Ultrasound Elastography as a Diagnostic Tool for Peyronie’s Disease: A State-of-the-Art Review
Abstract
:1. Introduction
1.1. Peyronie’s Disease (PD)
1.2. Elastography
1.3. The Aim of this Study
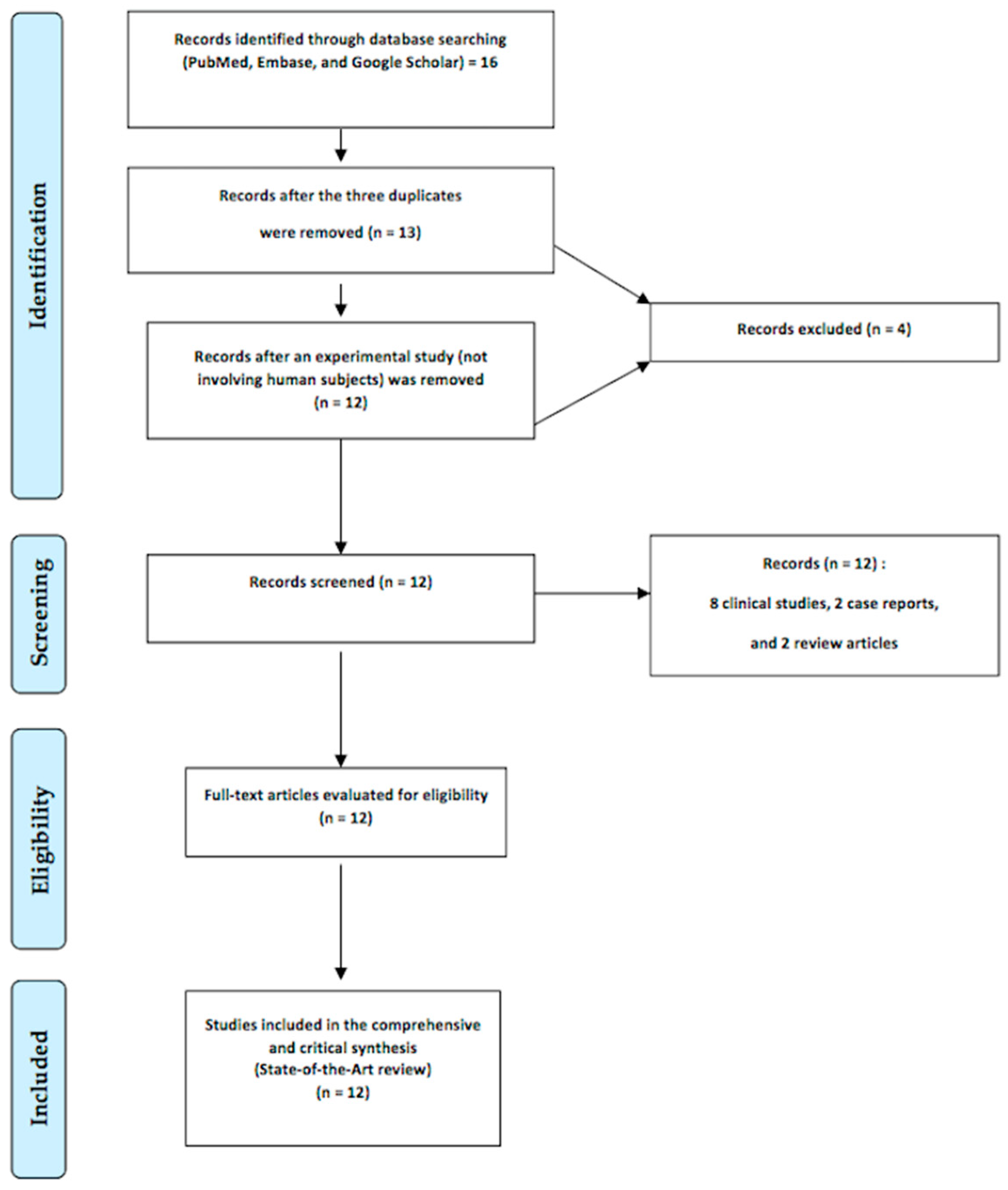
2. Relevant Sections
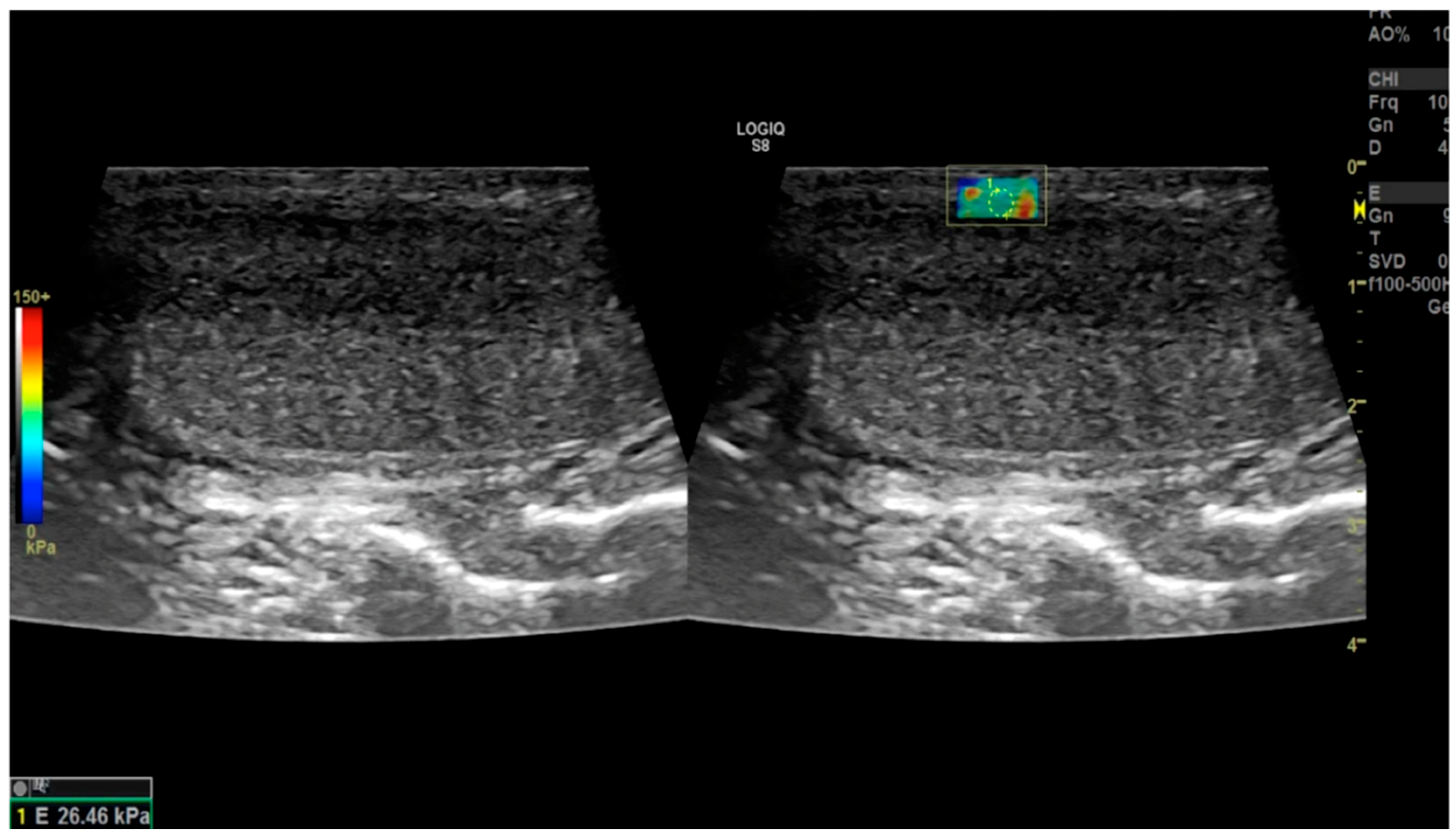
2.1. Strain Elastography
2.2. Transient Elastography
2.3. Acoustic Radiation Force Impulse
2.3.1. Point Shear-Wave Elastography (p-SWE)
2.3.2. Shear-Wave Elastography
2.3.3. Supersonic Shear Imaging
2.3.4. Vibro-Elastography and Vibro-Acoustography
2.4. Literature Review
2.4.1. Lahme et al. (2009) [38]
2.4.2. Morana et al. (2010) [39]
2.4.3. Riversi et al. (2012) [40]
2.4.4. Zhang et al. (2018) [41]
2.4.5. Trama et al. (2018) [42]
2.4.6. Tyloch et al. (2020) [43]
2.4.7. Trama et al. (2022) [44]
2.4.8. Zhao et al. (2024) [45]
2.4.9. Richards et al. (2014) [46]
2.4.10. Dhawan et al. (2022) [47]
2.4.11. Parmar et al. (2020) [37]
- -
- To assess the presence of concurrent ED to determine the need for a penile prosthesis for comprehensive treatment;
- -
- To determine whether plaques involve the neurovascular bundle or cavernosal artery, aiding in surgical preparation;
- -
- To conduct ultrasound assessments in the office, saving both time and money.
Sonoelastography
2.4.12. Simon et al. (2022) [48]
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herati, A.S.; Pastuszak, A.W. The Genetic Basis of Peyronie’s Disease: A Review. Sex Med. Rev. 2016, 4, 85–94. [Google Scholar] [CrossRef]
- Bias, W.B.; Nyberg, L.M., Jr.; Hochberg, M.C.; Walsh, P.C.; Opitz, J.M. Peyronie’s disease: A newly recognized autosomal-dominant trait. Am. J. Med. Genet. 1982, 12, 227–235. [Google Scholar] [CrossRef] [PubMed]
- DiBenedetti, D.B.; Nguyen, D.; Zografos, L.; Ziemiecki, R.; Zhou, X. A Population-Based Study of Peyronie’s Disease: Prevalence and Treatment Patterns in the United States. Adv. Urol. 2011, 2011, 282503. [Google Scholar] [CrossRef] [PubMed]
- Stuntz, M.; Perlaky, A.; des Vignes, F.; Kyriakides, T.; Glass, D. The Prevalence of Peyronie’s Disease in the United States: A Population-Based Study. PLoS ONE 2016, 11, e0150157. [Google Scholar] [CrossRef] [PubMed]
