Abstract
Background: Each year, millions of Americans develop truncal pressure ulcers (PUs) which can persist for months, years, or until the end of life. Despite the negative impact on quality of life and escalating costs associated with PUs, there is sparse evidence supporting validated and efficacious treatment options. As a result, treatment is based on opinion and extrapolation from other wound etiologies. The ideal reconstructive plan maximizes the patient’s nutritional status, incorporates the basic tenets of wound bed preparation (debridement, offloading, proper moisture balance, reduction of bacterial burden), and employs diagnostics to guide therapeutic intervention. The use of combination therapies can potentially overcome several of the barriers to wound healing. Negative pressure wound therapy (NPWT), a commonly used modality in the management of PUs, facilitates healing by stimulating the formation of granulation tissue and promoting wound contraction; however, NPWT alone is not always effective. Clinical studies examining microbial bioburden in PUs determined that most ulcers contain bacteria at levels that impede wound healing (>104 CFU/g). Objective: Thus, we hypothesized that adding an anti-microbial agent to decrease both planktonic and biofilm bacteria in the wound would increase the efficacy of NPWT. Method: In this prospective study, twenty patients with recalcitrant PUs that previously failed NPWT were treated with a biofilm-disrupting agent (Blast-X, Next Science, Jacksonville, FL, USA) in combination with NPWT. Fluorescence imaging was used to follow bacterial burden and guide therapy. Results: In total, 45% of the PUs reduced in size over the course of the four-week study, with a resolution of bacterial fluorescence in the NPWT dressing and wound bed seen in an average of three weeks. Conclusion: The combination of an antibiofilm agent and NPWT reduced bacterial levels and improved wound healing in recalcitrant PUs.
1. Introduction
Pressure ulcers (PUs) afflict millions of Americans. The incidence rate of PUs ranges from 4 to 38% within hospital settings, with a 68% mortality rate in the elderly attributable to PUs and associated secondary complications [1]. According to a study from the US Wound Registry, less than 40% of pressure ulcers are healed at three months and the average reduction in surface area over a four-week period is less than 10% [2]. Truncal ulcers are not amendable to self-care, requiring the assistance of family members and home health nurses. In addition to the stress on patients and family members, PUs burden the health care system. In 2020, the cost of caring for PUs in the United States was approximately USD 11 billion [1].
Despite the human and financial toll inflicted by this debilitating disease, there is limited quality research on treatment [3]. Most treatment plans are founded on expert opinion due to the lack of clinical data. In addition, wound care practice has favored single therapies that are either continued or discontinued based on wound improvement. Although episodes of care in which a reconstructive plan is outlined for the entire course of therapy have been discussed, there is little supporting evidence for their implementation. The authors contend that combination therapies instituted within episodes of care that are driven by relevant diagnostics, rather than separate intervals of single therapy, may improve PU healing rates.
