuberculosis: The complementary role of thoracic ultrasound. Front. Med. 2021, 8, 753821. [Google Scholar] [CrossRef]
- Nhat, P.T.H.; Van Hao, N.; Tho, P.V.; Kerdegari, H.; Pisani, L.; Thu, L.N.M.; Phuong, L.T.; Duong, H.T.H.; Thuy, D.B.; McBride, A.; et al. Clinical benefit of AI-assisted lung ultrasound in a resource-limited intensive care unit. Crit. Care 2023, 27, 257. [Google Scholar] [CrossRef]

| Pulmonary TB | Non-Pulmonary TB | Total | p-Values | |
|---|---|---|---|---|
| Total (%) | 58 (70.7) | 24 (29.3) | 82 (100) | |
| Males (%) | 42 (68.9) | 9 (37.5) | 51 (62.2) | 0.131 |
| Origin (%) | ||||
| Italy | 18 (31) | 10 (41.6) | 28 (34.1) | 0.523 |
| Other countries | 40 (69) | 14 (58.4) | 54 (65.9) | 0.670 |
| Risk Factors (%) | ||||
| Smoking | 20 (34.5) | 10 (41.6) | 30 (36.6) | 0.678 |
| HIV | 1 (1.7) | 0 (0) | 1 (1.2) | |
| COPD | 9 (15.5) | 3 (12.5) | 12 (14.6) | 0.760 |
| Active cancer | 2 (3.4) | 2 (8.3) | 4 (4.9) | 0.378 |
| Immunosuppression | 8 (13.7) | 3 (12.5) | 11 (13.4) | 0.891 |
| TB contact | 3 (5.2) | 0 (0) | 3 (3.6) | |
| Previous TB | 8 (13.7) | 4 (16.6) | 12 (14.6) | 0.773 |
| Homeless | 2 (3.4) | 1 (4.1) | 3 (3.6) | 0.879 |
| Other Diagnosis | Patients (%) |
|---|---|
| Pneumonia (bacterial) | 11 (45.8) |
| COPD | 4 (16.6) |
| Previous TB | 3 (12.5) |
| Pneumonia (viral) | 2 (8.3) |
| Lung cancer | 2 (8.3) |
| Organizing pneumonia | 2 (8.3) |
| Total | 24 (100%) |
| Pulmonary TB | Non-Pulmonary TB | p-Values | |
|---|---|---|---|
| Computed tomography | 32/58 (55.2%) | 6/24 (25%) | 0.112 |
| Chest X-ray | 23/58 (40%) | 4/24 (16.7%) | 0.135 |
| Lung ultrasound | 11/58 (19%) | 4/24 (16.7%) | 0.838 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzi, D.; Bartolucci, M.; Giannelli, F.; Cavigli, E.; Campolmi, I.; Rinaldi, F.; Miele, V. Parenchymal Cavitations in Pulmonary Tuberculosis: Comparison between Lung Ultrasound, Chest X-ray and Computed Tomography. Diagnostics 2024, 14, 522. https://doi.org/10.3390/diagnostics14050522
Cozzi D, Bartolucci M, Giannelli F, Cavigli E, Campolmi I, Rinaldi F, Miele V. Parenchymal Cavitations in Pulmonary Tuberculosis: Comparison between Lung Ultrasound, Chest X-ray and Computed Tomography. Diagnostics. 2024; 14(5):522. https://doi.org/10.3390/diagnostics14050522
Chicago/Turabian StyleCozzi, Diletta, Maurizio Bartolucci, Federico Giannelli, Edoardo Cavigli, Irene Campolmi, Francesca Rinaldi, and Vittorio Miele. 2024. "Parenchymal Cavitations in Pulmonary Tuberculosis: Comparison between Lung Ultrasound, Chest X-ray and Computed Tomography" Diagnostics 14, no. 5: 522. https://doi.org/10.3390/diagnostics14050522
APA StyleCozzi, D., Bartolucci, M., Giannelli, F., Cavigli, E., Campolmi, I., Rinaldi, F., & Miele, V. (2024). Parenchymal Cavitations in Pulmonary Tuberculosis: Comparison between Lung Ultrasound, Chest X-ray and Computed Tomography. Diagnostics, 14(5), 522. https://doi.org/10.3390/diagnostics14050522






