Advancements in Glaucoma Diagnosis: The Role of AI in Medical Imaging
Abstract
:1. Introduction
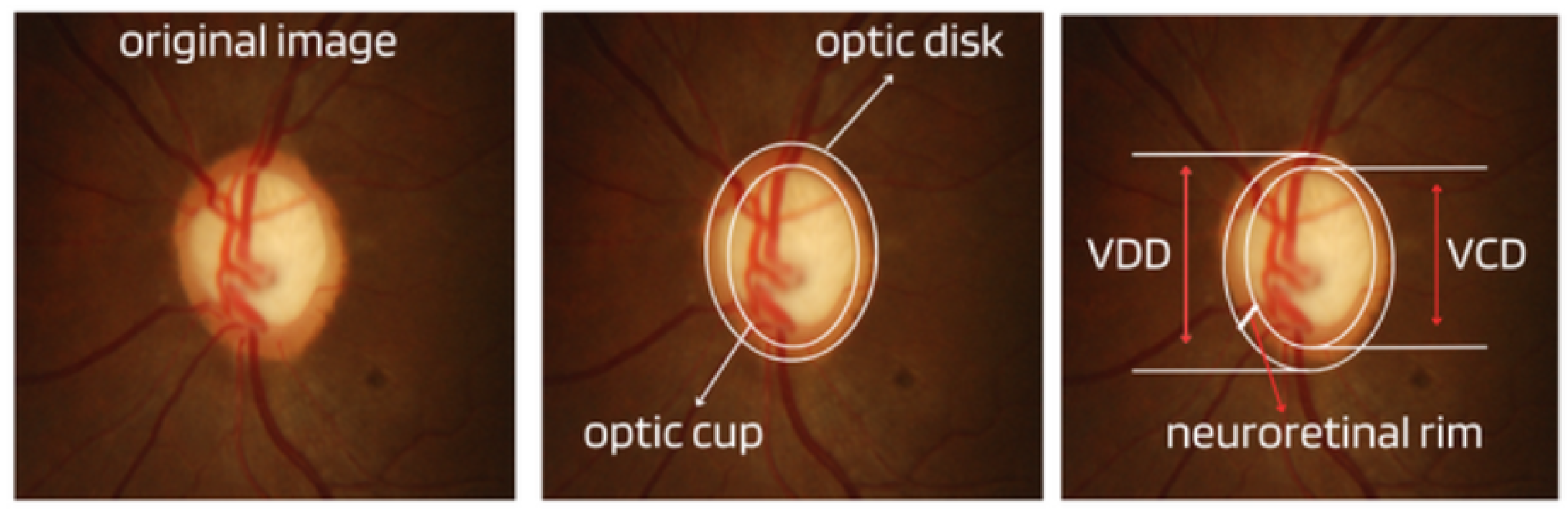
- Cup-to-Disc Ratio (CDR): An abnormal increase in disc cupping is important in the diagnosis of glaucoma; however, many people may have increased nerve cupping and not necessarily have glaucoma. This is especially true for myopic people, who tend to have a larger optical disc and consequently a larger optical cup. Therefore, during the diagnosis of glaucoma, it is important to assess not only the optical cup but also the cup-to-disc ratio (CDR). For better understanding, the CDR measurement is calculated from the relationship between the vertical diameter of the excavation (VCD) and the vertical diameter of the disc (VDD), as shown in Figure 2.To calculate the CDR ratio, the optical disc must be divided into 10 equal parts, as in Figure 3, and then the excavation scope must be taken into account in each division made. Therefore, it is considered a fractional percentage measurement, generally made horizontally, and can vary greatly between normal individuals. However, optical excavations greater than 0.65 indicate possible abnormalities, suggesting further investigation [2,12].
