Recent Technologies for Transcutaneous Oxygen and Carbon Dioxide Monitoring
Abstract
1. Introduction
2. Physiology
2.1. Heating
3. Overview of Available Techniques
3.1. Arterial Blood Gas Analysis
3.2. End-Tidal Monitoring
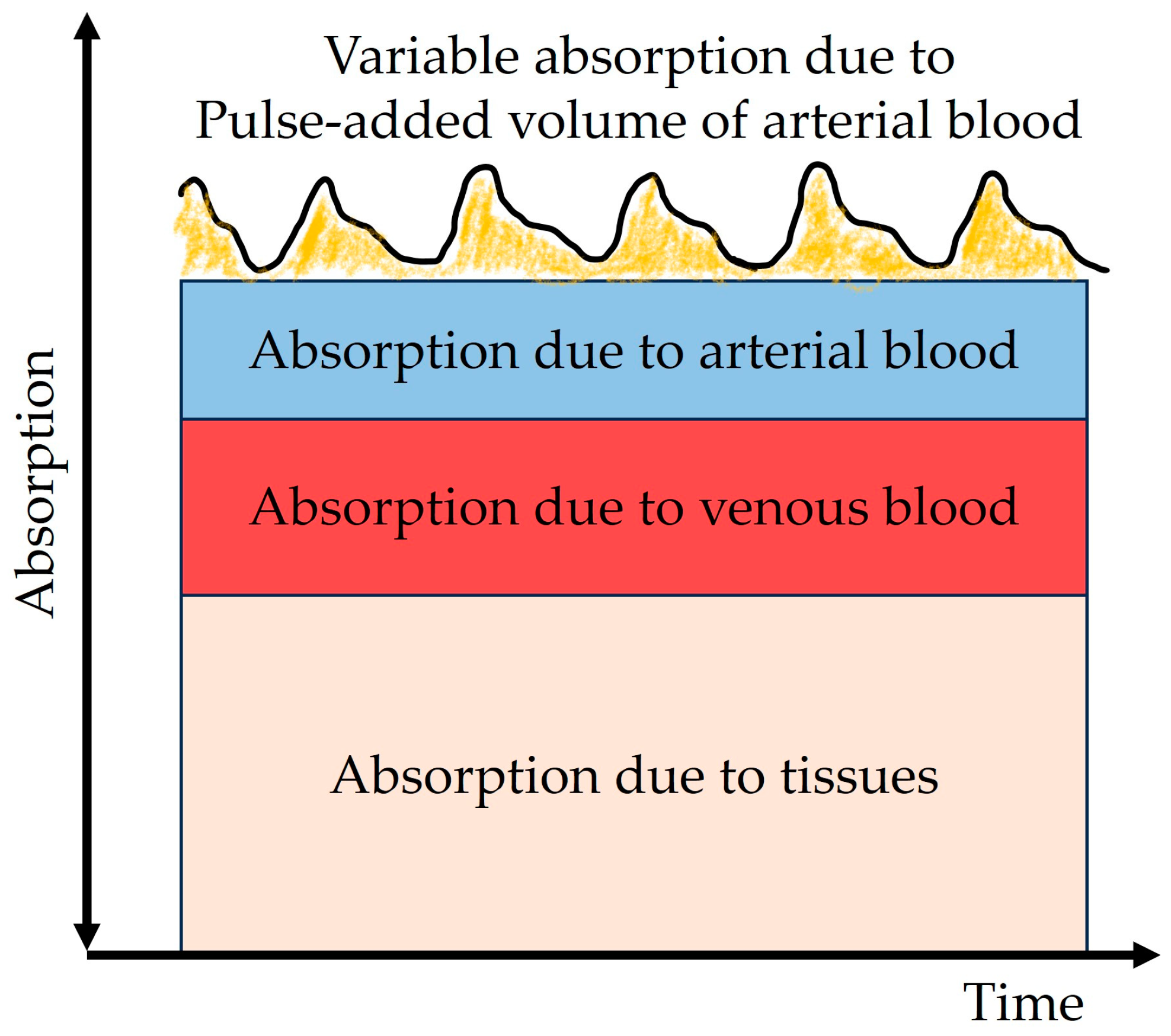
3.3. Pulse Oximetry
3.4. Transcutaneous Monitoring
- Non-invasive
- Continuous
- Fast readings
- Early recognition of poor tissue perfusion
- Early recognition of respiratory complications
- Estimation of systemic perfusion
- Decreased risk of operator error
- Not influenced by ventilation–perfusion disorders
- Possible combination for multiparametric sensors (for example pulse oximetry).
