CT Perfusion Derived rCBV < 42% Lesion Volume Is Independently Associated with Followup FLAIR Infarct Volume in Anterior Circulation Large Vessel Occlusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Data Collection
2.4. CTP Image Acquisition
2.5. Image Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arenillas, J.F.; Cortijo, E.; García-Bermejo, P.; Levy, E.I.; Jahan, R.; Liebeskind, D.; Goyal, M.; Saver, J.L.; Albers, G.W. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J. Cereb. Blood Flow Metab. 2018, 38, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.W.; Park, H.S.; Cha, J.K.; Kim, D.H.; Kang, M.J.; Choi, J.H.; Nah, H.W.; Huh, J.T. Relative CBV ratio on perfusion-weighted MRI indicates the probability of early recanalization after IV t-PA administration for acute ischemic stroke. J. Neurointerv. Surg. 2016, 8, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Wang, J.H.; Yang, S.; Wang, D.Z.; Zhang, Q.; Cheng, X.D.; Yu, N.W.; Guo, F.Q. Cerebral blood volume index may be a predictor of independent outcome of thrombectomy in stroke patients with low ASPECTS. J. Clin. Neurosci. 2022, 103, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Karamchandani, R.R.; Strong, D.; Rhoten, J.B.; Prasad, T.; Selig, J.; Defilipp, G.; Asimos, A.W. Cerebral blood volume index as a predictor of functional independence after basilar artery thrombectomy. J. Neuroimaging 2022, 32, 171–178. [Google Scholar] [CrossRef]
- Imaoka, Y.; Shindo, S.; Miura, M.; Terasaki, T.; Mukasa, A.; Todaka, T. Hypop erfusion intensity ratio and CBV index as predictive parameters to identify underlying intracranial atherosclerotic stenosis in endovascular thrombectomy. J. Neuroradiol. 2023, 50, 424–430. [Google Scholar] [CrossRef]
- Cortijo, E.; Calleja, A.I.; García-Bermejo, P.; Mulero, P.; Pérez-Fernández, S.; Reyes, J.; Muñoz, M.F.; Martínez-Galdámez, M.; Arenillas, J.F. Relative cerebral blood volume as a marker of durable tissue-at-risk viability in hyperacute ischemic stroke. Stroke 2014, 45, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, D.A.; Balar, A.B.; Koneru, M.; Hoseinyazdi, M.; Hyson, N.; Cho, A.; Greene, C.; Xu, R.; Luna, L.; Caplan, J.; et al. Pretreatment CT perfusion collateral parameters correlate with penumbra salvage in middle cerebral artery occlusion. J. Neuroimaging 2024, 34, 44–49. [Google Scholar] [CrossRef]