- Bella, A.J.; Lee, J.C.; Grober, E.D.; Carrier, S.; Bénard, F.; Brock, G.B. 2018 Canadian Urological Association guideline for Peyronie’s disease and congenital penile curvature. Can. Urol. Assoc. J. 2018, 12, E197–E209. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.M.; Weerakoon, P.; Stricker, P.D. The incidence, aetiology, and presentation of Peyronie’s disease in Sydney, Australia. J. Sex. Disabil. 2002, 20, 109–116. [Google Scholar] [CrossRef]
- La Pera, G.; Pescatori, E.S.; Calabrese, M.; Boffini, A.; Colombo, F.; Andriani, E.; Natali, A.; Vaggi, L.; Catuogno, C.; Giustini, M.; et al. Peyronie’s disease: Prevalence and association with cigarette smoking. A multicenter population-based study in men aged 50–69 years. Eur Urol. 2001, 40, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, U.; Sommer, F.; Klotz, T.; Braun, M.; Reifenrath, B.; Engelmann, U. The prevalence of Peyronie’s disease: Results of a large survey. BJU Int. 2001, 88, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Rhoden, E.L.; Teloken, C.; Ting, H.Y.; Lucas, M.L.; Teodósio da Ros, C.; Ary Vargas Souto, C. Prevalence of Peyronie’s disease in men over 50-y-old from Southern Brazil. Int. J. Impot. Res. 2001, 13, 291–293. [Google Scholar] [CrossRef]
- Shiraishi, K.; Shimabukuro, T.; Matsuyama, H. The prevalence of Peyronie’s disease in Japan: A study in men undergoing maintenance hemodialysis and routine health checks. J. Sex. Med. 2012, 9, 2716–2723. [Google Scholar] [CrossRef]
- Wong, A.; Tsang, S.S.; Ray, Y.M.O.; Chun, S.; Tsang, C.-F.; Ho, B.S.; Ng, A.T.; Tsu, J.H.; Lam, W. Mp33-12 Prevalence of Peyronie’s disease and its psychosexual impact in the chinese population: A large cohort population-based cross-sectional study. J. Urol. 2020, 203, e499. [Google Scholar]
- Kyei, M.Y.; Mensah, J.E.; Asante, E.; Bray, L.D.; Awuku-Asabre, J. Peyronie’s Disease in People of African Origin: A Mini Review. J. Gerontol. Aging Res. 2017, 1, 104. Available online: https://www.researchgate.net/publication/327751134_Peyronie%27s_Disease_in_People_of_African_origin_A._Mini_Review (accessed on 16 March 2024).
- Lindsay, M.B.; Schain, D.M.; Grambsch, P.; Benson, R.C.; Beard, C.M.; Kurland, L.T. The incidence of Peyronie’s disease in Rochester, Minnesota, 1950 through 1984. J. Urol. 1991, 146, 1007–1009. [Google Scholar] [CrossRef]