| Working Principle | Device | Technical Specification (Examples) | Advantages | Disadvantages | Future Development | Target Gas |
|---|---|---|---|---|---|---|
| Electrochemical | Clark’s electrode | Drift: 1–2 mmHg/h [30] Response time to 99%: 40 s (with polyethylene membrane, ref. [58]) | Accuracy | Heating Oxygen consumption Calibration | - | O2 |
| Severinghaus electrode | Sensitivity: linear (range 1.38–11.37% CO2) [58] Response time: 2 min after a rise in CO2, 4 min after a fall in CO2 [58] | Accuracy | Heating Oxygen consumption Calibration | - | CO2 | |
| ISFET [34,59] | Drift < 0.23 mV/h [59] Response time: <1 min [59], 60 s [34] Sensitivity dependence on temperature: 0.26 ± 0.1 mV/log(PCO2)°C [59] | Miniaturized | Drift of the reference electrode Temperature dependency Few hours or days of use | - | CO2 | |
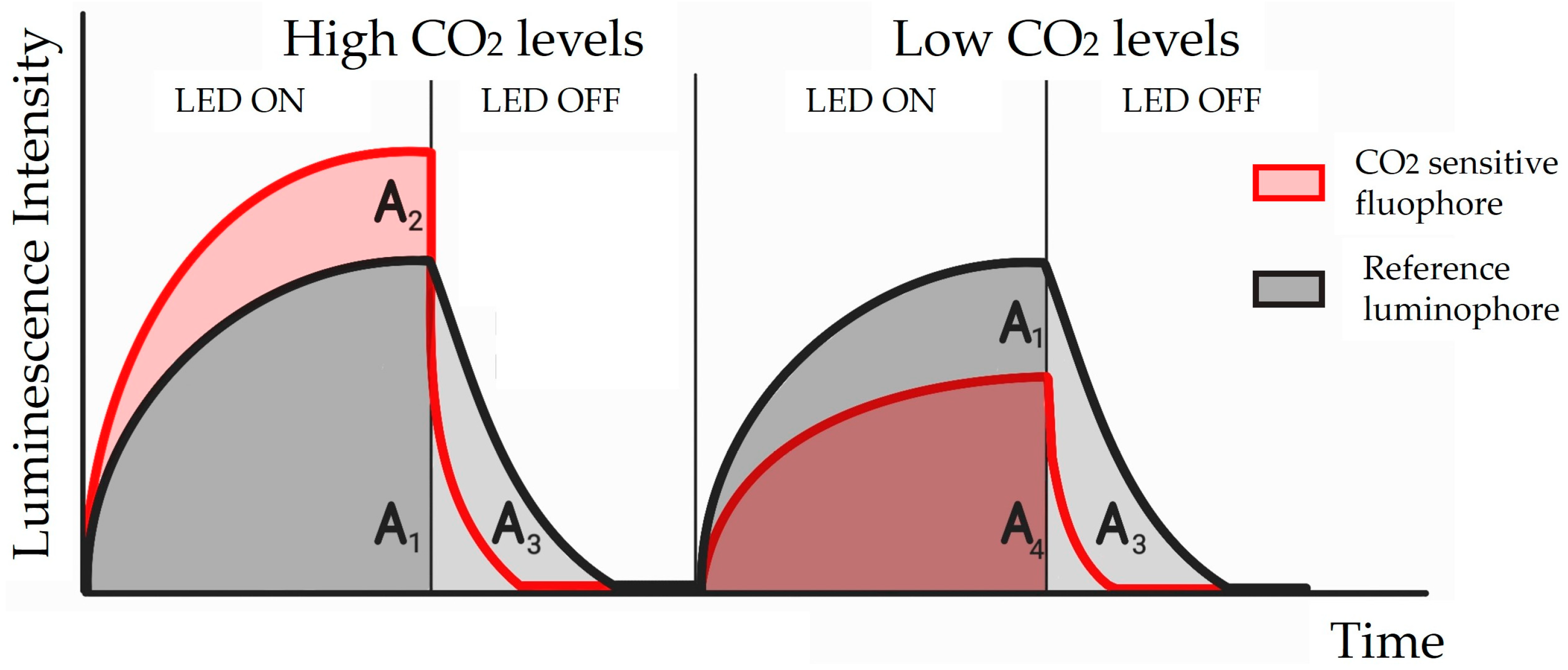
| Luminescence-based | Bandage-like sensor (2019) [60] | Response time: 1.5 faster respect to a commercially available tcpO2 sensor Sensitivity (=I0/I30): 1.61 Data fitting correlation coefficient (R2) = 0.9951 | Fast response Accurate Sensitive Wearable Flexible | Heating One hour of use | Increase time of use | O2 |
| Integrated readout circuit [18] | Measurement range: 0–150 mmHg of PO2 Power consumption (LED driver): 20 mW | No heating Low power consumption | Requires a stronger LED driver than PPG sensors | - | O2 | |
| Intensity- and lifetime-based sensor [61,62,63] | Measurement range: 0–160 mmHg of PO2 [64] Response time: 15 µs in room air (PO2 = 160 mmHg) Calibration time: 20/30 min [63] | No heating Real-time Insensitive to motion artifacts | Temperature-dependent Long calibration | - | O2 | |
| Fluorescent thin-film-based [3] | Measurement range: 0–75 mmHg PCO2 Power consumption of the circuit board: 64.33 mW Response time (depending on the PCO2 level): 6–20 min | Miniaturized No heating | Need a saline solution for stability | - | CO2 | |
| Dual lifetime referencing [65] | Measurement range: 0–75 mmHg CO2 Robustness against excitation length variation: luminescent ratio ∆% ∼1.6% across the PCO2 range Power: 541.25 mW | No heating No common noise | After 76 mmHg f-LDR becomes useless | Use a square wave for f-DLR technique Methods to compensate for the errors | CO2 | |
| Wearable prototype device [55] | Photostability: 120 min (under air conditions) Measurement range: 0–50 mmHg CO2 Model fitting: R2: 0.9808 Sensitivity: 0.13/mmHg | Reduced volume and thickness Photostable Biocompatible | Dependency on temperature | - | CO2 | |
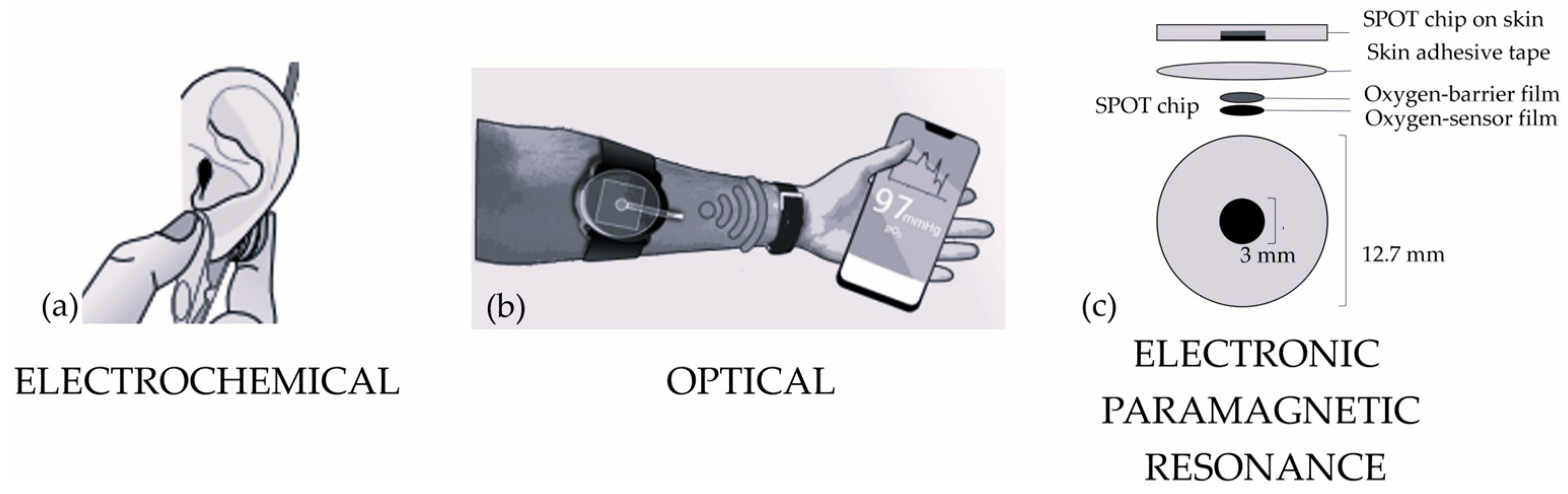
| Electronic Paramagnetic Resonance (EPR) | SPOT chip [7] | Sensitivity (mG/mmHg): 16.0–0.161 × T(C) Precision: SD = 5.5 mmHg Measurement range: 0–160 mmHg PO2 Reproducibility: 1 year | Robust No heating Highly sensitive Reproducible measurements | Validated on a small number of volunteers Temperature-dependent | More in vivo validations | O2 |
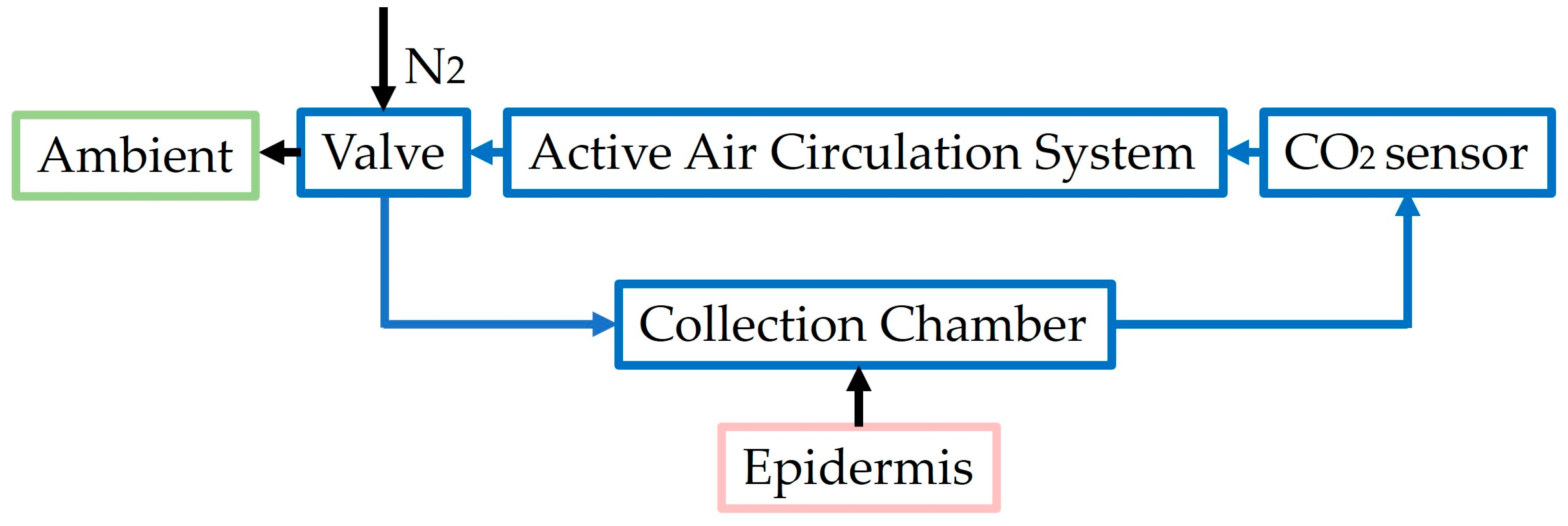
| Non-Dispersive Infra-Red (NDIR) | Photoreaction chamber with pyroelectric sensor [66] | Response time: <2 s Sensitivity: 4.3 mV/mm Measurement range: 1000–20,000 ppm of CO2 | Sensitive even at reduced dimensions Fast response time (<2 s) | Only in vitro Not developed yet to be applied on human skin | Include a heating wire to collect CO2 from the skin Include a vacuum pump | CO2 |