- Salim, H.; Lakhani, D.A.; Balar, A.; Musmar, B.; Adeeb, N.; Hoseinyazdi, M.; Luna, L.; Deng, F.; Hyson, N.Z.; Mei, J.; et al. Follow-up infarct volume on fluid attenuated inversion recovery (FLAIR) imaging in distal medium vessel occlusions: The role of cerebral blood volume index. J. Neurol. 2024, 1–9. [Google Scholar] [CrossRef]
- Rex, N.B.; McDonough, R.V.; Ospel, J.M.; Kashani, N.; Sehgal, A.; Fladt, J.C.; McTaggart, R.A.; Nogueira, R.; Menon, B.; Demchuk, A.M.; et al. CT Perfusion Does Not Modify the Effect of Reperfusion in Patients with Acute Ischemic Stroke Undergoing Endovascular Treatment in the ESCAPE-NA1 Trial. AJNR Am. J. Neuroradiol. 2023, 44, 1045–1049. [Google Scholar] [CrossRef]
- Waqas, M.; Mokin, M.; Primiani, C.T.; Gong, A.D.; Rai, H.H.; Chin, F.; Rai, A.T.; Levy, E.I.; Siddiqui, A.H. Large Vessel Occlusion in Acute Ischemic Stroke Patients: A Dual-Center Estimate Based on a Broad Definition of Occlusion Site. J. Stroke Cerebrovasc. Dis. 2020, 29, 104504. [Google Scholar] [CrossRef]
- Lakhani, D.A.; Balar, A.B.; Koneru, M.; Wen, S.; Hoseinyazdi, M.; Greene, C.; Xu, R.; Luna, L.; Caplan, J.; Dmytriw, A.A.; et al. The Compensation Index Is Better Associated with DSA ASITN Collateral Score Compared to the Cerebral Blood Volume Index and Hypoperfusion Intensity Ratio. J. Clin. Med. 2023, 12, 7365. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, D.A.; Balar, A.B.; Koneru, M.; Wen, S.; Ozkara, B.B.; Wang, R.; Hoseinyazdi, M.; Nabi, M.; Mazumdar, I.; Cho, A.; et al. CT perfusion based rCBF <38% volume is independently and negatively associated with digital subtraction angiography collateral score in anterior circulation large vessel occlusions. Neuroradiol. J. 2024. [Google Scholar] [CrossRef]
- Murphy, B.D.; Fox, A.J.; Lee, D.H.; Sahlas, D.J.; Black, S.E.; Hogan, M.J.; Coutts, S.B.; Demchuk, A.M.; Goyal, M.; Aviv, R.I.; et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke 2006, 37, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Levy, E.I.; Saver, J.L.; Siddiqui, A.H.; Goyal, M.; Bonafé, A.; Cognard, C.; Jahan, R.; Albers, G.W.; Investigators, S.P. Predictive Value of RAPID Assessed Perfusion Thresholds on Final Infarct Volume in SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke 2017, 48, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Nannoni, S.; Ricciardi, F.; Strambo, D.; Sirimarco, G.; Wintermark, M.; Dunet, V.; Michel, P. Correlation between ASPECTS and Core Volume on CT Perfusion: Impact of Time since Stroke Onset and Presence of Large-Vessel Occlusion. AJNR Am. J. Neuroradiol. 