- Tefekli, A.; Kandirali, E.; Erol, H.; Alp, T.; Köksal, T.; Kadioğlu, A. Peyronie’s disease in men under age 40: Characteristics and outcome. Int. J. Impot. Res. 2001, 13, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.A.; Estrada, C.R.; Storm, D.W.; Matkov, T.G. Peyronie disease in younger men: Characteristics and treatment results. J. Androl. 2003, 24, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Deveci, S.; Hopps, C.V.; O’Brien, K.; Parker, M.; Guhring, P.; Mulhall, J.P. Defining the clinical characteristics of Peyronie’s disease in young men. J. Sex. Med. 2007, 4, 485–490. [Google Scholar] [CrossRef]
- Cilio, S.; Fallara, G.; Capogrosso, P.; Candela, L.; Belladelli, F.; Pozzi, E.; Corsini, C.; Raffo, M.; Schifano, N.; Boeri, L.; et al. The symptomatic burden of Peyronie’s disease at presentation according to patient age: A critical analysis of the Peyronie’s disease questionnaire (PDQ) domains. Andrology 2023, 11, 501–507. [Google Scholar] [CrossRef]
- Paulis, G.; Cavallini, G.; Barletta, D.; Turchi, P.; Vitarelli, A.; Fabiani, A. Clinical and epidemiological characteristics of young patients with Peyronie’s disease: A retrospective study. Res. Rep. Urol. 2015, 7, 107–111. [Google Scholar]
- Hellstrom, W.J.; Bivalacqua, T.J. Peyronie’s disease: Etiology, medical, and surgical therapy. J. Androl. 2000, 21, 347–354. [Google Scholar] [CrossRef]
- Weidner, W.; Schroeder-Printzen, I.; Weiske, W.H.; Vosshenrich, R. Sexual dysfunction in Peyronie’s disease: An analysis of 222 patients without previous local plaque therapy. J. Urol. 1997, 157, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Paulis, G.; Romano, G.; Paulis, A. Prevalence, psychological impact, and risk factors of erectile dysfunction in patients with Peyronie’s disease: A retrospective analysis of 309 cases. Res. Rep. Urol. 2016, 8, 95–103. [Google Scholar]
- Nelson, C.J.; Diblasio, C.; Kendirci, M.; Hellstrom, W.; Guhring, P.; Mulhall, J.P. The Chronology of Depression and Distress in Men with Peyronie’s Disease. J. Sex. Med. 2008, 5, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Kuja-Halkola, R.; Henningsohn, L.; D’Onofrio, B.M.; Mills, J.; Adolfsson, A.; Larsson, H.; Cederlöf, M. Mental Disorders in Peyronie’s Disease: A Swedish Cohort Study of 3.5 Million Men. J. Urol. 2021, 205, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Devine, C.J., Jr.; Somers, K.D.; Ladaga, L.E. Peyronie’s disease: Pathophysiology. Prog. Clin. Biol. Res. 1991, 370, 355–358. [Google Scholar] [PubMed]