Most truncal PUs develop in patients with multiple comorbidities and chronic diseases [4]. Numerous systemic and local factors contribute to poor wound healing in this population [4]. Failure to address any one of these factors can result in a nonhealing wound. The ideal reconstructive plan maximizes the patient’s nutritional status, incorporates the basic tenets of wound bed preparation (debridement, offloading, proper moisture balance, reduction of bacterial burden), and employs diagnostics to guide therapeutic interventions [5].
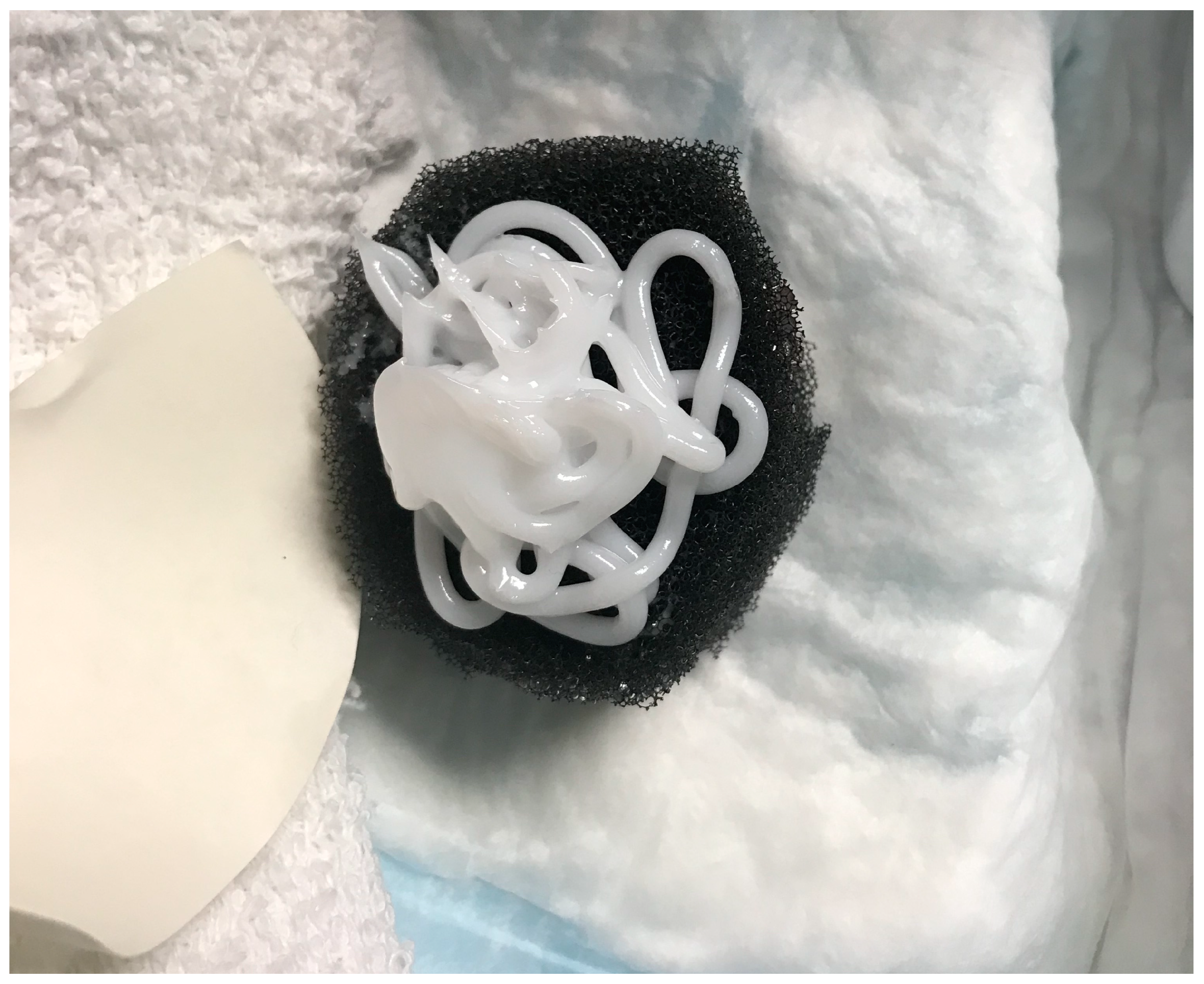
The presence of large amounts of bacteria (>104 CFU/g) inhibits wound healing [6]. In a recent clinical trial (FLAAG), nearly 91% (20/22) of PUs had bacterial loads greater than 104 CFU/g [6]. In chronic wounds, bacteria favor a biofilm phenotype [7]. Biofilm bacteria attach to the wound surface, aggregate, and produce a film: the extapolymeric substance (EPS), which protects them from antiseptics and antibiotics [8]. It has been shown that disrupting the biofilm promotes healing in acute and chronic wounds [9]. The key to a biofilm-based approach to wound care includes aggressive debridement and the use of a biofilm-disrupting topical agent [10,11]. In this trial, investigators sharply debrided the wounds weekly and applied a biofilm-disrupting polyethylene glycol-based hydrogel (BlastX, Next Science, Jacksonville, FL, USA) with every negative pressure wound therapy dressing change. The gel contains a pH buffer system and a benzalkonium chloride surfactant that destabilizes the biofilm EPS and subsequently aids in killing the exposed bacteria [12]. The white gel was placed on a negative pressure sponge, as shown in Figure 1.
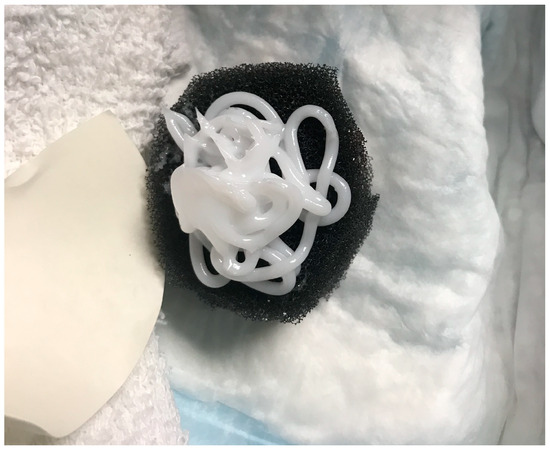
Figure 1.
Biofilm-disrupting gel on negative pressure sponge dressing.
2. Materials and Methods
This study is a continuation of the parent study: “Evaluation of the combination of a biofilm-disrupting agent and negative pressure wound therapy: a case series” (9 August 2023, clinicaltrials.gov #NCT04265170) [13]. Patient demographics and results from the parent study (patients 1–10 in Table 1) were included. Doubling the sample size through the inclusion of patients from the parent study allowed for more robust analysis and refining of the primary and secondary endpoints. The quantitative secondary analysis “allows for the generation of new knowledge without the costs of administration and implementation of additional data collection and maximizes the output of large-scale studies” [14]. For detailed materials, methods, inclusion, and exclusion criteria, see the parent study in the Journal of Wound Care [13].

Table 1.
Patient demographics.
3. Results
Table 1 provides demographics for patients in both groups. The difference between each cohort is the withdrawal rate and trial length. Group 1 had a withdrawal rate of 40% (4/10) and group 2 had a withdrawal rate of 10% (1/10). Group 1 also completed the study on treatment day 30 while group 2 completed the study on treatment day 28. Therefore, all results for treatment day 28 are derived from group 2 and all results for treatment day 30 are derived from group 1. The study enrolled nine male (45%), ten female (50%), and one patient without gender information (5%). The age of the enrolled patients ranged from 22 years old to 82 years old, with a mean age of 65.5 years old. Group 2 had twice as many patients with less than 20 percent area reduction (PAR) of the wound compared to group 1. Group 1 had four patients with a PAR greater than 20% and two patients with a PAR less than 20%. Group 2 had five patients with a PAR greater than 20% and four patients with a PAR less than 20%. There were nine patients between both groups (45%) with PAR greater than 20%.
