Correlation between Structural and Functional Changes in Patients with Raised Intraocular Pressure Due to Graves’ Orbitopathy
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
3.1. Clinical Features of the Examined Patient Groups
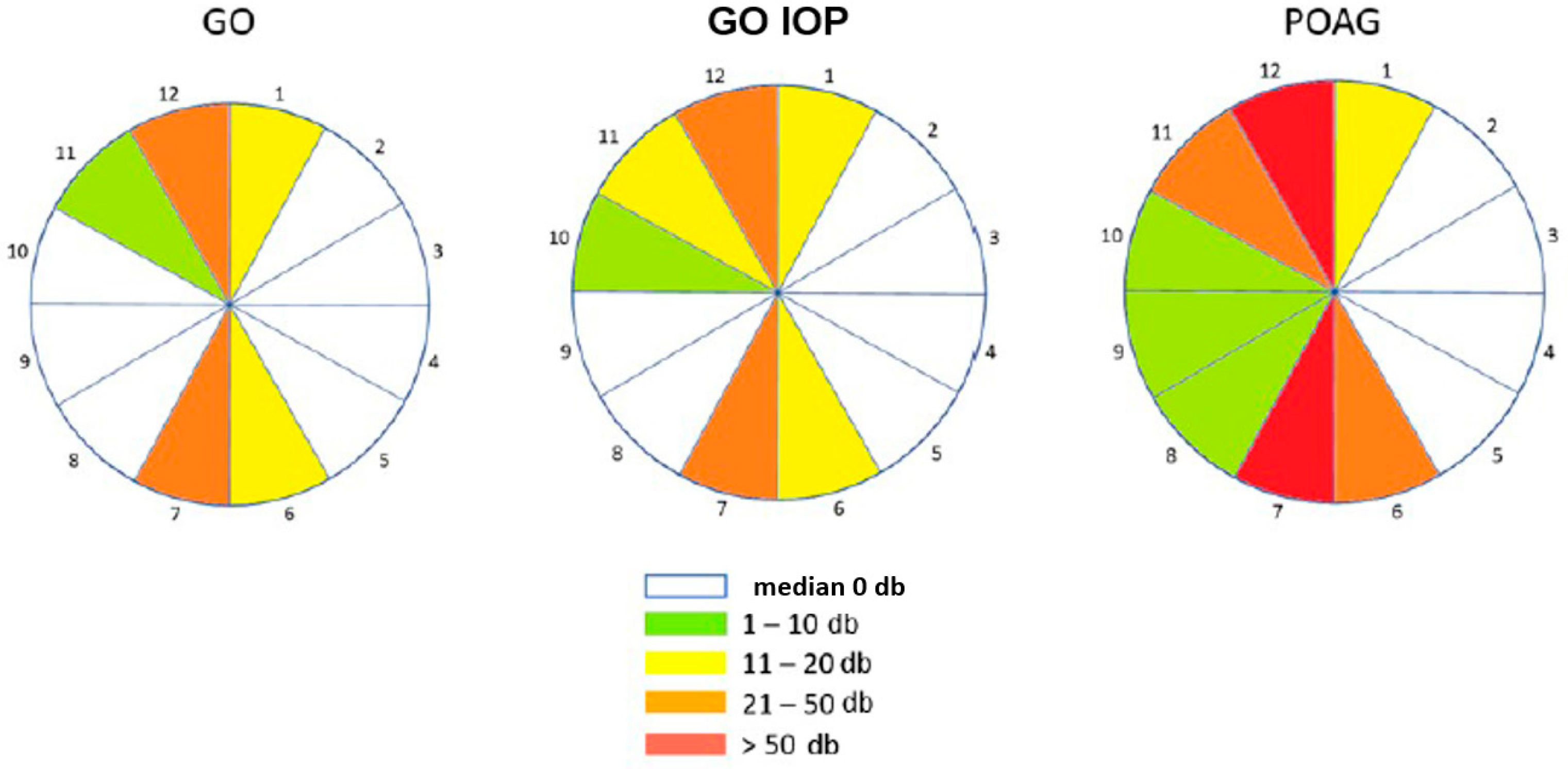
3.2. Structural–Functional Changes
3.3. Risk Factors for Elevated IOP in Subjects with GO
4. Discussion
4.1. Clinical Features of the Examined Patient Groups
4.2. Structural and Functional Changes
4.3. Risk Factors for Elevated IOP in Subjects with GO
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahaly, G.J.; Wüster, C.; Olivo, P.D.; Diana, T. High Titers of Thyrotropin Receptor Antibodies Are Associated With Orbitopathy in Patients With Graves Disease. J. Clin. Endocrinol. Metab. 2019, 104, 2561–2568. [Google Scholar] [CrossRef]
- Delavar, A.; Radha Saseendrakumar, B.; Lee, T.C.; Topilow, N.J.; Ting, M.A.; Liu, C.Y.; Korn, B.S.; Weinreb, R.N.; Kikkawa, D.O.; Baxter, S.L. Associations Between Thyroid Eye Disease and Glaucoma Among Those Enrolled in the National Institutes of Health All of Us Research Program. Ophthalmic Plast. Reconstr. Surg. 2023, 39, 336–340. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, J.; Woo, Y.J.; Bae, H.W.; Yoon, J.S. Prevalence of Ocular Hypertension and Glaucoma as Well as Associated Factors in Graves’ Orbitopathy. J. Glaucoma 2018, 27, 464–469. [Google Scholar] [CrossRef]
- Longo, C.M.; Higgins, P.J. Molecular biomarkers of Graves’ ophthalmopathy. Exp. Mol. Pathol. 2019, 106, 1–6. [Google Scholar] [CrossRef]
- Youngblood, H.; Hauser, M.A.; Liu, Y. Update on the genetics of primary open-angle glaucoma. Exp. Eye Res. 2019, 188, 107795. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, Y.; Li, J.; Li, H. Prognostic value of extraocular muscles for intraocular pressure in thyroid-associated ophthalmopathy patients. Quant. Imaging Med. Surg. 2023, 13, 5727–5736. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Rabiolo, A.; Fu, Q.; Morales, E.; Coleman, A.L.; Law, S.K.; Caprioli, J.; Nouri-Mahdavi, K. Longitudinal Macular Structure-Function Relationships in Glaucoma. Ophthalmology 2020, 127, 888–900. [Google Scholar] [CrossRef]
- Denniss, J.; Turpin, A.; McKendrick, A.M. Relating optical coherence tomography to visual fields in glaucoma: Structure-function mapping, limitations and future applications. Clin. Exp. Optom. 2019, 102, 291–299. [Google Scholar] [CrossRef]