| Wearable CO2 monitor [40] | (Cozir® NDIR CO2 [67]) Response time: 30 s Accuracy: ±70 ppm Dynamic range: 0–5% Lifetime > 15 years | No heating Wearable Accurate Good longevity Low power consumption | - | Self-calibration Diffusion optimization Signal processing Algorithms | CO2 | |
| Prototype for a miniaturized monitor [43] | Measurement range: 0–120 mmHg PCO2 Response time: 4 s Noise floor: 30 mVRMS | No heating Precision Response time 4 s | Only in vitro | - | CO2 | |
| Rate-based monitors [4,15,27] | O2 resolution: 1-ppm [27]; CO2 resolution: 2-ppm [27] S/N O2: 1.5 Δppm [27]; S/N CO2: 3 Δppm [27] | No heating Short response time (<2 min) | Bulky and high power requirements to be wearable Calibration | Test on adults | CO2 | |
| Innovative design for a wristband wireless device [68] | Power autonomy: 6 h Correlation coefficient (VS Sentec, Essex, UK): 0.47 Correlation coefficient (VS Lifesense Nonin, Plymouth, MA, USA): 0.32 | Overestimation of CO2 Wearable Autonomous Correction of humidity and temperature | Heating Calibration | - | CO2 | |
| CAPNO device [69] | - | Correction of humidity temperature | Heating Calibration | - | CO2 | |
| Photoacoustic spectroscopy | Photoacoustic [70] | SNR: 254.04 Minimum detection limit: 2.6 ppmv (at integration time 365 s) Sensitivity: 636.9 ppmv/V | Long-term stability Sensitivity Selectivity No heating | Temperature influences the f0 of the QTF | - | CO2 |
3.5. Implantable Sensors
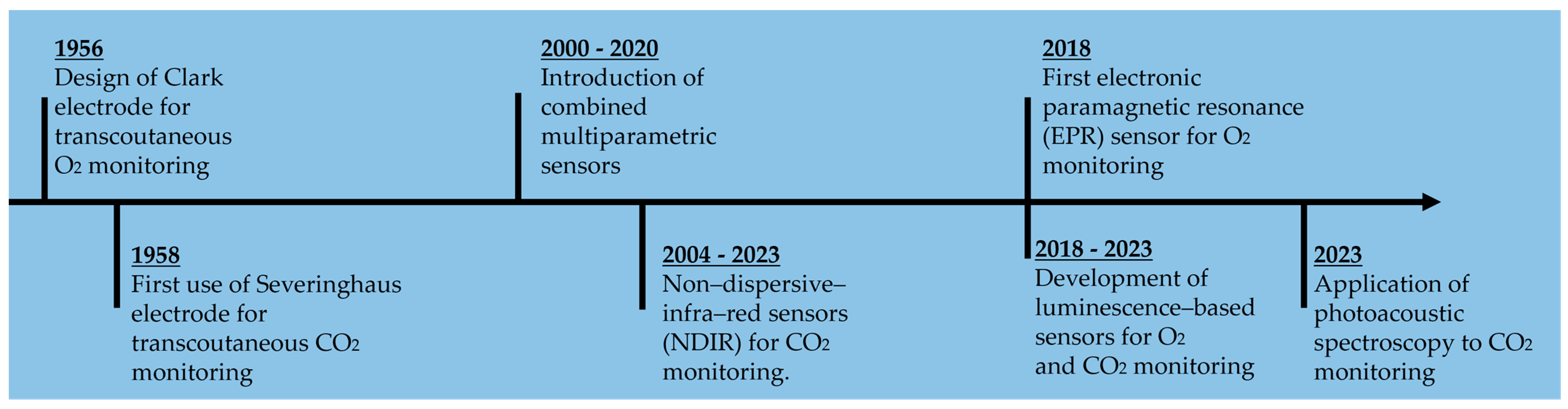
4. Transcutaneous Oxygen-Sensing Technologies
4.1. Electrochemical Sensors
4.2. Optical Sensors
4.3. Electronic Paramagnetic Resonance Sensors
5. Transcutaneous Carbon-Dioxide-Sensing Techniques
5.1. Electrochemical Sensors
5.1.1. Severinghaus-Type Electrode
5.1.2. Ion Selective Field Effect Transistor (ISFET)
5.2. Optical Sensors
5.2.1. Luminescence-Based Sensors
5.2.2. Non-Dispersive Infrared Sensors
5.2.3. Rate-Based Methods
5.2.4. Quartz-Enhanced Photoacoustic Spectroscopy
6. Combined Transcutaneous Oxygen- and Carbon-Dioxide-Sensing Techniques
6.1. SpO2/PtCO2 Sensors
6.2. SpO2/PtO2/PtCO2 Sensors
6.3. O2/CO2 Concentrations
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costanzo, I.M.; Sen, D.; Rhein, L.; Guler, U. Respiratory Monitoring: Current State of the Art and Future Roads. IEEE Rev. Biomed. Eng. 2020, 15, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, I.; Sen, D.; Adegite, J.; Rao, P.M.; Guler, U. A Noninvasive Miniaturized Transcutaneous Oxygen Monitor. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Tufan, T.B.; Guler, U. A Fluorescent Thin Film-Based Miniaturized Transcutaneous Carbon Dioxide Monitor. In Proceedings of the 2021 IEEE Biomedical Circuits and Systems Conference (BioCAS), Berlin, Germany, 7–9 October 2021; pp. 1–5. [Google Scholar]
- Chatterjee, M.; Ge, X.; Kostov, Y.; Tolosa, L.; Rao, G. A novel approach toward noninvasive monitoring of transcutaneous CO2. Med. Eng. Phys. 2014, 36, 136–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitsubayashi, K.; Wakabayashi, Y.; Murotomi, D.; Yamada, T.; Kawase, T.; Iwagaki, S.; Karube, I. Wearable and flexible oxygen sensor for transcutaneous oxygen monitoring. Sens. Actuators B Chem. 2003, 95, 373–377. [Google Scholar] [CrossRef]