2021, 42, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Puetz, V.; Dzialowski, I.; Hill, M.D.; Demchuk, A.M. The Alberta Stroke Program Early CT Score in clinical practice: What have we learned? Int. J. Stroke 2009, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Blasco, J.; López, A.; Amaro, S.; Román, L.S.; Llull, L.; Renú, A.; Rudilosso, S.; Laredo, C.; Obach, V.; et al. Complete reperfusion is required for maximal benefits of mechanical thrombectomy in stroke patients. Sci. Rep. 2017, 7, 11636. [Google Scholar] [CrossRef] [PubMed]
- Latchaw, R.E.; Alberts, M.J.; Lev, M.H.; Connors, J.J.; Harbaugh, R.E.; Higashida, R.T.; Hobson, R.; Kidwell, C.S.; Koroshetz, W.J.; Mathews, V.; et al. Recommendations for imaging of acute ischemic stroke: A scientific statement from the American Heart Association. Stroke 2009, 40, 3646–3678. [Google Scholar] [CrossRef]
- Yoshie, T.; Yu, Y.; Jiang, H.; Honda, T.; Trieu, H.; Scalzo, F.; Saver, J.L.; Liebeskind, D.S.; Investigators, U.R.T. Perfusion Parameter Thresholds That Discriminate Ischemic Core Vary with Time from Onset in Acute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2020, 41, 1809–1815. [Google Scholar] [CrossRef]
- Bendszus, M.; Fiehler, J.; Subtil, F.; Bonekamp, S.; Aamodt, A.H.; Fuentes, B.; Gizewski, E.R.; Hill, M.D.; Krajina, A.; Pierot, L.; et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: Multicentre, open-label, randomised trial. Lancet 2023, 402, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
| Study Demographics (n = 158) | Median (Interquatile Range) or Number (Percentage) |
|---|---|
| Age | 68 (60–77) |
| Sex | |
| Female | 83 (52.53%) |
| Male | 75 (47.47%) |
| Race | |
| African American | 70 (44.30%) |
| Caucasian | 79 (50.00%) |
| Asian | 4 (2.53%) |
| Others | 5 (3.16%) |
| Comorbidities | |
| Hypertension | 126 (79.75%) |
| Hyperlipidemia | 79 (50.00%) |
| Diabetes Mellitus | 41 (25.95%) |
| Heart Disease | 77 (48.73%) |
| Atrial Fibrillation | 57 (36.08%) |
| Smoking | 74 (46.84%) |
| Prior stroke or transient Ischemic Attack | 30 (18.99%) |
| Study Cohort (n = 158) | Median (Interquatile Range) or Number (Percentage) |