- Bartalena, L.; Tanda, M.L. Current concepts regarding Graves’ orbitopathy. J. Intern. Med. 2022, 292, 692–716. [Google Scholar] [CrossRef]
- European Glaucoma Society. Terminology and Guidelines for Glaucoma, 5th ed.; European Glaucoma Society: Zug, Switzerland, 2021. [Google Scholar]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; EUGOGO. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Hsu, C.K.; Yao, H.Y.; Lin, C.M.; Chang, H.C.; Lu, D.W.; Chen, Y.H.; Chien, K.H. Characteristics of Optic Disc and Visual Field Changes in Patients with Thyroid-Associated Orbitopathy and Open-Angle Glaucoma. J. Clin. Med. 2021, 10, 3839. [Google Scholar] [CrossRef] [PubMed]
- Tanuj, D.; Saurabh, V.; Meghal, G.; Shibal, B.; Nidhi, C.; Kanchan, S.; Namrata, S. Ocular and Systemic Factors Associated with Glaucoma. J. Curr. Glaucoma Pract. 2022, 16, 179–191. [Google Scholar] [CrossRef]
- Betzler, B.K.; Young, S.M.; Sundar, G. Intraocular Pressure and Glaucoma in Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Waisbourd, M.; Spaeth, G.L.; Katz, L.J. Improvement in structure and visual function in patients with glaucoma: The possible key to better treatment? Surv. Ophthalmol. 2021, 66, 644–652. [Google Scholar] [CrossRef]
- Sayin, O.; Yeter, V.; Arıtürk, N. Optic Disc, Macula, and Retinal Nerve Fiber Layer Measurements Obtained by OCT in Thyroid-Associated Ophthalmopathy. J. Ophthalmol. 2016, 2016, 9452687. [Google Scholar] [CrossRef]
- Forte, R.; Bonavolontà, P.; Vassallo, P. Evaluation of retinal nerve fiber layer with optic nerve tracking optical coherence tomography in thyroid-associated orbitopathy. Ophthalmologica 2010, 224, 116–121. [Google Scholar] [CrossRef]
- Gautam Adhikari, P.; Shrestha, G.B. Evaluation of Intraocular Pressure in Thyroid-associated Orbitopathy. J. Nepal. Health Res. Counc. 2022, 19, 824–829. [Google Scholar] [PubMed]
- Naghizadeh, F.; Holló, G. Detection of early glaucomatous progression with octopus cluster trend analysis. J. Glaucoma 2014, 23, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, F.; Garas, A.; Vargha, P.; Holló, G. Structure-function relationship between the octopus perimeter cluster mean sensitivity and sector retinal nerve fiber layer thickness measured with the RTVue optical coherence tomography and scanning laser polarimetry. J. Glaucoma 2014, 23, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, W.; Wang, D.; Bai, H. Structure-Function Relationship between Cluster Mean Defect and Sector Peripapillary Retinal Nerve Fiber Layer Thickness in Primary Open Angle Glaucoma. J. Ophthalmol. 2022, 2022, 5231545. [Google Scholar] [CrossRef] [PubMed]