For the analysis, responders were classified as patients who saw a percentage area reduction greater than 20% at the end of the four-week study. Non-responders were classified as patients who did not see a percentage area reduction greater than 20% at the end of the four-week study. The overall group was classified as both responders and non-responders at the end of the four-week study. Since the length of observation was not equal for both groups (group 1 ended on treatment day 30 and group 2 ended on treatment day 28), the results for treatment days 28 and 30 were not plotted.
- Primary Endpoint (wound size):
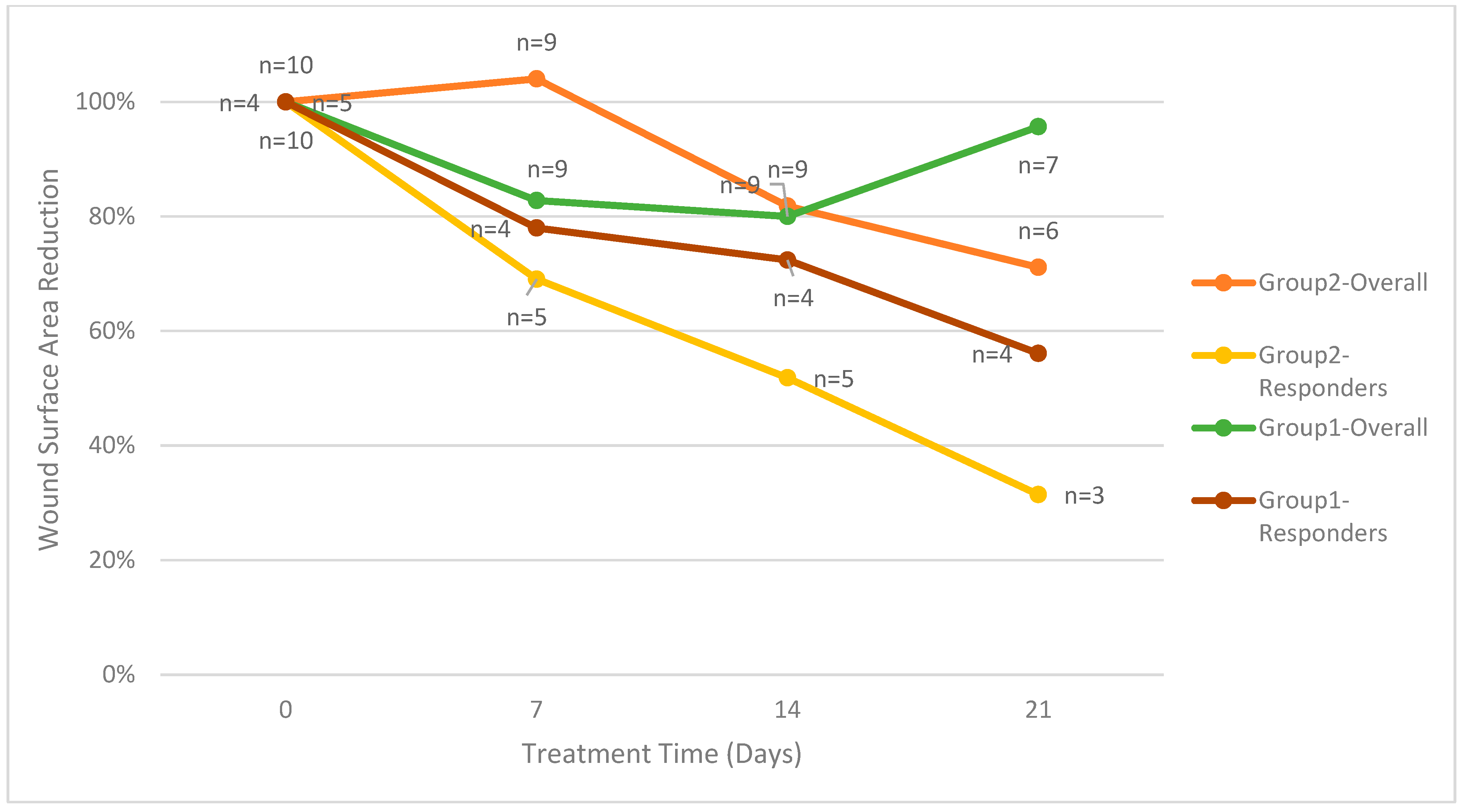
The primary endpoint is the reduction in wound surface area, with the first group evaluated through day 30 and the second group evaluated through day 28. For the ten patients enrolled in group 2, four patients (40%) had a PAR greater than 20% at four weeks. One patient in group 2 who continued treatment through day 49 had a PAR greater than 20%. As shown in Figure 2, the five patients in group 2 with a PAR greater than 20% had an average percent wound area reduction of 74%. For group 1, the four patients with a PAR greater than 20% had an average percent wound area reduction of 49%. On treatment day 28, the average percent wound area reduction for all patients in group 2 was 29%. On treatment day 30, the average percent wound area reduction for all patients in group 1 was 34%.