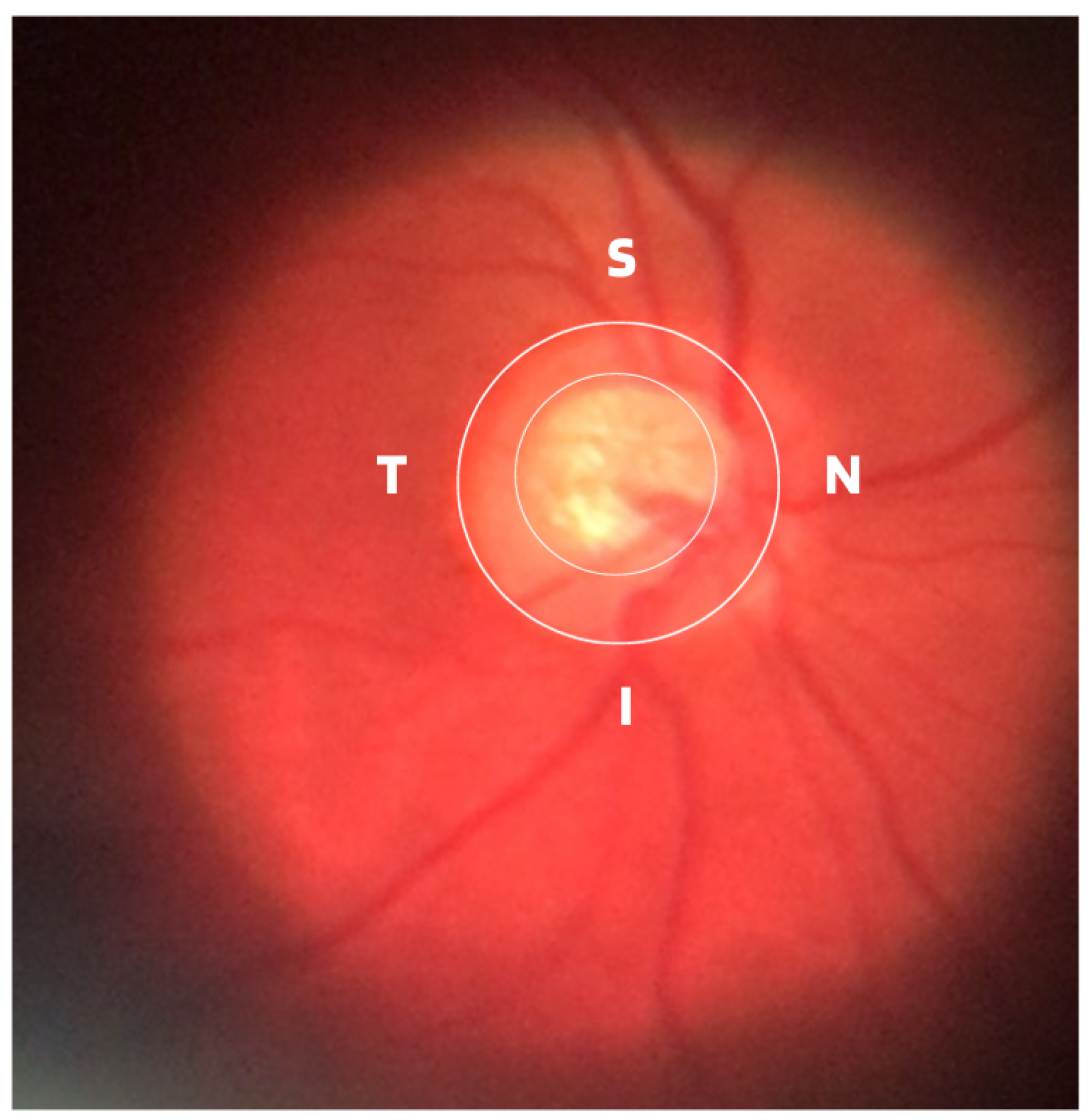
- ISNT Rule: The border formed between the optic cup and the optic disc, called the neuroretinal ring or neural ring, is also considered an indication of glaucoma, for which there is a rule called ISNT, which alludes to the orientation (inferior, superior, nasal, and temporal) of the edges in the image of the fundus, as shown in Figure 1. When considering the ISNT rule, in nonglaucomatous eyes, it is suggested that the thickness of the neural ring should be greatest in the inferior quadrant, followed by the superior, nasal, and temporal quadrants. Misalignment in the guidelines of this rule leads to suspicion of glaucoma [13].
- Cup-to-disc ratio (CDR) asymmetry: The CDR relationship between both eyes is symmetric in most people, and asymmetry is an important sign of suspected glaucomatous damage. This is due to the observation that 1% to 6% normal adults may have a discrepancy of 0.2 in the cup/disc ratio, while 1% of the general population may have an asymmetry of 0.3. Therefore, cup asymmetry is a finding on ophthalmological examination that requires additional tests to rule out the presence of glaucoma or other possible complications [14,15].
- Other structural damage to the optic disc: The main descriptions of these types of damage related to glaucoma are as follows [2,16,17]:
- Changes in RNFL: the presence of defects located in the retinal nerve fiber layer is called Hoyt’s sign and is characterized by a dark area that extends and widens from the optic disc, exhibiting an arched shape.