- Hollo, G. Comparison of structure–function relationship between corresponding retinal nerve fibre layer thickness and Octopus visual field cluster defect values determined by normal and tendency-oriented strategies. Br. J. Ophthalmol. 2017, 101, 150–154. [Google Scholar] [CrossRef]
- Monsalve, B.; Ferreras, A.; Khawaja, A.P.; Calvo, P.; Ara, M.; Fogagnolo, P.; Iester, M. The relationship between structure and function as measured by OCT and Octopus perimetry. Br. J. Ophthalmol. 2015, 99, 1230–1235. [Google Scholar] [CrossRef]
- Sen, E.; Berker, D.; Elgin, U.; Tutuncu, Y.; Ozturk, F.; Guler, S. Comparison of optic disc topography in the cases with Graves disease and healthy controls. J. Glaucoma 2012, 21, 586–589. [Google Scholar] [CrossRef]
- Xu, B.; Wang, S.; Chen, L.; Tan, J. The early diagnostic value of optical coherence tomography (OCT) and OCT angiography in thyroid-associated ophthalmopathy. Ther. Adv. Chronic Dis. 2023, 14, 20406223231166802. [Google Scholar] [CrossRef]
- Holló, G.; Naghizadeh, F. Evaluation of Octopus Polar Trend Analysis for detection of glaucomatous progression. Eur. J. Ophthalmol. 2014, 24, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Yun, Y.I.; Choi, H.J. Screening fundus photography predicts and reveals risk factors for glaucoma conversion in eyes with large optic disc cupping. Sci. Rep. 2023, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Lee, A.; Yoon, J.; Kim, K.E.; Kook, M.S. Comparison of the Circumpapillary Structure-Function and Vasculature-Function Relationships at Different Glaucoma Stages Using Longitudinal Data. Investig. Ophthalmol. Vis. Sci. 2024, 65, 30. [Google Scholar] [CrossRef] [PubMed]
- Ogm, B.E.; Ugurlu, N.; Bilginer, M.C.; Polat, S.B.; Genc, B.; Ersoy, R.; Cakir, B. Thicknesses of the retinal layers in patients with Graves’ disease with or without orbitopathy. Int. Ophthalmol. 2022, 42, 3397–3405. [Google Scholar] [CrossRef]