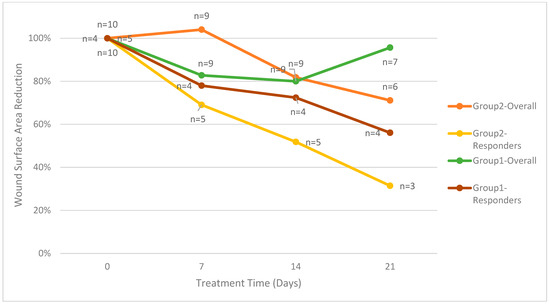
Figure 2.
Mean wound surface area reduction divided by groups.
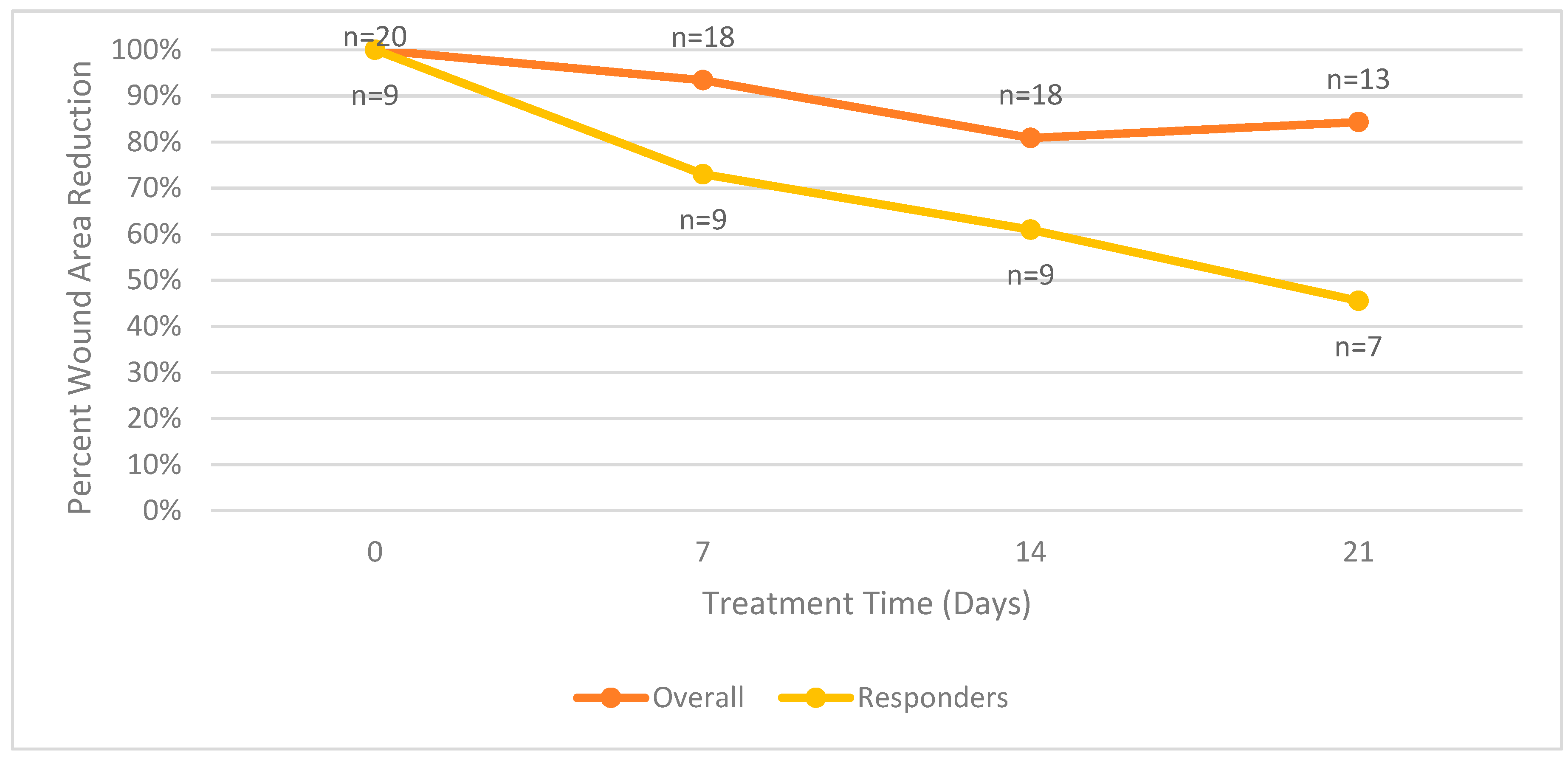
Figure 3 summarizes the mean surface area wound reduction for all patients in the trial. The mean surface area wound reduction for both groups combined is equal to the values reported in Figure 3.