- Peripapillary atrophy: According to the ophthalmological appearance, peripapillary atrophy can be divided into a peripheral alpha zone and a central beta zone. The alpha zone is characterized by patchy hypopigmentation and thinning of the layers of the chorioretinal tissue. It is laterally adjacent to the retina and medially in contact with the beta area, with the sclera and large choroidal vessels visible. In normal eyes, the alpha and beta areas are usually located in the temporal area, followed by the inferior and superior areas. In glaucomatous eyes, the beta area is more present in the temporal region and its extension is associated with thinning of the RNFL.
- Excavation of the optic disc: In addition to disc excavation, the neuroretinal ring or neural rim must also be observed, as excavation is influenced by the size of the optic disc.
- Disc hemorrhage: The presence of peripapillary hemorrhages is an important sign in both the diagnosis and the monitoring of glaucoma. Therefore, vessel deflection and nasal excavation must be examined.
- Denudation of the lamina, cribriform: the presence of visible extinction of the cribriform lamina to the edge of the optic disc is called a notch, which represents the evolution of a defect located in the neural rim until there is a complete absence of tissue in the region, which exposes the cribriform lamina and allows visualization of its pores. Although it is very suggestive of glaucoma, this sign is not characteristic of the disease.
2. Epidemiology
3. Scientific and Technological Advances in Artificial Intelligence
4. Related Works
4.1. Main Public Databases
4.2. Approaches Using Deep Learning
- Feature vector extraction and classification: In this type of application, various image processing and feature extraction techniques can be used on digital images; however, a classifier will be the part of the system responsible for the categorization task, or that is, it will apply the decision process on which category a given image belongs to. Among the algorithms that work in this way are SVM, KNN, Naive Bayes, etc. Works such as these have been published by several authors and have appeared in [54,55].
- Use of CNN networks: This approach eliminates the need to extract feature vectors, since CNN networks can extract such features through feature maps with their convolutional layers. Considered the gold standard of digital image processing, this methodology was applied in works such as those consulted in [38,56,57], using public and private databases.
- Use of multitechnologies: This type of modeling seeks to achieve the desired objective using a combination of techniques, such as KNN, SVM, CNN, etc. Numerous researchers, such as [61,62,63], have opted for this type of application, which is shown to be a valid way to recognize glaucomatous patterns.
5. Discussion and Conclusions
- The databases were obtained using high-resolution retinal cameras, except for the BrG set, which was obtained using a smartphone connected to a portable ophthalmoscope.
- With the exception of the refuge and Rim-one-dl datasets, which were formed using two digital fundus cameras, all other datasets were obtained using only one digital retinal camera.
- Most databases were labeled based on ophthalmological opinions solely by examining fundus images. Only a few databases were labeled with ophthalmic care and the gold standard for diagnosing glaucoma.
- All publicly available databases are considered too small to train classification algorithms from scratch, which means without using transfer learning.
- Publicly available databases generally have a homogeneous ethnic composition in the collected population.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, N.Y.; Friedman, D.S.; Stalmans, I.; Ahmed, I.I.K.; Sng, C.C. Current opinion in ophthalmology. Curr. Opin. Ophthalmol. 2020, 31, 91–100. [Google Scholar] [CrossRef]
- Bragança, C.P.; Torres, J.M.; De Almeida Soares, C.P. Inteligência artificial e diagnóstico do glaucoma. Braz. Appl. Sci. Rev. 2023, 7, 683–707. [Google Scholar] [CrossRef]
- Heijl, A.; Bengtsson, B.; Oskarsdottir, S.E. Prevalence and severity of undetected manifest glaucoma: Results from the early manifest glaucoma trial screening. Ophthalmology 2013, 120, 1541–1545. [Google Scholar] [CrossRef]
- Salmon, J.F. Clinical Ophthalmology: A Systematic Approach, 10th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2024. [Google Scholar]
- NIH National Library of Medicine. Medical Encyclopedia [Internet]. Medical Encyclopedia: Glaucoma. 2023. Available online: https://medlineplus.gov/ency/article/001620.htm (accessed on 20 February 2024).
- Giorgis, A.T.; Alemu, A.M.; Arora, S.; Gessesse, G.W.; Melka, F.; Woldeyes, A.; Amin, S.; Kassam, F.; Kurji, A.K.; Damji, K.F. Results from the first teleglaucoma pilot project in Addis Ababa, Ethiopia. J. Glaucoma 2019, 28, 701–707. [Google Scholar] [CrossRef]
- Smith, A.M.; Czyz, C.N. Neuroanatomy, cranial nerve 2 (Optic). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sociedade Brasileira de Glaucoma (SBC). Manual De Exame Em Glaucoma. 2015. Available online: https://www.sbglaucoma.org.br/medico/wp-content/uploads/2016/05/folder.pdf (accessed on 20 February 2024).