- Casini, G.; Marinò, M.; Rubino, M.; Licari, S.; Covello, G.; Mazzi, B.; Ionni, I.; Rocchi, R.; Sframeli, A.T.; Figus, M.; et al. Retinal, choroidal and optic disc analysis in patients with Graves’ disease with or without orbitopathy. Int. Ophthalmol. 2020, 40, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Ballae Ganeshrao, S.; Turpin, A.; Denniss, J.; McKendrick, A.M. Enhancing Structure-Function Correlations in Glaucoma with Customized Spatial Mapping. Ophthalmology 2015, 122, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Ray, M.; Jammalamadaka, S.R.; Senthil, S.; Srinivas, M.B.; Pyne, S. Focused analysis of RNFL decay in glaucomatous eyes using circular statistics on high-resolution OCT data. PLoS ONE 2023, 18, e0292915. [Google Scholar] [CrossRef] [PubMed]
- Dhabarde, K.A.; Kende, R.P.; Rahul, N.V.; Surabhi; Nangare, A.R. Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Indian J. Ophthalmol. 2023, 72, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, S.M.; Hospel, J.; Daumerie, C.; Alexopoulou, O.; Maiter, D.; Burlacu, M.C. Significance of thyroperoxidase and thyroglobulin antibodies in medically treated Graves’ disease. Eur. Thyroid. J. 2023, 12, e230193. [Google Scholar] [CrossRef] [PubMed]
- Katahira, M.; Tsunekawa, T.; Mizoguchi, A.; Yamaguchi, M.; Tsuru, K.; Takashima, H.; Terada, R. Clinical significance of thyroglobulin antibodies and thyroid peroxidase antibodies in Graves’ disease: A cross-sectional study. Hormones 2023, 22, 253–261. [Google Scholar] [CrossRef]
- Wall, J.R.; Lahooti, H.; El Kochairi, I. Thyroid Peroxidase and Thyroglobulin Antibodies and Ophthalmopathy in Patients with Graves’ disease, Hashimoto Thyroiditis and Transient Thyroiditis. J. Hum. Endocrinol. 2016, 1, 002. [Google Scholar] [CrossRef]
- Shanmuganathan, T.; Girgis, C.; Lahooti, H.; Champion, B.; Wall, J.R. Does autoimmunity against thyroglobulin play a role in the pathogenesis of Graves’ ophthalmopathy: A review. Clin. Ophthalmol. 2015, 9, 2271–2276. [Google Scholar] [CrossRef]
- Fan, S.X.; Zeng, P.; Li, Z.J.; Wang, J.; Liang, J.Q.; Liao, Y.R.; Hu, Y.X.; Xu, M.T.; Wang, M. The Clinical Features of Graves’ Orbitopathy with Elevated Intraocular Pressure. J. Ophthalmol. 2021, 2021, 9879503. [Google Scholar] [CrossRef]
- Porcu, E.; Medici, M.; Pistis, G.; Volpato, C.B.; Wilson, S.G.; Cappola, A.R.; Bos, S.D.; Deelen, J.; den Heijer, M.; Freathy, R.M.; et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013, 9, e1003266. [Google Scholar] [CrossRef]
- van Heemst, D. The ageing thyroid: Implications for longevity and patient care. Nat. Rev. Endocrinol. 2024, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Marasović Šušnjara, I.; Vejić, M. Prevalence of smoking in Croatia—How to solve the problem? Acta Med. Croatica 2020, 74, 189–196. [Google Scholar]