|---|---|
| Vessel Occluded | |
| M1 | 114 (72.15%) |
| Proximal M2 | 32 (20.25%) |
| Supraclinoid ICA | 12 (7.59%) |
| Alberta Stroke Program Early CT Score (ASPECTS) | |
| 0 | 2 (1.27%) |
| 1 | 1 (0.63%) |
| 2 | 4 (2.53%) |
| 3 | 3 (1.90%) |
| 5 | 10 (6.33%) |
| 6 | 8 (5.06%) |
| 7 | 12 (7.59%) |
| 8 | 27 (17.09%) |
| 9 | 22 (13.92%) |
| 10 | 69 (43.67%) |
| CT Perfusion Parameter | |
| rCBV < 42% (mL) | 6.00 (0.00–33.00) |
| rCBF < 30% (mL) | 7.50 (0.00–38.00) |
| Tmax > 6 s (mL) | 110.00 (66.00–161.00) |
| MRI Parameter | |
| FLAIR Volume (mL) | 32.25 (7.12–114.52) |
| Study Cohort (n = 158) | Median (Interquatile Range) or Number (Percentage) |
|---|---|
| Symptom onset to door time in minutes | 66.00 (44.00–105.00) |
| Door-to-CT time in minutes | 30.00 (21.00–46.00) |
| Door-to-needle time in minutes | 59.00 (47.50–82.50) |
| Door-to-groin puncture time in minutes | 178.00 (133.00–221.00) |
| Door-to-recanalization time in minutes | 32.00 (24.00–53.00) |
| Symptom onset to MRI time in days | 2.00 (1.00–3.00) |
| Study Cohort (n = 158) | Median (Interquatile Range) or Number (Percentage) |
|---|---|
| Intravenous tissue-type plasminogen activator (IV tPA) | 51 (32.28%) |
| Mechanical thrombectomy (MT) | 130 (82.28%) |
| Admission NIH Stroke Scale | 15 (10–20) |
| Premorbid Modified Rankin Score (mRS) | |
| 0 | 100 (65.79%) |
| 1 | 20 (13.16%) |
| 2 | 11 (7.24%) |
| 3 | 19 (12.50%) |
| 4 | 1 (0.66%) |
| 5 | 1 (0.66%) |
| Modified treatment in cerebral infarction (mTICI) | |
| 0 | 6 (4.96%) |
| 1 | 1 (0.83%) |
| 2A | 4 (3.31%) |
| 2B | 27 (22.31%) |
| 2C | 17 (14.05%) |
| 3 | 66 (54.55%) |
| Variables | Unstandardized Coefficients | Multivariable Regression Model | ||||
|---|---|---|---|---|---|---|
| Unadjusted Beta | Standard Error | Adjusted Beta | Lower Bound | Upper Bound | p Value | |
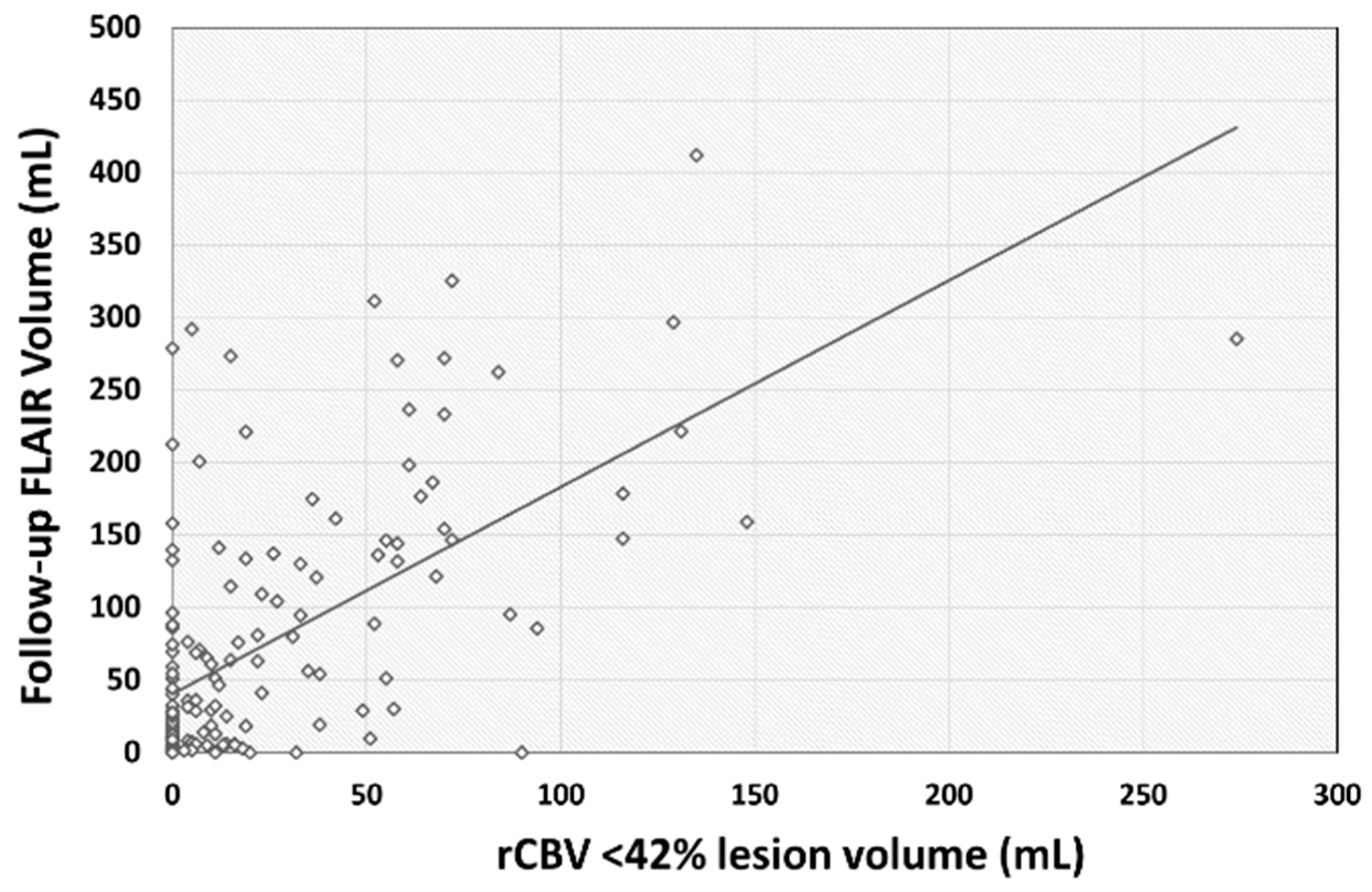
| rCBV < 42% | 1.48 | 0.18 | 0.60 | 1.133 | 1.83 | <0.001 |
| Age | −0.56 | 0.40 | −0.10 | −1.36 | 0.23 | 0.16 |
| Sex | −4.33 | 10.56 | −0.03 | −25.27 | 16.61 | 0.68 |
| Race | 3.78 | 7.74 | 0.03 | −11.58 | 19.13 | 0.63 |
| Hypertension | 18.14 | 14.21 | 0.09 | −10.06 | 46.34 | 0.21 |
| Hyperlipidemia | −11.68 | 10.75 | −0.07 | −33.01 | 9.65 | 0.28 |
| Diabetes Mellitus | 28.39 | 12.27 | 0.16 | 4.04 | 52.73 | <0.05 |
| Heart Disease | 0.67 | 12.08 | 0.004 | −23.29 | 24.63 | 0.96 |