- Guler, U.; Costanzo, I.; Sen, D. Emerging Blood Gas Monitors: How They Can Help With COVID-19. IEEE Solid-State Circuits Mag. 2020, 12, 33–47. [Google Scholar] [CrossRef]
- Kmiec, M.M.; Hou, H.; Kuppusamy, M.L.; Drews, T.M.; Prabhat, A.M.; Petryakov, S.V.; Demidenko, E.; Schaner, P.E.; Buckey, J.C.; Blank, A.; et al. Transcutaneous oxygen measurement in humans using a paramagnetic skin adhesive film. Magn. Reson. Med. 2018, 81, 781–794. [Google Scholar] [CrossRef]
- Eberhard, P. The Design, Use, and Results of Transcutaneous Carbon Dioxide Analysis: Current and Future Directions. Obstet. Anesthesia Dig. 2007, 105, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.; Tipton, E.; Liu, W.-H.; Conway, Z.; Soalheira, K.; Sutherland, J.; Fingleton, J. Accuracy and precision of transcutaneous carbon dioxide monitoring: A systematic review and meta-analysis. Thorax 2018, 74, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Van Weteringen, W.; Goos, T.G.; van Essen, T.; Ellenberger, C.; Hayoz, J.; de Jonge, R.C.J.; Reiss, I.K.M.; Schumacher, P.M. Novel transcutaneous sensor combining optical tcPO2 and electrochemical tcPCO2 monitoring with reflectance pulse oximetry. Med. Biol. Eng. Comput. 2019, 58, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Mandru, A. Enhancement of skin permeability with thermal ablation techniques: Concept to commercial products. Drug Deliv. Transl. Res. 2020, 11, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Maibach, H. Transcutaneous CO2 and O2 Diffusion. Ski. Pharmacol. Physiol. 1993, 6, 3–9. [Google Scholar] [CrossRef]
- Rabi, Y.; Kowal, D.; Ambalavanan, N. Blood Gases: Technical Aspects and Interpretation. In Assisted Ventilation of the Neonate: An Evidence-Based Approach to Newborn Respiratory Care, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 80–96.e3. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Z.; Tian, X.; Ma, X.; Xu, L.; Shang, G. Noninvasive carbon dioxide monitoring in pediatric patients undergoing laparoscopic surgery: Transcutaneous vs. end-tidal techniques. BMC Pediatr. 2023, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Ge, X.; Kostov, Y.; Luu, P.; Tolosa, L.; Woo, H.; Viscardi, R.; Falk, S.; Potts, R.; Rao, G. A rate-based transcutaneous CO2 sensor for noninvasive respiration monitoring. Physiol. Meas. 2015, 36, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1. [Google Scholar] [PubMed]
- Mari, A.; Nougue, H.; Mateo, J.; Vallet, B.; Vallée, F. Transcutaneous PCO2 monitoring in critically ill patients: Update and perspectives. J. Thorac. Dis. 2019, 11, S1558–S1567. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, I.; Sen, D.; Guler, U. An Integrated Readout Circuit for a Transcutaneous Oxygen Sensing Wearable Device. In Proceedings of the 2020 IEEE Custom Integrated Circuits Conference (CICC), Boston, MA, USA, 22–25 March 2020; pp. 1–4. [Google Scholar]
- Sharma, S.; Hashmi, M.F. Partial Pressure of Oxygen. StatPearls. December 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493219/ (accessed on 17 January 2024).
- Collins, J.-A.; Rudenski, A.; Gibson, J.; Howard, L.; O’driscoll, R. Relating oxygen partial pressure, saturation and content: The haemoglobin–oxygen dissociation curve. Breathe 2015, 11, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Lichtblau, M.; Saxer, S.; Schneider, S.R.; Appenzeller, P.; Bauer, M.; Hasler, E.D.; Schwarz, E.I.; Bloch, K.E.; Ulrich, S. Hyperoxia improves exercise capacity in cardiopulmonary disease: A series of randomised controlled trials. ERJ Open Res. 2023, 9, 2022. [Google Scholar] [CrossRef] [PubMed]