| Group | N | Min | Max | Centile | p | |||
|---|---|---|---|---|---|---|---|---|
| 25. | Median | 75. | ||||||
| Age (yrs) | GO | 48 | 22.00 | 81.00 | 45.50 | 55.50 | 63.50 | <0.001 |
| GO IOP | 50 | 28.00 | 77.00 | 52.50 | 61.50 | 68.25 | ||
| POAG | 84 | 18.00 | 90.00 | 57.25 | 65.00 | 73.00 | ||
| BCVA | GO | 94 | 0.80 | 1.00 | 1.00 | 1.00 | 1.00 | <0.001 |
| GO IOP | 97 | 0.80 | 1.00 | 0.90 | 1.00 | 1.00 | ||
| POAG | 153 | 0.80 | 1.00 | 0.80 | 1.00 | 1.00 | ||
| IOP (mmHg) | GO | 94 | 10.00 | 20.00 | 14.00 | 16.00 | 17.00 | <0.001 |
| GO IOP | 97 | 21.00 | 28.00 | 22.00 | 24.00 | 26.00 | ||
| POAG | 153 | 10.00 | 26.00 | 18.00 | 20.00 | 22.50 | ||
| CCT (μm) | GO | 94 | 465.00 | 617.00 | 531.75 | 555.50 | 575.25 | <0.001 |
| GO IOP | 97 | 433.00 | 658.00 | 521.00 | 541.00 | 563.50 | ||
| POAG | 153 | 450.00 | 669.00 | 506.00 | 535.00 | 558.50 | ||
| Exophthalmometry (mm) | GO | 94 | 10.00 | 27.00 | 16.00 | 18.00 | 21.00 | <0.001 |
| GO IOP | 97 | 11.00 | 29.00 | 17.00 | 20.00 | 23.00 | ||
| POAG | 153 | 13.00 | 19.00 | 15.00 | 16.00 | 17.00 | ||
| Group | N | Min | Max | Centile | p | |||
|---|---|---|---|---|---|---|---|---|
| 25. | Median | 75. | ||||||
| CAS | GO | 94 | 0.00 | 3.00 | 0.00 | 0.00 | 1.00 | <0.001 |
| GO IOP | 97 | 0.00 | 5.00 | 1.00 | 1.00 | 3.00 | ||
| NOSPECS classification | GO | 94 | 0.00 | 3.00 | 0.00 | 1.00 | 2.00 | <0.001 |
| GO IOP | 97 | 0.00 | 3.00 | 1.00 | 2.00 | 3.00 | ||
| Group | N | Min | Max | Centile | p | |||
|---|---|---|---|---|---|---|---|---|
| 25. | Median | 75. | ||||||
| RNFL (μm) | GO | 94 | 61.00 | 113.00 | 83.00 | 89.50 | 96.25 | <0.001 |
| GO IOP | 97 | 66.00 | 110.00 | 85.00 | 89.00 | 94.00 | ||
| POAG | 153 | 49.00 | 111.00 | 63.00 | 80.00 | 89.00 | ||
| Rim area (mm2) | GO | 94 | 1.00 | 2.13 | 1.30 | 1.51 | 1.66 | <0.001 |
| GO IOP | 97 | 0.87 | 2.09 | 1.20 | 1.35 | 1.53 | ||
| POAG | 153 | 0.21 | 2.80 | 0.87 | 1.09 | 1.32 | ||
| Disc area (mm2) | GO | 94 | 1.12 | 2.85 | 1.66 | 1.85 | 2.08 | 0.327 |
| GO IOP | 97 | 1.27 | 2.83 | 1.68 | 1.96 | 2.17 | ||
| POAG | 153 | 0.78 | 3.25 | 1.59 | 1.90 | 2.24 | ||
| Average c/d ratio | GO | 94 | 0.07 | 0.70 | 0.30 | 0.40 | 0.55 | <0.001 |
| GO IOP | 97 | 0.12 | 0.71 | 0.40 | 0.50 | 0.62 | ||
| POAG | 153 | 0.05 | 0.93 | 0.56 | 0.65 | 0.74 | ||
| Vertical c/d ratio | GO | 94 | 0.06 | 0.66 | 0.30 | 0.40 | 0.51 | <0.001 |