| Atrial Fibrillation | 5.17 | 12.24 | 0.03 | −19.12 | 29.46 | 0.67 |
| Occlusion Segment | −0.81 | 3.56 | −0.02 | −7.87 | 6.26 | 0.82 |
| Prior transient Ischemic Attack or stroke | 7.58 | 14.62 | 0.04 | −21.43 | 36.58 | 0.61 |
| Intravenous tissue-type plasminogen activator (IV tPA) | −22.00 | 11.69 | −0.13 | −45.15 | 1.14 | 0.06 |
| Admission NIH Stroke Scale | 0.89 | 0.83 | 0.08 | −0.76 | 2.55 | 0.29 |
| Premorbid Modified Rankin Score (mRS) | −0.48 | 5.29 | −0.007 | −10.98 | 10.02 | 0.93 |
| Alberta Stroke Program Early CT Score (ASPECTS) | −9.37 | 2.99 | −0.21 | −15.29 | −3.44 | <0.01 |
| The modified treatment in cerebral infarction (mTICI) score | −14.58 | 3.40 | −0.28 | −21.33 | −7.825 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakhani, D.A.; Balar, A.B.; Salim, H.; Koneru, M.; Wen, S.; Ozkara, B.; Lu, H.; Wang, R.; Hoseinyazdi, M.; Xu, R.; et al. CT Perfusion Derived rCBV < 42% Lesion Volume Is Independently Associated with Followup FLAIR Infarct Volume in Anterior Circulation Large Vessel Occlusion. Diagnostics 2024, 14, 845. https://doi.org/10.3390/diagnostics14080845
Lakhani DA, Balar AB, Salim H, Koneru M, Wen S, Ozkara B, Lu H, Wang R, Hoseinyazdi M, Xu R, et al. CT Perfusion Derived rCBV < 42% Lesion Volume Is Independently Associated with Followup FLAIR Infarct Volume in Anterior Circulation Large Vessel Occlusion. Diagnostics. 2024; 14(8):845. https://doi.org/10.3390/diagnostics14080845
Chicago/Turabian StyleLakhani, Dhairya A., Aneri B. Balar, Hamza Salim, Manisha Koneru, Sijin Wen, Burak Ozkara, Hanzhang Lu, Richard Wang, Meisam Hoseinyazdi, Risheng Xu, and et al. 2024. "CT Perfusion Derived rCBV < 42% Lesion Volume Is Independently Associated with Followup FLAIR Infarct Volume in Anterior Circulation Large Vessel Occlusion" Diagnostics 14, no. 8: 845. https://doi.org/10.3390/diagnostics14080845
APA StyleLakhani, D. A., Balar, A. B., Salim, H., Koneru, M., Wen, S., Ozkara, B., Lu, H., Wang, R., Hoseinyazdi, M., Xu, R., Nabi, M., Mazumdar, I., Cho, A., Chen, K., Sepehri, S., Hyson, N., Urrutia, V., Luna, L., Hillis, A. E., ... Yedavalli, V. S. (2024). CT Perfusion Derived rCBV < 42% Lesion Volume Is Independently Associated with Followup FLAIR Infarct Volume in Anterior Circulation Large Vessel Occlusion. Diagnostics, 14(8), 845. https://doi.org/10.3390/diagnostics14080845