- Oshika, T.; Yoshitomi, F.; Oki, K. The pachymeter guide: A new device to facilitate accurate corneal thickness measurement. Jpn. J. Ophthalmol. 1997, 41, 426–427. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Qu, G.; Song, D.; Yuan, Y.; Xu, Y.; Gao, K.; Luo, G.; Xiao, Z.; Lam, D.S.; et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med. Imaging 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Garway-Heath, D.F. Early diagnosis in glaucoma. Prog. Brain Res. 2008, 173, 47–57. [Google Scholar] [PubMed]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The diagnosis and treatment of glaucoma. Dtsch. äRzteblatt Int. 2020, 117, 225. [Google Scholar] [CrossRef] [PubMed]
- Khalil, T.; Usman Akram, M.; Khalid, S.; Jameel, A. Improved automated detection of glaucoma from fundus image using hybrid structural and textural features. IET Image Process. 2017, 11, 693–700. [Google Scholar] [CrossRef]
- Arvind, H.; George, R.; Raju, P.; Ve, R.S.; Mani, B.; Kannan, P.; Vijaya, L. Optic Disc Dimensions and Cup-Disc Ratios among Healthy South Indians: The Chennai Glaucoma Study. Ophthalmic Epidemiol. 2011, 18, 189–197. [Google Scholar] [CrossRef]
- Qiu, M.; Boland, M.V.; Ramulu, P.Y. Cup-to-Disc Ratio Asymmetry in U.S. Adults: Prevalence and Association with Glaucoma in the 2005–2008 National Health and Nutrition Examination Survey. Ophthalmology 2017, 124, 1229–1236. [Google Scholar] [CrossRef]
- Tinku, R.S.J.; Diniz Filho, A. Simplificando o Diagnóstico e Tratamento do Glaucoma; Cultura Médica: Rio de Janeiro, Brazil, 2019. [Google Scholar]
- Jung, K.I.; Jeon, S.; Park, C.K. Lamina Cribrosa Depth is Associated With the Cup-to-Disc Ratio in Eyes With Large Optic Disc Cupping and Cup-to-Disc Ratio Asymmetry. J. Glaucoma 2016, 25, e536–e545. [Google Scholar] [CrossRef]
- Tatham, A.J.; Weinreb, R.N.; Medeiros, F.A. Strategies for improving early detection of glaucoma: The combined structure–function index. Clin. Ophthalmol. 2014, 8, 611–621. [Google Scholar]
- Topouzis, F.; Anastasopoulos, E. Glaucoma—The Importance of Early Detection and Early Treatment. J.-Glaucoma Importance Early Detect. Early Treat. 2007, 1, 13. [Google Scholar]
- Camara, J.; Neto, A.; Pires, I.M.; Villasana, M.V.; Zdravevski, E.; Cunha, A. A Comprehensive Review of Methods and Equipment for Aiding Automatic Glaucoma Tracking. Diagnostics 2022, 12, 935. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Vision. 2019. Available online: https://www.who.int/docs/default-source/documents/publications/world-vision-report-accessible.pdf (accessed on 20 February 2024).