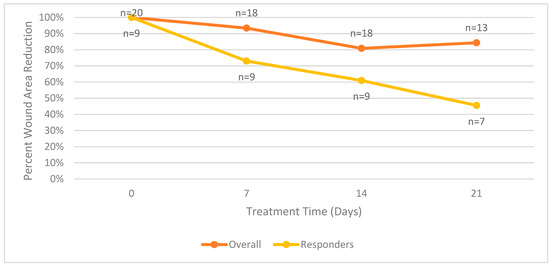
Figure 3.
Mean wound surface area reduction comparison between overall and responder groups.
- Secondary Endpoints:
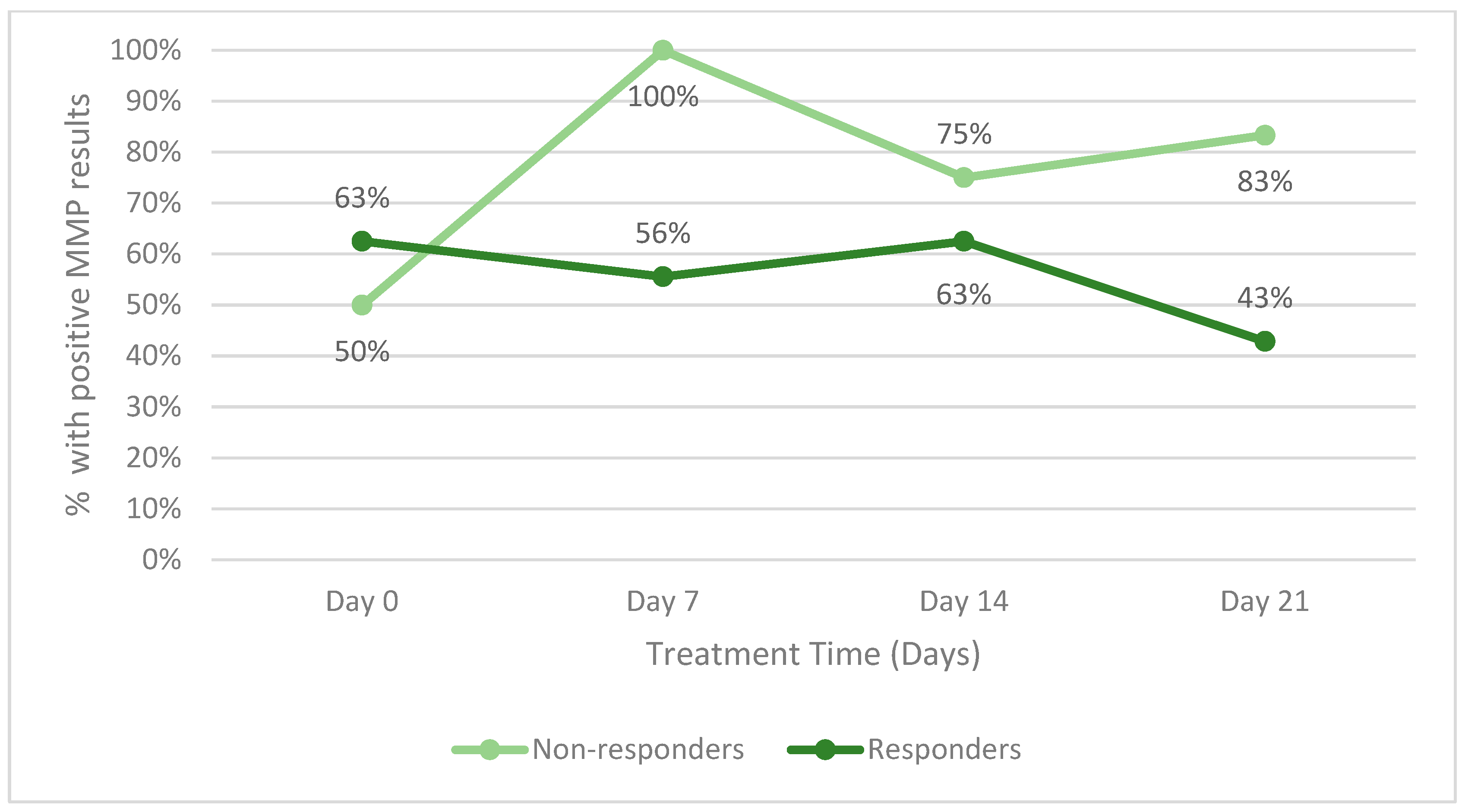
The secondary endpoint of the study was to identify changes in host proteases during therapy. Figure 4 illustrates the change in host matrix metalloprotease activity for all responders between the two groups and all patients (including those who did not respond). Patients who withdrew from the study in either group were not included. Throughout the study, a lower percentage of responders had positive MMP results than non-responders. Figure 5 is an example of the reduction in bacterial fluorescence following treatment with the biofilm disrupting agent.
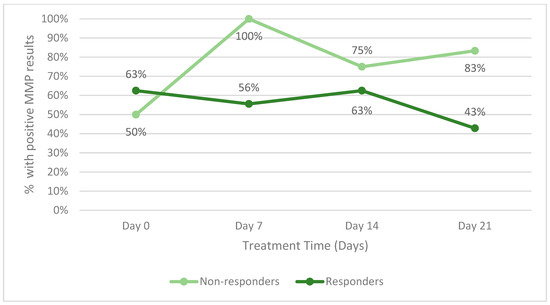
Figure 4.