- Laffey, J.G.; Kavanagh, B.P. Hypocapnia. N. Engl. J. Med. 2002, 347, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Angelucci, A.; Aliverti, A.; Gugliandolo, P.; Pezzuto, B.; Berna, G.; Romani, S.; Tedesco, C.C.; Agostoni, P. Comparison between PtCO2 and PaCO2 and Derived Parameters in Heart Failure Patients during Exercise: A Preliminary Study. Sensors 2021, 21, 6666. [Google Scholar] [CrossRef]
- Bhat, R.; Kim, W.D.; Shukla, A.; Vidyasagar, D. Simultaneous tissue pH and transcutaneous carbon dioxide monitoring in critically ill neonates. Crit. Care Med. 1981, 9, 744–749. [Google Scholar] [CrossRef]
- Urbano, J.; Cruzado, V.; López-Herce, J.; del Castillo, J.; Bellón, J.M.; Carrillo, A. Accuracy of three transcutaneous carbon dioxide monitors in critically ill children. Pediatr. Pulmonol. 2010, 45, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, P.; Hammacher, K.; Mindt, W. Elecrrochemical Elecrrode with Heating Means. U.S. Patent 3795239, 5 March 1974. [Google Scholar]
- Ge, X.; Adangwa, P.; Lim, J.Y.; Kostov, Y.; Tolosa, L.; Pierson, R.; Herr, D.; Rao, G. Development and characterization of a point-of care rate-based transcutaneous respiratory status monitor. Med. Eng. Phys. 2018, 56, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Kellogg, D.L.; Johnson, J.M. Highlighted Topic Mechanisms and Modulators of Temperature Regulation Local thermal control of the human cutaneous circulation. J. Appl. Physiol. 2010, 109, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Lübbers, D.W. Theoretical basis of the transcutaneous blood gas measurements. Crit. Care Med. 1981, 9, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, Y.; Peura, R.A. Noninvasive Transcutaneous Monitoring of Arterial Blood Gases. IEEE Trans. Biomed. Eng. 1984, BME-31, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Hayoz, J.; Wagner, R. Device and Methods for Transcutaneous Determination of Blood Gases. U.S. Patent 8527023, 3 September 2013. [Google Scholar]
- Gisiger, P.; Palma, J.; Eberhard, P. OxiCarbo®, a single sensor for the non-invasive measurement of arterial oxygen saturation and CO2 partial pressure at the ear lobe. Sens. Actuators B Chem. 2001, 76, 527–530. [Google Scholar] [CrossRef]
- Lermuzeaux, M.; Meric, H.; Sauneuf, B.; Girard, S.; Normand, H.; Lofaso, F.; Terzi, N. Superiority of transcutaneous CO2 over end-tidal CO2 measurement for monitoring respiratory failure in nonintubated patients: A pilot study. J. Crit. Care 2016, 31, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dervieux, E.; Théron, M.; Uhring, W. Carbon Dioxide Sensing—Biomedical Applications to Human Subjects. Sensors 2021, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Bromley, I. Transcutaneous monitoring—Understanding the principles. Infant 2008, 4, 95–98. [Google Scholar]
- Lundstrøm, K.E. Transcutaneous Monitoring of pO2 and pCO2 in Neonates—A Blessing or a Burden? Available online: https://acutecaretesting.org/en/articles/transcutaneous-monitoring-of-po2-and-pco2-in-neonates--a-blessing-or-a-burden (accessed on 5 October 2023).