- da Silva Negreiros, E.C.M.; dos Santos Silva, L.C.; de Araújo, A.C.R.; Dias, L.R.C.; de Moura, L.V.M.; Santa Rosa, I.M.; de Menezes Filho, J.M.; Marques, C.P.C. Mortalidade por Diabetes Mellitus no nordeste do Brasil no período de 2014 a 2018. Braz. J. Health Rev. 2023, 6, 14138–14155. [Google Scholar] [CrossRef]
- Reis, T.M.; de Moraes Ramos, Y.T.; da Silva, Y.R.M.; Silva, R.A.; de Araújo, M.R.A.; de Araújo, W.M.; Beserra, I.Â.; da Cunha, A.D.R.; Silva, M.B.A. Análise de um triênio dos casos de tracoma em escolares residentes do município de Moreno. Braz. J. Health Rev. 2019, 2, 2273–2286. [Google Scholar]
- Alvaredo, F.; Chancel, L.; Piketty, T.; Saez, E.; Zucman, G. World Inequality Report 2018; Belknap Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Vaahtoranta-Lehtonen, H.; Tuulonen, A.; Aronen, P.; Sintonen, H.; Suoranta, L.; Kovanen, N.; Linna, M.; Läärä, E.; Malmivaara, A. Cost effectiveness and cost utility of an organized screening programme for glaucoma. Acta Ophthalmol. Scand. 2007, 85, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Żmijewska, A.; Szaflik, J.P.; Borowiecki, P.; Pohnke, K.; Romaniuk, U.; Szopa, I.; Pniewski, J.; Szaflik, J. A new platform designed for glaucoma screening: Identifying the risk of glaucomatous optic neuropathy using fundus photography with deep learning architecture together with intraocular pressure measurements. Klin. Oczna/Acta Ophthalmol. Pol. 2020, 122, 1–6. [Google Scholar] [CrossRef]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Epling, J.W.; Jaén, C.R.; et al. Screening for primary open-angle glaucoma: US Preventive Services Task Force recommendation statement. JAMA 2022, 327, 1992–1997. [Google Scholar] [PubMed]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L. Primary open-angle glaucoma preferred practice pattern®. Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef]
- McCarthy, J.; Minsky, M.L.; Rochester, N.; Shannon, C.E. A proposal for the dartmouth summer research project on artificial intelligence, August 31, 1955. AI Mag. 2006, 27, 12. [Google Scholar]
- Russell, S.; Norvig, P. Artificial Intelligence: A Modern Approach, 4th ed.; Pearson: London, UK, 2020. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Saritas, M.M.; Yasar, A. Performance analysis of ANN and Naive Bayes classification algorithm for data classification. Int. J. Intell. Syst. Appl. Eng. 2019, 7, 88–91. [Google Scholar] [CrossRef]
- LeCun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. Imagenet large scale visual recognition challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial networks. Commun. ACM 2020, 63, 139–144. [Google Scholar] [CrossRef]
- Diaz-Pinto, A.; Morales, S.; Naranjo, V.; Köhler, T.; Mossi, J.M.; Navea, A. CNNs for automatic glaucoma assessment using fundus images: An extensive validation. Biomed. Eng. Online 2019, 18, 1–19. [Google Scholar] [CrossRef]
- Carmona, E.J.; Rincón, M.; García-Feijoó, J.; Martínez-de-la Casa, J.M. Identification of the optic nerve head with genetic algorithms. Artif. Intell. Med. 2008, 43, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Sivaswamy, J.; Krishnadas, S.; Joshi, G.D.; Jain, M.; Tabish, A.U.S. Drishti-gs: Retinal image dataset for optic nerve head (onh) segmentation. In Proceedings of the 2014 IEEE 11th International Symposium on Biomedical Imaging (ISBI), Beijing, China, 29 April–2 May 2014; pp. 53–56. [Google Scholar]
- Staal, J.; Abràmoff, M.D.; Niemeijer, M.; Viergever, M.A.; Van Ginneken, B. Ridge-based vessel segmentation in color images of the retina. IEEE Trans. Med. Imaging 2004, 23, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ramani, R.G.; Shanthamalar, J.J. Improved image processing techniques for optic disc segmentation in retinal fundus images. Biomed. Signal Process. Control 2020, 58, 101832. [Google Scholar] [CrossRef]