- Devine, C.J.J.; Somers, K.D.; Jordan, G.H.; Schlossberg, S.M. Proposal: Trauma as a cause of Peyronie’s lesion. J. Urol. 1997, 157, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Jarow, J.P.; Lowe, F.C. Penile trauma: An etiologic factor in Peyronie’s disease and erectile dysfunction. J. Urol. 1997, 158, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- El-Sakka, A.I.; Salabas, E.; Dinçer, M.; Kadioglu, A. The pathophysiology of Peyronie’s disease. Arab J. Urol. 2013, 11, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Paulis, G.; Romano, G.; Paulis, L.; Barletta, D. Recent Pathophysiological Aspects of Peyronie’s Disease: Role of Free Radicals, Rationale, and Therapeutic Implications for Antioxidant Treatment-Literature Review. Adv. Urol. 2017, 2017, 4653512. [Google Scholar] [CrossRef]
- Paulis, G.; De Giorgio, G.; Paulis, L. Role of Oxidative Stress in Peyronie’s Disease: Biochemical Evidence and Experiences of Treatment with Antioxidants. Int. J. Mol. Sci. 2022, 23, 15969. [Google Scholar] [CrossRef]
- Sikka, S.C.; Hellstrom, W.J. Role of oxidative stress and antioxidants in Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 353–360. [Google Scholar] [CrossRef]
- Paulis, G.; Brancato, T. Inflammatory mechanisms and oxidative stress in Peyronie’s disease: Therapeutic “rationale” and related emerging treatment strategies. Inflamm. Allergy Drug Targets 2012, 11, 48–57. [Google Scholar] [CrossRef]
- Davila, H.H.; Magee, T.R.; Vernet, D.; Rajfer, J.; Gonzalez-Cadavid, N.F. Gene transfer of inducible nitric oxide synthase complementary DNA regresses the fibrotic plaque in an animal model of Peyronie’s disease. Biol. Reprod. 2004, 71, 1568–1577. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Champion, H.C.; Hellstrom, W.J. Implications of nitric oxide synthase isoforms in the pathophysiology of Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cadavid, N.F.; Magee, T.R.; Ferrini, M.; Qian, A.; Vernet, D.; Rajfer, J. Gene expression in Peyronie’s disease. Int. J. Impot. Res. 2002, 14, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Kelâmi, A. Autophotography in evaluation of functional penile disorders. Urology 1983, 21, 628–629. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.F.; Dean, R.C. Diagnostic utility of penile ultrasound in Peyronie’s disease. World. J. Urol. 2020, 38, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Masterson, J.M.; Masterson, T.A., 3rd. The role of imaging in the diagnosis and management of Peyronie’s disease. Curr. Opin. Urol. 2020, 30, 283–289. [Google Scholar] [CrossRef]