Percentage of patients with positive host matrix metalloprotease (MMP) activity within.
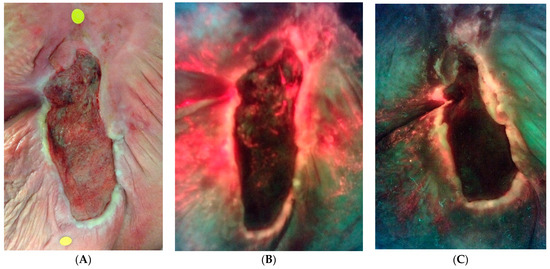
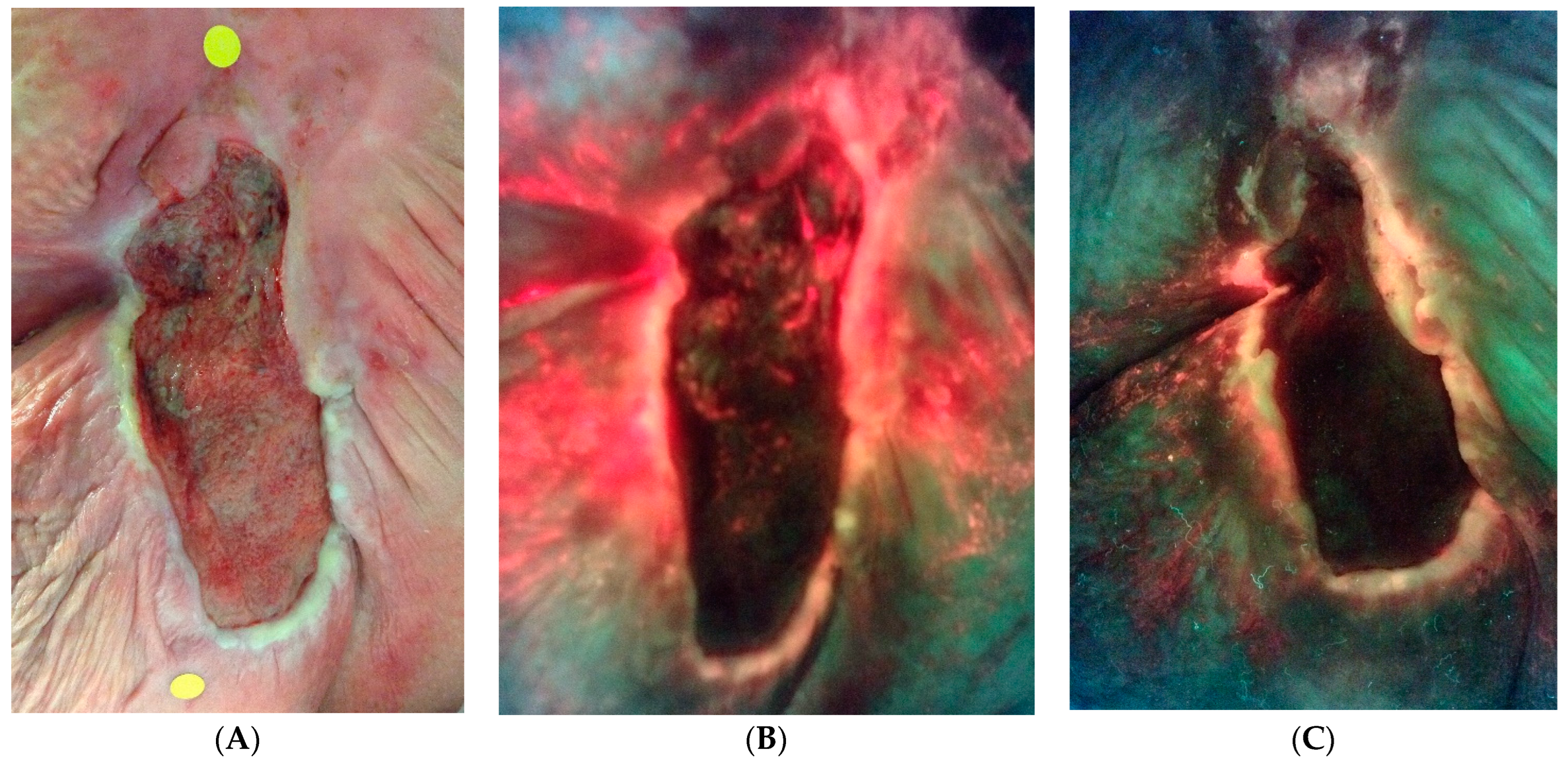
Figure 5.
An example of bacterial fluorescence at the time of enrollment after failed negative pressure. (A) Standard image. (B) Fluorescence image with extensive bacterial fluorescence. (C) Reduction in ulcer surface area and marked reduction in bacterial fluorescence at 4 weeks.
4. Discussion
Pressure ulcers afflict roughly three million people in the United States annually [15]. Despite the gravity of the problem, there is sparse evidence supporting any given treatment regimen. There is less evidence for using a combination of therapies throughout the course of treatment.