- Budai, A.; Bock, R.; Maier, A.; Hornegger, J.; Michelson, G. Robust vessel segmentation in fundus images. Int. J. Biomed. Imaging 2013, 2013, 154860. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q. Glaucoma-Deep: Detection of Glaucoma Eye Disease on Retinal Fundus Images using Deep Learning. Int. J. Adv. Comput. Sci. Appl. 2017, 8. [Google Scholar] [CrossRef]
- Decencière, E.; Zhang, X.; Cazuguel, G.; Lay, B.; Cochener, B.; Trone, C.; Gain, P.; Ordonez, R.; Massin, P.; Erginay, A.; et al. Feedback on a publicly distributed image database: The Messidor database. Image Anal. Stereol. 2014, 33, 231–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, F.S.; Liu, J.; Wong, W.K.; Tan, N.M.; Lee, B.H.; Cheng, J.; Wong, T.Y. Origa-light: An online retinal fundus image database for glaucoma analysis and research. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3065–3068. [Google Scholar]
- Kovalyk, O.; Morales-Sánchez, J.; Verdú-Monedero, R.; Sellés-Navarro, I.; Palazón-Cabanes, A.; Sancho-Gómez, J.L. PAPILA: Dataset with fundus images and clinical data of both eyes of the same patient for glaucoma assessment. Sci. Data 2022, 9, 291. [Google Scholar] [CrossRef]
- Orlando, J.I.; Fu, H.; Breda, J.B.; Van Keer, K.; Bathula, D.R.; Diaz-Pinto, A.; Fang, R.; Heng, P.A.; Kim, J.; Lee, J.; et al. Refuge challenge: A unified framework for evaluating automated methods for glaucoma assessment from fundus photographs. Med. Image Anal. 2020, 59, 101570. [Google Scholar] [CrossRef]
- Bajwa, M.N.; Singh, G.A.P.; Neumeier, W.; Malik, M.I.; Dengel, A.; Ahmed, S. G1020: A benchmark retinal fundus image dataset for computer-aided glaucoma detection. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 19–24 July 2020; pp. 1–7. [Google Scholar]
- Bragança, C.P.; Torres, J.M.; Soares, C.P.d.A.; Macedo, L.O. Detection of glaucoma on fundus images using deep learning on a new image set obtained with a smartphone and handheld ophthalmoscope. Healthcare 2022, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.J.F.; Diaz-Aleman, T.; Sigut, J.; Alayon, S.; Arnay, R.; Angel-Pereira, D. Rim-one dl: A unified retinal image database for assessing glaucoma using deep learning. Image Anal. Stereol. 2020, 39, 161–167. [Google Scholar] [CrossRef]
- Sevastopolsky, A.; Drapak, S.; Kiselev, K.; Snyder, B.M.; Keenan, J.D.; Georgievskaya, A. Stack-u-net: Refinement network for image segmentation on the example of optic disc and cup. arXiv 2018, arXiv:1804.11294. [Google Scholar]
- Gupta, N.; Garg, H.; Agarwal, R. A robust framework for glaucoma detection using CLAHE and EfficientNet. Vis. Comput. 2021, 38, 2315–2328. [Google Scholar] [CrossRef]
- Singh, L.K.; Pooja; Garg, H.; Khanna, M.; Bhadoria, R.S. An enhanced deep image model for glaucoma diagnosis using feature-based detection in retinal fundus. Med. Biol. Eng. Comput. 2021, 59, 333–353. [Google Scholar] [CrossRef]
- Shiny Christobel, J.; Vimala, D.; Joshan Athanesious, J.; Christopher Ezhil Singh, S.; Murugan, S. Effectiveness of Feature Extraction by PCA-Based Detection and Naive Bayes Classifier for Glaucoma Images. Int. J. Digit. Multiméd. Broadcast. 2022, 2022, 5. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Liu, H.; Li, Y.; Wang, X.; Jiang, L.; Wang, Z.; Fan, X.; Wang, N. A large-scale database and a CNN model for attention-based glaucoma detection. IEEE Trans. Med. Imaging 2019, 39, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, C.Y.L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y.; et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Rashwan, H.; Akram, F.; Pandey, N.; Sarker, M.M.K.; Saleh, A.; Abdulwahab, S.; Maaroof, N.; Romani, S.; Puig, D. Retinal Optic Disc Segmentation using Conditional Generative Adversarial Network. arXiv 2018, arXiv:cs.CV/1806.03905. [Google Scholar]
- Chang, C.W.; Chang, C.Y.; Lin, Y.Y.; Su, W.W.; Chen, H.S.L. A Glaucoma Detection System Based on Generative Adversarial Network and Incremental Learning. Appl. Sci. 2023, 13, 2195. [Google Scholar] [CrossRef]