- Miller, J.S.S. SenTec Digital Monitoring System Noninvasive Ventilation and Oxygenation Monitoring. Available online: www.sentec.ch (accessed on 26 May 2023).
- Roussakis, E.; Li, Z.; Nichols, A.J.; Evans, C.L. Oxygen-Sensing Methods in Biomedicine from the Macroscale to the Microscale. Angew. Chem. Int. Ed. 2015, 54, 8340–8362. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, D. Transcutaneous carbon dioxide monitoring: Literature review. Oral Health Dent Manag. 2014, 13, 453–457. [Google Scholar] [PubMed]
- Tipparaju, V.V.; Mora, S.J.; Yu, J.; Tsow, F.; Xian, X. Wearable Transcutaneous CO2; Monitor Based on Miniaturized Nondispersive Infrared Sensor. IEEE Sens. J. 2021, 21, 17327–17334. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, I.; Sen, D.; Giri, B.; Pratt, N.; Rao, P.; Guler, U. Fluorescent Intensity and Lifetime Measurement of Platinum-Porphyrin Film for Determining the Sensitivity of Transcutaneous Oxygen Sensor. In Proceedings of the 2020 IEEE International Symposium on Circuits and Systems (ISCAS), Seville, Spain, 12–14 October 2020; pp. 1–5. [Google Scholar]
- Sankaran, D.; Zeinali, L.; Iqbal, S.; Chandrasekharan, P.; Lakshminrusimha, S. Non-invasive carbon dioxide monitoring in neonates: Methods, benefits, and pitfalls. J. Perinatol. 2021, 41, 2580–2589. [Google Scholar] [CrossRef] [PubMed]
- Tufan, T.B.; Sen, D.; Guler, U. An Infra-Red-Based Prototype for a Miniaturized Transcutaneous Carbon Dioxide Monitor. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Mexico City, Mexico, 1–5 November 2021; pp. 7132–7135. [Google Scholar]
- Capnography: End Tidal CO2 Monitoring Facts. Available online: https://www.medtronic.com/covidien/en-gb/respiratory-and-monitoring-solutions/patient-monitoring-respiratory-interventions-blog/emergency-room/key-facts-in-end-tidal-co2-monitoring.html# (accessed on 22 September 2023).
- Inspiratory End-Tidal Oxygen Content Difference: A Sensitive: Critical Care Medicine. Available online: https://journals.lww.com/ccmjournal/abstract/1989/04000/inspiratory_end_tidal_oxygen_content_difference__a.9.aspx (accessed on 18 January 2024).
- Nassar, B.S.; Schmidt, G.A. Estimating Arterial Partial Pressure of Carbon Dioxide in Ventilated Patients: How Valid Are Surrogate Measures? Ann. Am. Thorac. Soc. 2017, 14, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Aminiahidashti, H.; Shafiee, S.; Kiasari, Z.Z.; Sazgar, A. Applications of End-Tidal Carbon Dioxide (ETCO 2) Monitoring in Emergency Department; a Narrative Review. Emergency 2018, 6, 5. [Google Scholar]
- Sakuraya, M.; Douno, E.; Iwata, W.; Takaba, A.; Hadama, K.; Kawamura, N.; Maezawa, T.; Iwamoto, K.; Yoshino, Y.; Yoshida, K. Accuracy evaluation of mainstream and sidestream end-tidal carbon dioxide monitoring during noninvasive ventilation: A randomized crossover trial (MASCAT-NIV trial). J. Intensiv. Care 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.Q.; Tremper, K.K.; Lee, S.E.; Barker, S.J. Noninvasive monitoring of carbon dioxide: A comparison of the partial pressure of transcutaneous and end-tidal carbon dioxide with the partial pressure of arterial carbon dioxide. J. Clin. Monit. Comput. 1987, 3, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Malarvili, M.B.; Howe, T.A.; Ramanathan, S.; Alexie, M.; Singh, O.P. Design of carbon dioxide sensor for capnography. In Systems and Signal Processing of Capnography as a Diagnostic Tool for Asthma Assessment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 131–156. [Google Scholar] [CrossRef]
- Beck, S.E.; Marcus, C.L. Pediatric Polysomnography. Sleep. Med. Clin. 2009, 4, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.K.; Crotwell, D.N.; Restrepo, R.D. Capnography/Capnometry During Mechanical Ventilation: 2011. Respir. Care 2011, 56, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Poets, C.F. Pulse Oximetry vs. Transcutaneous Monitoring in Neonates: Practical Aspects, 2003. Available online: https://acutecaretesting.org/en/articles/pulse-oximetry-vs-transcutaneous-monitoring-in-neonates-practical-aspects (accessed on 15 January 2024).