- Lahme, S.; Zimmermanns, V.; Liske, P.; Ober, P. Real-Time Elastography (RTE) in Patients with Peyronie’s Disease: First Results of a New Imaging Technique for the Detection and Mesurement of Plaques. J. Urol. 2009, 181, 280. [Google Scholar] [CrossRef]
- Morana, C.; Loiero, G.; Sangiorgio, A.; Zani, T.; Catalano, G. Elastosonography in the Peyronie’s disease: Our preliminary experience. Arch. Ital. Urol. Androl. 2010, 82, 269–270. [Google Scholar]
- Riversi, V.; Tallis, V.; Trovatelli, S.; Belba, A.; Volterrani, L.; Iacoponi, F.; Ponchietti, R. Realtime-elastosonography of the penis in patients with Peyronie’s disease. Arch. Ital. Urol. Androl. 2012, 84, 174–177. [Google Scholar]
- Zhang, X.; Zhou, B.; Miranda, A.F.; Trost, L.W. A Novel Noninvasive Ultrasound Vibro-elastography Technique for Assessing Patients with Erectile Dysfunction and Peyronie Disease. Urology 2018, 116, 99–105. [Google Scholar] [CrossRef]
- Trama, F.; Riccardo, F.; Ruffo, A.; Celentano, G.; Romeo, G.; Russo, A. Elastosonographic Changes in Patients with Peyronie’s Disease, before and after Treatment with a Compound Based on Ecklonia bicyclis, Tribulus terrestris, and Water-Soluble Chitosan. OJU J. 2018, 8, 77–87. Available online: https://www.scirp.org/pdf/OJU_2018032914374831.pdf (accessed on 16 March 2024). [CrossRef]
- Tyloch, J.F.; Tyloch, D.J.; Adamowicz, J.; Warsiński, P.; Ostrowski, A.; Nowikiewicz, M.; Drewa, T. Application of three-dimensional ultrasonography (3D ultrasound) to pretreatment evaluation of plastic induration of the penis (Peyronie’s disease). Med. Ultrason. 2020, 22, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Trama, F.; Illiano, E.; Iacono, F.; Ruffo, A.; di Lauro, G.; Aveta, A.; Crocetto, F.; Manfredi, C.; Costantini, E. Use of penile shear wave elastosonography for the diagnosis of Peyronie’s Disease: A prospective case-control study. Basic Clin. Androl. 2022, 32, 15. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, X.; Zhang, Y.; Zhang, C. Role of Shear Wave Elastography in the Diagnosis of Peyronie Disease. J. Ultrasound Med. 2024, 43, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Goldenberg, E.; Pek, H.; Gilbert, B.R. Penile sonoelastography for the localization of a non-palpable, non-sonographically visualized lesion in a patient with penile curvature from Peyronie’s disease. J. Sex. Med. 2014, 11, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Dhok, A.; Phatak, S.; Mitra, K.; Ansari, A. Peyronie’s Disease Presenting as Curvature of the Penis: A Case Report. Cureus 2022, 14, e32055. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Dudea, S.M.; Crisan, N.; Stanca, V.D.; Dudea-Simon, M.; Andras, I.; Mihaly, Z.A.; Coman, I. Elastography in the Urological Practice: Urinary and Male Genital Tract, Prostate Excluded-Review. Diagnostics 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed]
- Ophir, J.; Mehta, D. Elimination of diffraction error in acoustic attenuation estimation via axial beam translation. Ultrason. Imaging 1988, 10, 139–152. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar]
- Cui, X.W.; Li, K.N.; Yi, A.J.; Wang, B.; Wei, Q.; Wu, G.G.; Dietrich, C.F. Ultrasound elastography. Endosc. Ultrasound 2022, 11, 252–274. [Google Scholar] [CrossRef]
- Kwon, S.J.; Jeong, M.K. Advances in ultrasound elasticity imaging. Biomed. Eng. Lett. 2017, 7, 71–79. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Arndt, R.; Schmidt, S.; Loddenkemper, C.; Grünbaum, M.; Zidek, W.; van der Giet, M.; Westhoff, T.H. Noninvasive evaluation of renal allograft fibrosis by transient elastography—A pilot study. Transpl. Int. 2010, 23, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Sommerer, C.; Scharf, M.; Seitz, C.; Millonig, G.; Seitz, H.K.; Zeier, M.; Mueller, S. Assessment of renal allograft fibrosis by transient elastography. Transpl. Int. 2013, 26, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lukenda, V.; Mikolasevic, I.; Racki, S.; Jelic, I.; Stimac, D.; Orlic, L. Transient elastography: A new noninvasive diagnostic tool for assessment of chronic allograft nephropathy. Int. Urol. Nephrol. 2014, 46, 1435–1440. [Google Scholar] [CrossRef]
- Lee, Y.J. Shear Wave Elastography: A Reliable and Outperforming Diagnostic Tool for Liver Fibrosis Assessment in Chronic Hepatitis. A Literature Review. Available online: https://www.konicaminolta.jp/healthcare/products/us/aixplorer/pdf/whitepaper_liver-fibrosis-literature-review_eng.pdf (accessed on 16 March 2024).