| GO IOP | 97 | 0.09 | 0.74 | 0.41 | 0.50 | 0.60 | ||
| POAG | 153 | 0.05 | 0.91 | 0.55 | 0.62 | 0.75 | ||
| Cup volume (mm3) | GO | 94 | 0.00 | 0.66 | 0.02 | 0.06 | 0.15 | <0.001 |
| GO IOP | 97 | 0.00 | 0.51 | 0.03 | 0.09 | 0.23 | ||
| POAG | 153 | 0.00 | 1.43 | 0.14 | 0.25 | 0.40 | ||
| r2 = 50.3%; p = 0.001 | OR | 95% CI | p | |
|---|---|---|---|---|
| Low | High | |||
| Age (yrs) | 1.08 | 1.01 | 1.15 | 0.026 * |
| Female sex | 2.96 | 0.51 | 17.19 | 0.226 |
| Smoking | 2.40 | 0.47 | 12.30 | 0.294 |
| Arterial Hypertension | 3.51 | 0.55 | 22.29 | 0.183 |
| DM | 1.36 | 0.11 | 16.57 | 0.812 |
| Stress at the time of diagnosis of thyroid disease | 0.90 | 0.18 | 4.37 | 0.892 |
| Thyroid function on diagnosis of GO: hipo | 0.305 | |||
| Thyroid function on diagnosis of GO: eu | 6.96 | 0.57 | 84.56 | 0.128 |
| Thyroid function on diagnosis of GO: hiper | 2.97 | 0.36 | 24.14 | 0.309 |
| TSH < 0.35 mIU/L | 0.498 | |||
| TSH 0.35–4.94 mIU/L | 2.62 | 0.26 | 25.96 | 0.411 |
| TSH > 4.94 mIU/L | 0.77 | 0.04 | 14.84 | 0.860 |
| fT3 < 3.8 pmol/L | 0.141 | |||
| FT3 3.8–6.0 pmol/L | 0.57 | 0.12 | 2.69 | 0.481 |
| FT3 > 6 pmol/L | 22.95 | 0.89 | 591.99 | 0.059 |
| fT4 < 9.0 pmol/L | 0.114 | |||
| FT4 9.0–19.0 pmol/L | 0.00 | 0.00 | 0.999 | |
| FT4 > 19.0 pmol/L | 0.00 | 0.00 | 0.999 | |
| Anti-TSHR elevated values | 0.41 | 0.11 | 1.52 | 0.184 |
| Anti-TPO elevated values | 1.46 | 0.26 | 8.13 | 0.663 |
| Anti-TG elevated values | 21.00 | 1.59 | 278.06 | 0.021 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagatin, F.; Prpić, A.; Škunca Herman, J.; Zrinšćak, O.; Iveković, R.; Vatavuk, Z. Correlation between Structural and Functional Changes in Patients with Raised Intraocular Pressure Due to Graves’ Orbitopathy. Diagnostics 2024, 14, 649. https://doi.org/10.3390/diagnostics14060649
Bagatin F, Prpić A, Škunca Herman J, Zrinšćak O, Iveković R, Vatavuk Z. Correlation between Structural and Functional Changes in Patients with Raised Intraocular Pressure Due to Graves’ Orbitopathy. Diagnostics. 2024; 14(6):649. https://doi.org/10.3390/diagnostics14060649
Chicago/Turabian StyleBagatin, Freja, Ante Prpić, Jelena Škunca Herman, Ognjen Zrinšćak, Renata Iveković, and Zoran Vatavuk. 2024. "Correlation between Structural and Functional Changes in Patients with Raised Intraocular Pressure Due to Graves’ Orbitopathy" Diagnostics 14, no. 6: 649. https://doi.org/10.3390/diagnostics14060649