In the United States, more than 2 million patients develop pressure ulcers annually [16], and hospital-acquired pressure ulcers account for over 60,000 deaths [17]. Despite the personal and financial cost of treating pressure ulcers, there is a paucity of evidence supporting the regimens used in treating this wound type. Treatment, largely based on experience, opinion, and extrapolation of evidence from studies on other wound types, has resulted in a healing rate of less than 30% at 3 months and a percent area reduction of approximately 10% at 4 weeks [18]. It is estimated that the annual cost to treat pressure ulcers in the US is USD 11 billion [19].
Pressure ulcers are complex wounds and there are several underlying abnormalities that lead to poor wound healing [3]. The authors hypothesize that one factor contributing to the poor healing rates is the use of a single modality rather than combination therapy. Addressing several deficits at once might promote more rapid closure. In addition, the recent advent of point-of-care diagnostics such as fluorescence imaging allows clinicians to focus treatment on specific abnormalities in the nonhealing wound. In this study, point-of-care diagnostics were used to follow combination therapy in the treatment of recalcitrant pressure ulcers.
NPWT has become a mainstay in the treatment of pressure ulcers. The evidence for NPWT does not include large randomized clinical trials; however, a meta-analysis of 16 clinical trials with a total of 629 patients demonstrated that NPWT increased healing rates and more rapid time to healing compared to the standard of care [20]. Like most therapies in wound care, NPWT does not work in every patient. Recent studies using fluorescence imaging showed that pressure ulcers contain greater than the chronic inhibitory bacterial load (CIBL) in over 80% of wounds [21]. The results from this trial demonstrated increased bacterial burden, above CIBL, in all wounds that previously failed NPWT. In addition, the NPWT sponge dressings fluoresced brightly on the first dressing changes. These findings suggested that excessive bacteria inhibited healing in these recalcitrant wounds.
Aggressive debridement and the addition of an antibiofilm agent applied at every NPWT dressing change eliminated bacterial fluorescence in all cases. Novel antibiofilm agents represent the latest advance in topical antiseptics for nonhealing wounds. In most wounds, it took 3 to 4 weeks to eliminate the fluorescence. The reduction of bacterial burden below CIBL promoted wound healing. The average PAR was 45% in wounds that had failed to heal for months.
Several studies have evaluated therapies to improve the success of NPWT [22,23]. Instillation therapy with NPWT, iNPWT (Veraflow®, 3M, St. Paul, MN, USA), has gained popularity in hospitals across the US [24]. In this technique, normal saline or an antiseptic solution are instilled into the wound and allowed to dwell for 10 to 20 min, after which time sub-atmospheric pressure is reapplied to the wound. The cycle is repeated three to four times per day [25]. Research suggests that iNPWT promotes wound healing and decreases bacterial burden [22]; however, iNPWT has several disadvantages: inpatient monitoring is required; additional equipment is needed to administer the installation solution; it requires greater nursing time; and it adds cost to the use of NPWT [26]. The results from this study suggest that the use of antibiofilm agents in combination with NPWT may reduce the need for this expensive therapy. Further studies are needed to confirm this supposition.
Matrix metalloprotease and human neutrophil elastase are markers of inflammation. Studies suggest that reducing bacteria in the wound will result in a concomitant fall in inflammatory markers [27]. A fall in inflammatory markers in this study corresponds to a fall in bacterial burden and more rapid healing. Further research into the relationship between host proteases and bacterial levels is warranted.
5. Limitations
The primary limitation of this study is the small number of patients enrolled. This trial highlights some of the challenges in conducting pressure ulcer clinical trials: most patients in the study were immobile, several patients resided in nursing homes, and many potential patients were excluded due to their inability to properly offload the ulcer and secondary to malnutrition. Several potential patients did not want to try NPWT again after failing the therapy in the past. Although patient withdrawal and drop-out are to be expected, a withdrawal rate of 20% (5/20) between the two groups resulted in a sample size that was further reduced. Statistical analysis of primary and secondary endpoints was not possible due to these factors.
6. Conclusions
Aggressive debridement, application of antibiofilm agents directed by point-of-care fluorescence imaging in combination with NPWT may improve healing rates in pressure ulcers that have previously failed NPWT.
Author Contributions
T.E.S. and L.S., conceptualization of the clinical trial; methodology, all authors; validation, K.B., T.E.S. and M.F.M.; formal analysis, M.F.M.; investigation, L.S., K.B. and O.A.-J.; data curation, K.B.; writing—original draft preparation, T.E.S.; writing—review and editing, all authors; project administration, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an unrestricted grant from Next Science.
Institutional Review Board Statement
This protocol and informed consent were approved by the central IRB, WCG. IRB Approval Code: 20192645 Approval Date: 22 October 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data from the trial are included in the manuscript and available on request.
Conflicts of Interest
This research was sponsored by a grant from Next Science LLC. Matthew Myntti is the Chief Technology Officer for Next Science. The authors have no other conflicts of interest.