- Angelucci, A.; Bernasconi, S.; D’andrea, M.; Contini, M.; Gugliandolo, P.; Agostoni, P.; Aliverti, A. Integration of a body sensor network of wearable devices for cardio-respiratory monitoring. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar]
- Cascales, J.P.; Li, X.; Roussakis, E.; Evans, C.L. A Patient-Ready Wearable Transcutaneous CO2 Sensor. Biosensors 2022, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-J.; Lee, S.; Kim, J.-H.; Kil, H.-J.; Kim, Y.-C.; Park, J.-W. Wearable, Luminescent Oxygen Sensor for Transcutaneous Oxygen Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 41026–41034. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Severinghaus, J.W.; Bradley, A.F. Electrodes for Blood pO2 and pCO2 Determination. J. Appl. Physiol. 1958, 13, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Vlekkert, H.V.D.; De Rooij, N. Carbon dioxide gas-sensing electrode based on a pH-ISFET with back-side contacts. Sens. Actuators 1989, 17, 275–278. [Google Scholar] [CrossRef]
- Lim, C.-J.; Park, J.-W. Wearable transcutaneous oxygen sensor for health monitoring. Sens. Actuators A Phys. 2019, 298, 1607. [Google Scholar] [CrossRef]
- Sandoval, J.P.C.; Roussakis, E.; Witthauer, L.; Goss, A.; Li, X.; Greenfield, D.; Chen, Y.; Marks, H.L.; Evans, C.L. Wearable device for remote monitoring of transcutaneous tissue oxygenation. In Proceedings of the Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables II, Online, 6–12 March 2021. [Google Scholar]
- Sandoval, J.P.C.; Greenfield, D.L.; Roussakis, E.; Li, X.; Goss, A.; Witthauer, L.; Kelley, L.; Evans, C.L. Wearable device and methods for non-invasive measurements of tissue oxygenation. In Proceedings of the Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables III, San Francisco, CA, USA, 22 January–28 February 2022; p. PC1195603. [Google Scholar] [CrossRef]
- Sandoval, J.P.C.; Roussakis, E.; Li, X.; Greenfield, D.A.; Witthauer, L.; Goss, A.; Keshishian, H.; Nguyen, J.Q.; Marks, H.L.; Draghici, A.E.; et al. Transcutaneous monitoring of oxygen and carbon dioxide using wearable devices. In Proceedings of the Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables IV, San Francisco, CA, USA, 28 January–3 February 2023; p. PC1237503. [Google Scholar] [CrossRef]
- Cascales, J.P.; Roussakis, E.; Witthauer, L.; Goss, A.; Li, X.; Chen, Y.; Marks, H.L.; Evans, C.L. Wearable device for remote monitoring of transcutaneous tissue oxygenation. Biomed. Opt. Express 2020, 11, 6989–7002. [Google Scholar] [CrossRef] [PubMed]
- Tufan, T.B.; Guler, U. A Miniaturized Transcutaneous Carbon Dioxide Monitor Based on Dual Lifetime Referencing. In BioCAS 2022—IEEE Biomedical Circuits and Systems Conference: Intelligent Biomedical Systems for a Better Future, Proceedings; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022; pp. 144–148. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, D.-E.; Kwon, D.-H.; Lee, S.-H.; Kang, S.-W. Development of non-invasive optical transcutaneous pCO/sub 2/ gas sensor and analytic equipment. IEEE Sens. 2004, 17, 249–257. [Google Scholar]
- Datasheet of COZIR Sensor. OPTIONAL SPECIFICATIONS Temperature & Humidity Measurement—CO2 Sensors Low Power Range—COZIR®, 2018. Available online: https://www.sstsensing.com/wp-content/uploads/2018/04/DS0113rev13_CO2_Cozir-A.pdf (accessed on 16 January 2024).
- Grangeat, P.; Gharbi, S.; Accensi, M.; Grateau, H. First Evaluation of a Transcutaneous Carbon Dioxide Monitoring Wristband Device during a Cardiopulmonary Exercise Test. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3352–3355. [Google Scholar]
- Grangeat, P.; Gharbi, S.; Koenig, A.; Comsa, M.-P.; Accensi, M.; Grateau, H.; Ghaith, A.; Chacaroun, S.; Doutreleau, S.; Verges, S. Evaluation in Healthy Subjects of a Transcutaneous Carbon Dioxide Monitoring Wristband during Hypo and Hypercapnia Conditions. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) in Conjunction with the 43rd Annual Conference of the Canadian Medical and Biological Engineering Society, Montreal, QC, Canada, 20–24 July 2020; pp. 4640–4643. [Google Scholar]
- Zhang, Y.; Xie, Y.; Lu, J.; Zhao, J.; Wu, Y.; Tong, J.; Shao, J. Continuous real-time monitoring of carbon dioxide emitted from human skin by quartz-enhanced photoacoustic spectroscopy. Photoacoustics 2023, 30, 100488. [Google Scholar] [CrossRef] [PubMed]
- Marland, J.R.; Gray, M.E.; Dunare, C.; Blair, E.O.; Tsiamis, A.; Sullivan, P.; González-Fernández, E.; Greenhalgh, S.N.; Gregson, R.; Clutton, R.E.; et al. Real-time measurement of tumour hypoxia using an implantable microfabricated oxygen sensor. Sens. Bio-Sensing Res. 2020, 30, 100375. [Google Scholar] [CrossRef]
- Mitra, S.; Cumming, D.R. Implantable microsystems for personalised anticancer therapy. In CMOS Circuits for Biological Sensing and Processing; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 259–286. [Google Scholar] [CrossRef]
- Sonmezoglu, S.; Maharbiz, M.M. 34.4 A 4.5mm3 Deep-Tissue Ultrasonic Implantable Luminescence Oxygen Sensor. In Proceedings of the 2020 IEEE International Solid-State Circuits Conference—(ISSCC), San Francisco, CA, USA, 16–20 February 2020; pp. 454–456. [Google Scholar]
- Clark, J.L.C. Electrochemical Device for Chemical Analysis. U.S. Patent 2913386, 17 November 1959. [Google Scholar]
- Stetter, J.R.; Li, J. Amperometric Gas SensorsA Review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. BioEssays 2015, 37, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Garlick, G.F.J. Luminescence. In Light and Matter II/Licht und Materie II. Encyclopedia of Physics/Handbuch der Physik; Flügge, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1958; pp. 1–128. [Google Scholar] [CrossRef]
- Murthy, K.; Virk, H.S. Luminescence Phenomena: An Introduction. Defect. Diffus. Forum 2013, 347, 1–34. [Google Scholar] [CrossRef]
- Demas, J.N.; DeGraff, B.A.; Coleman, P.B. Peer Reviewed: Oxygen Sensors Based on Luminescence Quenching. Anal. Chem. 1999, 71, 793A–800A. [Google Scholar] [CrossRef] [PubMed]
- Bambot, S.B.; Holavanahali, R.; Lakowicz, J.R.; Carter, G.M.; Rao, G. Phase fluorometric sterilizable optical oxygen sensor. Biotechnol. Bioeng. 1994, 43, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Holst, G.A.; Köster, T.; Voges, E.; Lübbers, D.W. FLOX—An oxygen-flux-measuring system using a phase-modulation method to evaluate the oxygen-dependent fluorescence lifetime. Sens. Actuators B Chem. 1995, 29, 231–239. [Google Scholar] [CrossRef]
- Perimed Periflux System 5000. Available online: https://rimuhc.ca/-/perimed-periflux-system-5000 (accessed on 24 September 2023).