- Dong, Z.; Kim, J.; Huang, C.; Lowerison, M.R.; Lok, U.W.; Chen, S.; Song, P. Three-Dimensional Shear Wave Elastography Using a 2D Row Column Addressing (RCA) Array. BME Front. 2022, 2022, 9879632. [Google Scholar] [CrossRef]
- Cantisani, V.; David, E.; Grazhdani, H.; Rubini, A.; Radzina, M.; Dietrich, C.F.; Durante, C.; Lamartina, L.; Grani, G.; Valeria, A.; et al. Prospective Evaluation of Semiquantitative Strain Ratio and Quantitative 2D Ultrasound Shear Wave Elastography (SWE) in Association with TIRADS Classification for Thyroid Nodule Characterization. Ultraschall Med. 2019, 40, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Veyrieres, J.B.; Albarel, F.; Lombard, J.V.; Berbis, J.; Sebag, F.; Oliver, C.; Petit, P. A threshold value in Shear Wave elastography to rule out malignant thyroid nodules: A reality? Eur. J. Radiol. 2012, 81, 3965–3972. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Bercoff, J.; Athanasiou, A.; Deffieux, T.; Gennisson, J.L.; Montaldo, G.; Muller, M.; Tardivon, A.; Fink, M. Quantitative assessment of breast lesion viscoelasticity: Initial clinical results using supersonic shear imaging. Ultrasound Med. Biol. 2008, 34, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Rivaz, H.; Rohling, R. A hand-held probe for vibro-elastography. Med. Image Comput. Comput. Assist. Interv. 2005, 8, 613–620. [Google Scholar]
- Fatemi, M.; Greenleaf, J.F. Ultrasound-stimulated vibro-acoustic spectrography. Science 1998, 280, 82–85. [Google Scholar] [CrossRef]
- Mitri, F.G.; Davis, B.J.; Urban, M.W.; Alizad, A.; Greenleaf, J.F.; Lischer, G.H.; Wilson, T.M.; Fatemi, M. Vibro-acoustography imaging of permanent prostate brachytherapy seeds in an excised human prostate–preliminary results and technical feasibility. Ultrasonics 2009, 49, 389–394. [Google Scholar] [CrossRef]
- Urban, M.W.; Chalek, C.; Kinnick, R.R.; Kinter, T.M.; Haider, B.; Greenleaf, J.F.; Thomenius, K.E.; Fatemi, M. Implementation of vibro-acoustography on a clinical ultrasound system. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1169–1181. [Google Scholar] [CrossRef]
- Alizad, A.; Whaley, D.H.; Urban, M.W.; Carter, R.E.; Kinnick, R.R.; Greenleaf, J.F.; Fatemi, M. Breast vibro-acoustography: Initial results show promise. Breast Cancer Res. 2012, 14, R128. [Google Scholar] [CrossRef]
- Nelson, C.J.; Mulhall, J.P. Psychological impact of Peyronie’s disease: A review. J. Sex. Med. 2013, 10, 653–660. [Google Scholar] [CrossRef]
- Chung, E.; Clendinning, E.; Lessard, L.; Brock, G. Five-year follow-up of Peyronie’s graft surgery: Outcomes and patient satisfaction. J. Sex. Med. 2011, 8, 594–600. [Google Scholar] [CrossRef]