References
- Afzali Borojeny, L.; Albatineh, A.N.; Hasanpour Dehkordi, A.; Ghanei Gheshlagh, R. The Incidence of Pressure Ulcers and its Associations in Different Wards of the Hospital: A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2020, 11, 171. [Google Scholar]
- Sheehan, P.; Jones, P.; Caselli, A.; Giurini, J.M.; Veves, A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003, 26, 1879–1882. [Google Scholar] [CrossRef]
- Mervis, J.S.; Phillips, T.J. Pressure ulcers: Prevention and management. J. Am. Acad. Dermatol. 2019, 81, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Jaul, E.; Barron, J.; Rosenzweig, J.P.; Menczel, J. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr. 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.S.; Khoo, T.L.; Saad, A.Z. Wound bed preparation from a clinical perspective. Indian J. Plast. Surg. 2012, 45, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv. Wound Care 2021, 10, 123–136. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D. A study of biofilm-based wound management in subjects with critical limb ischaemia. J. Wound Care 2008, 17, 145–148+150–152+154–155. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C.; Ward, L.S.; James, G.A. Antibiofilm efficacy of a lactoferrin/xylitol wound hydrogel used in combination with silver wound dressings. Int. Wound J. 2011, 8, 268–273. [Google Scholar] [CrossRef]
- Werdin, F.; Tenenhaus, M.; Rennekampff, H.O. Chronic wound care. Lancet 2008, 372, 1860–1862. [Google Scholar] [CrossRef]
- Wolcott, R. Disrupting the biofilm matrix improves wound healing outcomes. J. Wound Care 2015, 24, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.G.; Tran, P.L.; Haley, C.L.; Kruzek, C.; Colmer-Hamood, J.A.; Myntti, M.; Hamood, A.N. Next science wound gel technology, a novel agent that inhibits biofilm development by gram-positive and gram-negative wound pathogens. Antimicrob. Agents Chemother. 2014, 58, 3060–3072. [Google Scholar] [CrossRef]
- Serena, T.E.; Jalodi, O.; Serena, L.; Patel, K.; Myntti, M. Evaluation of the combination of a biofilm-disrupting agent and negative pressure wound therapy: A case series. J. Wound Care 2021, 30, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ruggiano, N.; Perry, T.E. Conducting secondary analysis of qualitative data: Should we, can we, and how? Qual. Soc. Work. 2019, 18, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Preventing Pressure Ulcers in Hospitals. 2014. Available online: https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html (accessed on 1 June 2020).
- Reddy, M.; Gill, S.S.; Kalkar, S.R.; Wu, W.; Anderson, P.J.; Rochon, P.A. Treatment of pressure ulcers: A systematic review. JAMA 2008, 300, 2647–2662. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Rock, K.; Nazzal, M.; Jones, O.; Qu, W. Pressure ulcers in the United States’ inpatient population from 2008 to 2012: Results of a retrospective nationwide study. Ostomy Wound Manag. 2016, 62, 30–38. [Google Scholar]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Maggi, J.; Nierman, D.; Rolnitzky, L.; Bell, D.; Rennert, R.; Golinko, M.; Yan, A.; Lyder, C.; Vladeck, B. High cost of stage IV pressure ulcers. Am. J. Surg. 2010, 200, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Wang, L.; Yuan, B.F.; Shen, H.W.; Du, L.; Cai, J.Y.; Chen, H.L. Negative pressure wound therapy for III/IV pressure injuries: A meta-analysis. Wound Repair Regen. 2021, 29, 20–33. [Google Scholar] [CrossRef]
- Andersen, C.A.; McLeod, K.; Steffan, R.; Serena, T.E. Underappreciated bacterial burden in pressure ulcers: Clinical trial and real-world evidence. J. Wound Manag. 2023, 24, 80. [Google Scholar]
- Goss, S.G.; Schwartz, J.A.; Facchin, F.; Avdagic, E.; Gendics, C.; Lantis, J.C., 2nd. Negative Pressure Wound Therapy with Instillation (NPWTi) Reduces Post-debridement Bioburden in Chronically Infected Lower Extremity Wounds Than NPWT Alone. J. Am. Coll. Clin. Wound Spec. 2014, 4, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Shores, J.; Heinrich, C.; Baqai, W.; Kalina, S.; Sogioka, N.; Gupta, S. Negative pressure wound therapy with instillation: A pilot study describing a new method for treating infected wounds. Int. Wound J. 2008, 5, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.J.; Attinger, C.E.; Steinberg, J.S.; Evans, K.K.; Lehner, B.; Willy, C.; Lavery, L.; Wolvos, T.; Orgill, D.; Ennis, W.; et al. Negative-pressure wound therapy with instillation: International consensus guidelines. Plast. Reconstr. Surg. 2013, 132, 1569–1579. [Google Scholar] [CrossRef]
- Gupta, S.; Gabriel, A.; Lantis, J.; Téot, L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int. Wound J. 2016, 13, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, K.; Nagakumar, J.S.; Hongaiah, D. Negative Pressure Wound Therapy with Flap Reconstruction for Extensive Soft Tissue Loss in the Foot: A Case Report. Cureus 2020, 12, e10116. [Google Scholar] [CrossRef]
- Serena, T.E.; Cullen, B.M.; Bayliff, S.W.; Gibson, M.C.; Carter, M.J.; Chen, L.; Yaakov, R.A.; Samies, J.; Sabo, M.; DeMarco, D.; et al. Defining a New Diagnostic Assessment Parameter for Wound Care: Elevated Protease Activity, an Indicator of Nonhealing, for Targeted Protease-modulating Treatment. Wound Repair. Regen. 2016, 24, 589–595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).