- Beran, A.V.; Huxtable, R.F.; Sperling, D.R. Electrochemical sensor for continuous transcutaneous PCO2 measurement. J. Appl. Physiol. 1976, 41, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J.; Perrin, E.; Bennani, I.; DE Muralt, B.; Titelion, V.; Picaud, C. Is continuous transcutaneous monitoring of PCO2 (TcPCO2) over 8 h reliable in adults? Respir. Med. 2001, 95, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Storre, J.H.; Steurer, B.; Kabitz, H.-J.; Dreher, M.; Windisch, W. Transcutaneous P co 2 Monitoring During Initiation of Noninvasive Ventilation. Chest 2007, 132, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Tufan, T.B.; Rhein, L.; Guler, U. Implementation Techniques for Transcutaneous Carbon Dioxide Monitoring: Approaches for Wearable Smart Health Applications. IEEE Trans. Biomed. Eng. 2023, 71, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Materials for fluorescence-based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Mills, A. Optical sensors for carbon dioxide and their applications. In Sensors for Environment, Health and Security: Advanced Materials and Technologies; Springer: Berlin/Heidelberg, Germany, 2009; pp. 347–370. [Google Scholar] [CrossRef]
- Vafaei, M.; Amini, A.; Siadatan, A. Breakthrough in CO2 Measurement with a Chamberless NDIR Optical Gas Sensor. IEEE Trans. Instrum. Meas. 2019, 69, 2258–2268. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Aliverti, A.; Angelucci, A.; Bernasconi, S.; Moro, F.L.; Froio, D. Wearable Device for Noninvasive Measuring The Partial Pressure of Transcutaneous CO2 of A Person And Related Method of Measurement 2022. Available online: https://re.public.polimi.it/handle/11311/1208187 (accessed on 3 January 2024).
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Angelucci, A.; Bernasconi, S.; Colombo, L.; Contini, M.; Gugliandolo, P.; Agostoni, P.; Aliverti, A. A Wearable Device to Monitor the Partial Pressure of Transcutaneous Carbon Dioxide. IEEE Trans. Instrum. Meas. 2024, 73, 1–12. [Google Scholar] [CrossRef]
- WO2023180948A1—Wearable Device for Noninvasive Measuring the Partial Pressure of Transcutaneous CO2 of a Person and Related Method of Measurement—Google Patents. Available online: https://patents.google.com/patent/WO2023180948A1/en (accessed on 26 February 2024).
- Hazenberg, A.; Zijlstra, J.; Kerstjens, H.; Wijkstra, P. Validation of a Transcutaneous CO2 Monitor in Adult Patients with Chronic Respiratory Failure. Respiration 2011, 81, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Manual of OxiVenT Sensor. SENTEC DIGITAL MONITOR with OxiVenTTM Sensor. Available online: https://www.sentec.com/info/legal- (accessed on 27 May 2023).
- Wijk, J.J.; Weteringen, W.; Hoeks, S.E.; Staals, L.M. Validation of a new combined transcutaneous tcPCO 2 and tcPO 2 sensor in children in the operating theater. Pediatr. Anesthesia 2021, 32, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Marasco, C.; Perez, A.; Ehrman, J.; Hoilett, O. Metabolic Rate Measurement Apparatus and Method Thereof 20200113516, 2020. Available online: https://www.freepatentsonline.com/y2020/0113516.html (accessed on 27 May 2023).
- Jansen, E.D.; van Leeuwen, T.G.; Motamedi, M.; Borst, C.; Welch, A.J. Temperature dependence of the absorption coefficient of water for midinfrared laser radiation. Lasers Surg. Med. 1994, 14, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Huong, A.; Ngu, X. Skin Tissue Oxygen Saturation Prediction: A Comparison Study of Artificial Intelligence Techniques. Adv. Sci. Technol. Eng. Syst. J. 2020, 5, 334–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernasconi, S.; Angelucci, A.; De Cesari, A.; Masotti, A.; Pandocchi, M.; Vacca, F.; Zhao, X.; Paganelli, C.; Aliverti, A. Recent Technologies for Transcutaneous Oxygen and Carbon Dioxide Monitoring. Diagnostics 2024, 14, 785. https://doi.org/10.3390/diagnostics14080785
Bernasconi S, Angelucci A, De Cesari A, Masotti A, Pandocchi M, Vacca F, Zhao X, Paganelli C, Aliverti A. Recent Technologies for Transcutaneous Oxygen and Carbon Dioxide Monitoring. Diagnostics. 2024; 14(8):785. https://doi.org/10.3390/diagnostics14080785
Chicago/Turabian StyleBernasconi, Sara, Alessandra Angelucci, Anastasia De Cesari, Aurora Masotti, Maurizio Pandocchi, Francesca Vacca, Xin Zhao, Chiara Paganelli, and Andrea Aliverti. 2024. "Recent Technologies for Transcutaneous Oxygen and Carbon Dioxide Monitoring" Diagnostics 14, no. 8: 785. https://doi.org/10.3390/diagnostics14080785
APA StyleBernasconi, S., Angelucci, A., De Cesari, A., Masotti, A., Pandocchi, M., Vacca, F., Zhao, X., Paganelli, C., & Aliverti, A. (2024). Recent Technologies for Transcutaneous Oxygen and Carbon Dioxide Monitoring. Diagnostics, 14(8), 785. https://doi.org/10.3390/diagnostics14080785