- Bercoff, J.; Chaffai, S.; Tanter, M.; Sandrin, L.; Catheline, S.; Fink, M.; Gennisson, J.L.; Meunier, M. In vivo breast tumor detection using transient elastography. Ultrasound Med. Biol. 2003, 29, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Adhoute, X.; Foucher, J.; Laharie, D.; Terrebonne, E.; Vergniol, J.; Castéra, L.; Lovato, B.; Chanteloup, E.; Merrouche, W.; Couzigou, P.; et al. Diagnosis of liver fibrosis using FibroScan and other noninvasive methods in patients with hemochromatosis: A prospective study. Gastroenterol. Clin. Biol. 2008, 32, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Nguyen, M.; Ju, I.; Brancatisano, A.; Ryan, B.; van der Poorten, D. Utility of Fibroscan XL to assess the severity of non-alcoholic fatty liver disease in patients undergoing bariatric surgery. Sci. Rep. 2021, 11, 14006. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Tanter, M.; Catheline, S.; Fink, M. Shear modulus imaging with 2-D transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Bercoff, J.; Sandrin, L.; Fink, M. Ultrafast compound imaging for 2-D motion vector estimation: Application to transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Gallotti, A.; Mucelli, R.P. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am. J. Roentgenol. 2010, 195, 132–136. [Google Scholar] [CrossRef]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghina, A.M.; Peck-Radosavljevic, M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Tinelli, C.; Lissandrin, R.; Zicchetti, M.; Bernuzzi, S.; Salvaneschi, L.; Filice, C. Elastography Study Group Ultrasound point shear wave elastography assessment of liver and spleen stiffness: Effect of training on repeatability of measurements. Eur. Radiol. 2014, 24, 1283–1289. [Google Scholar] [CrossRef]
- Ferraioli, G.; Parekh, P.; Levitov, A.B.; Filice, C. Shear wave elastography for evaluation of liver fibrosis. J. Ultrasound Med. 2014, 33, 197–203. [Google Scholar] [CrossRef]
- Cassinotto, C.; Lapuyade, B.; Mouries, A.; Hiriart, J.B.; Vergniol, J.; Gaye, D.; Castain, C.; Le Bail, B.; Chermak, F.; Foucher, J.; et al. Non-invasive assessment of liver fibrosis with impulse elastography: Comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 2014, 61, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Andresen, R.; Wegner, H.E.; Miller, K.; Banzer, D. Imaging modalities in Peyronie’s disease. An intrapersonal comparison of ultrasound sonography, X-ray in mammography technique, computerized tomography, and nuclear magnetic resonance in 20 patients. Eur. Urol. 1998, 34, 128–135. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulis, G.; De Giorgio, G.; Paulis, A. Ultrasound Elastography as a Diagnostic Tool for Peyronie’s Disease: A State-of-the-Art Review. Diagnostics 2024, 14, 665. https://doi.org/10.3390/diagnostics14060665
Paulis G, De Giorgio G, Paulis A. Ultrasound Elastography as a Diagnostic Tool for Peyronie’s Disease: A State-of-the-Art Review. Diagnostics. 2024; 14(6):665. https://doi.org/10.3390/diagnostics14060665
Chicago/Turabian StylePaulis, Gianni, Giovanni De Giorgio, and Andrea Paulis. 2024. "Ultrasound Elastography as a Diagnostic Tool for Peyronie’s Disease: A State-of-the-Art Review" Diagnostics 14, no. 6: 665. https://doi.org/10.3390/diagnostics14060665
APA StylePaulis, G., De Giorgio, G., & Paulis, A. (2024). Ultrasound Elastography as a Diagnostic Tool for Peyronie’s Disease: A State-of-the-Art Review. Diagnostics, 14(6), 665. https://doi.org/10.3390/diagnostics14060665