- Jain, S.; Indora, S.; Atal, D.K. Rider manta ray foraging optimization-based generative adversarial network and CNN feature for detecting glaucoma. Biomed. Signal Process. Control 2022, 73, 103425. [Google Scholar] [CrossRef]
- Shinde, R. Glaucoma detection in retinal fundus images using U-Net and supervised machine learning algorithms. Intell.-Based Med. 2021, 5, 100038. [Google Scholar] [CrossRef]
- Dos Santos Ferreira, M.V.; de Carvalho Filho, A.O.; de Sousa, A.D.; Silva, A.C.; Gattass, M. Deep learning for optic disc segmentation and glaucoma diagnosis on retinal images. Appl. Sci. 2020, 10, 4916. [Google Scholar]
- Vinícius dos Santos Ferreira, M.; Oseas de Carvalho Filho, A.; Dalília de Sousa, A.; Corrêa Silva, A.; Gattass, M. Convolutional neural network and texture descriptor-based automatic detection and diagnosis of glaucoma. Expert Syst. Appl. 2018, 110, 250–263. [Google Scholar] [CrossRef]
- Zedan, M.J.; Zulkifley, M.A.; Ibrahim, A.A.; Moubark, A.M.; Kamari, N.A.M.; Abdani, S.R. Automated glaucoma screening and diagnosis based on retinal fundus images using deep learning approaches: A comprehensive review. Diagnostics 2023, 13, 2180. [Google Scholar] [CrossRef] [PubMed]
- Zulfira, F.Z.; Suyanto, S.; Septiarini, A. Segmentation technique and dynamic ensemble selection to enhance glaucoma severity detection. Comput. Biol. Med. 2021, 139, 104951. [Google Scholar] [CrossRef] [PubMed]
- Yunitasari, D.A.; Sigit, R.; Harsono, T. Glaucoma detection based on cup-to-disc ratio in retinal fundus image using support vector machine. In Proceedings of the 2021 International Electronics Symposium (IES), Surabaya, Indonesia, 29–30 September 2021; pp. 368–373. [Google Scholar]
- Wang, P.; Yuan, M.; He, Y.; Sun, J. 3D augmented fundus images for identifying glaucoma via transferred convolutional neural networks. Int. Ophthalmol. 2021, 41, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Gheisari, S.; Shariflou, S.; Phu, J.; Kennedy, P.J.; Agar, A.; Kalloniatis, M.; Golzan, S.M. A combined convolutional and recurrent neural network for enhanced glaucoma detection. Sci. Rep. 2021, 11, 1945. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yan, L.; Wang, Y.; Shi, J.; Chen, H.; Zhang, X.; Jiang, M.; Wu, Z.; Zhou, K. Deep learning-based automated detection of glaucomatous optic neuropathy on color fundus photographs. Graefe’S Arch. Clin. Exp. Ophthalmol. 2020, 258, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, L.; Wormstone, I.M.; Qiao, C.; Zhang, C.; Liu, P.; Li, S.; Wang, H.; Mou, D.; Pang, R.; et al. Development and validation of a deep learning system to detect glaucomatous optic neuropathy using fundus photographs. JAMA Ophthalmol. 2019, 137, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Nazir, T.; Javed, A.; Tariq, U.; Yong, H.S.; Khan, M.A.; Cha, J. An efficient deep learning approach to automatic glaucoma detection using optic disc and optic cup localization. Sensors 2022, 22, 434. [Google Scholar] [CrossRef]
- Kim, M.; Han, J.C.; Hyun, S.H.; Janssens, O.; Van Hoecke, S.; Kee, C.; De Neve, W. Medinoid: Computer-aided diagnosis and localization of glaucoma using deep learning. Appl. Sci. 2019, 9, 3064. [Google Scholar] [CrossRef]
- Hemelings, R.; Elen, B.; Barbosa-Breda, J.; Lemmens, S.; Meire, M.; Pourjavan, S.; Vandewalle, E.; Van de Veire, S.; Blaschko, M.B.; De Boever, P.; et al. Accurate prediction of glaucoma from colour fundus images with a convolutional neural network that relies on active and transfer learning. Acta Ophthalmol. 2020, 98, e94–e100. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.; Abdel-Mottaleb, M. A comparative study of deep learning models for diagnosing glaucoma from fundus images. IEEE Access 2021, 9, 23894–23906. [Google Scholar] [CrossRef]
- Aamir, M.; Irfan, M.; Ali, T.; Ali, G.; Shaf, A.; Al-Beshri, A.; Alasbali, T.; Mahnashi, M.H. An adoptive threshold-based multi-level deep convolutional neural network for glaucoma eye disease detection and classification. Diagnostics 2020, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Phene, S.; Dunn, R.C.; Hammel, N.; Liu, Y.; Krause, J.; Kitade, N.; Schaekermann, M.; Sayres, R.; Wu, D.J.; Bora, A.; et al. Deep Learning and Glaucoma Specialists: The Relative Importance of Optic Disc Features to Predict Glaucoma Referral in Fundus Photographs. Ophthalmology 2019, 126, 1627–1639. [Google Scholar] [CrossRef]
- Lee, E.B.; Wang, S.Y.; Chang, R.T. Interpreting deep learning studies in glaucoma: Unresolved challenges. Asia-Pac. J. Ophthalmol. 2021, 10, 261–267. [Google Scholar] [CrossRef]
- Camara, J.; Neto, A.; Pires, I.M.; Villasana, M.V.; Zdravevski, E.; Cunha, A. Literature Review on Artificial Intelligence Methods for Glaucoma Screening, Segmentation, and Classification. J. Imaging 2022, 8, 19. [Google Scholar] [CrossRef]


| Database | Glaucoma | Normal | Total | Viewing Angle |
|---|---|---|---|---|
| Acrima [38] | 396 | 396 | 700 | 30 a 50° |
| Drions [39] | 55 | 55 | 110 | 30 a 50° |
| Drishti-Gs1 [40] | 50 | 51 | 101 | 30° |
| Drive [41] | 34 | 6 | 40 | 45° |
| Glaucoma DB [42] | 85 | 35 | 120 | 30 a 50° |
| Hrf [43] | 15 | 15 | 30 | 45° |
| sjchoi86-Frf [44] | 101 | 300 | 401 | 30 a 50° |
| Messidor [45] | 28 | 72 | 100 | 45° |
| Origa [46] | 168 | 482 | 650 | 30 a 50° |
| Papila [47] | 155 | 333 | 488 | 30 a 50° |
| Refuge [48] | 120 | 1080 | 1200 | 30 a 50° |
| G1020 [49] | 296 | 724 | 1020 | 45° |
| BrG [50] | 1000 | 1000 | 2000 | 25° |
| Rim-one DL [51] | 172 | 313 | 485 | 30 a 50° |
| Paper | Algorithm | Dataset | Accuracy/Precision |
|---|---|---|---|
| Dias et al. [38] | multilevel CNN | Private | 99.4% |
| Bragança et al. [50] | Ensemble CNN | BrG | 90.0% |
| Singh et al. [54] | SVM, KNN e Naive Bayes | STARE e MESSIDOR | 95.0% |
| Shiny et al. [55] | SVM | DRISHTI | 95.3% |
| Shinde et al. [61] | Le-Net e modelo U-Net CNN | RIM-ONE, DRISHTI-GS, DRIONS-DB, JSIEC e DRIVE | 100% |
| Sreng et al. [62] | VGG16-19,Xception, ResNet50 e InceptionV3 | ACRIMA, DRISHTI GS1, HRF, RIM-ONE, | 96.5% |
| Santos et al. [63] | DeepLabv3+ and MobileNet | RIM-ONE, ORIGA, ACRIMA, DRISHTI-GS1 and REFUGE | 95.59 |
| Zulfira et al. [65] | SVM, KNN e Naive Bayes | DRIONS-DB | 98.6% |
| Yunitasari et al. [66] | Dynamic Ensemble | RIM-ONE | 91.0% |
| Wang et al. [67] | SVM | DRISHTI | 95.0% |
| Gheisari et al. [68] | VGG e AlexNet | DRIONS-DB, HRF, RIM-ONE e DRISHTI-GS1 | 94.3% |
| Li et al. [69] | VGG, ResNet e RNN | Private | 95.0% |
| Liu et al. [70] | ResNet | Private | 95.0% |
| Nawaz et al. [71] | ResNet | Private | 96.2% |
| Kim et al. [72] | EficienteNet-B0 | ORIGA | 97.2% |
| Hemelings et al. [73] | VGG, Inception e ResNet | Private | 96.2% |
| Alghamdi et al. [74] | ResNet | Private | 98.0% |
| Aamir et al. [75] | VGG-16 | RIM-ONE e RIGA | 93.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragança, C.P.; Torres, J.M.; Macedo, L.O.; Soares, C.P.d.A. Advancements in Glaucoma Diagnosis: The Role of AI in Medical Imaging. Diagnostics 2024, 14, 530. https://doi.org/10.3390/diagnostics14050530
Bragança CP, Torres JM, Macedo LO, Soares CPdA. Advancements in Glaucoma Diagnosis: The Role of AI in Medical Imaging. Diagnostics. 2024; 14(5):530. https://doi.org/10.3390/diagnostics14050530
Chicago/Turabian StyleBragança, Clerimar Paulo, José Manuel Torres, Luciano Oliveira Macedo, and Christophe Pinto de Almeida Soares. 2024. "Advancements in Glaucoma Diagnosis: The Role of AI in Medical Imaging" Diagnostics 14, no. 5: 530. https://doi.org/10.3390/diagnostics14050530
APA StyleBragança, C. P., Torres, J. M., Macedo, L. O., & Soares, C. P. d. A. (2024). Advancements in Glaucoma Diagnosis: The Role of AI in Medical Imaging. Diagnostics, 14(5), 530. https://doi.org/10.3390/diagnostics14050530










