Human Papillomavirus Carcinogenicity and the Need of New Perspectives: Thoughts from a Retrospective Analysis on Human Papillomavirus Outcomes Conducted at the Hospital University of Bari, Apulia, Italy, between 2011 and 2022
Abstract
1. Introduction
The HPV Epidemiology and the Different Infectivity Pathways
2. Material and Methods
2.1. Samples Collection
2.2. DNA Extraction and Multiplex Real-Time PCR
2.3. Statistical Analysis
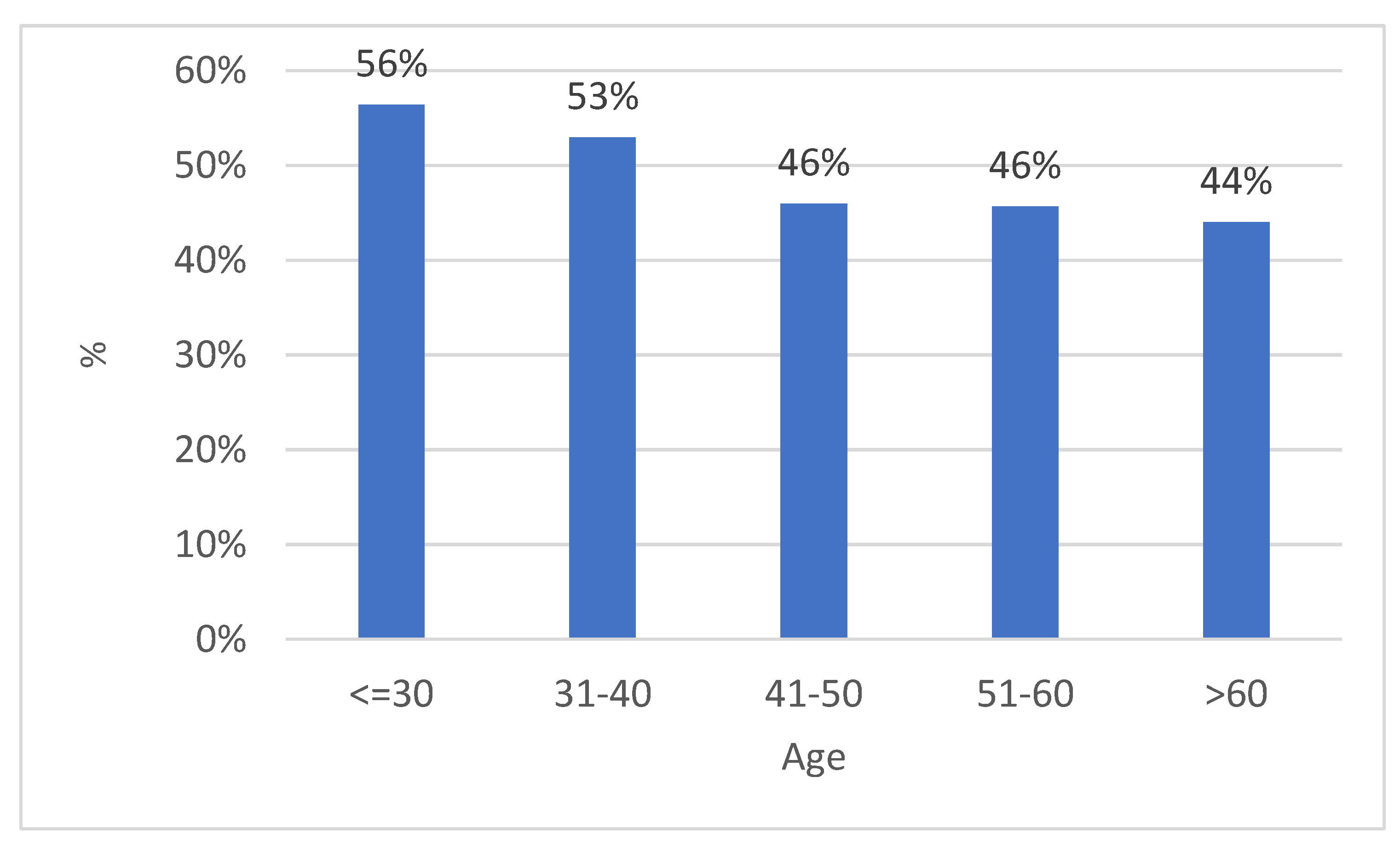
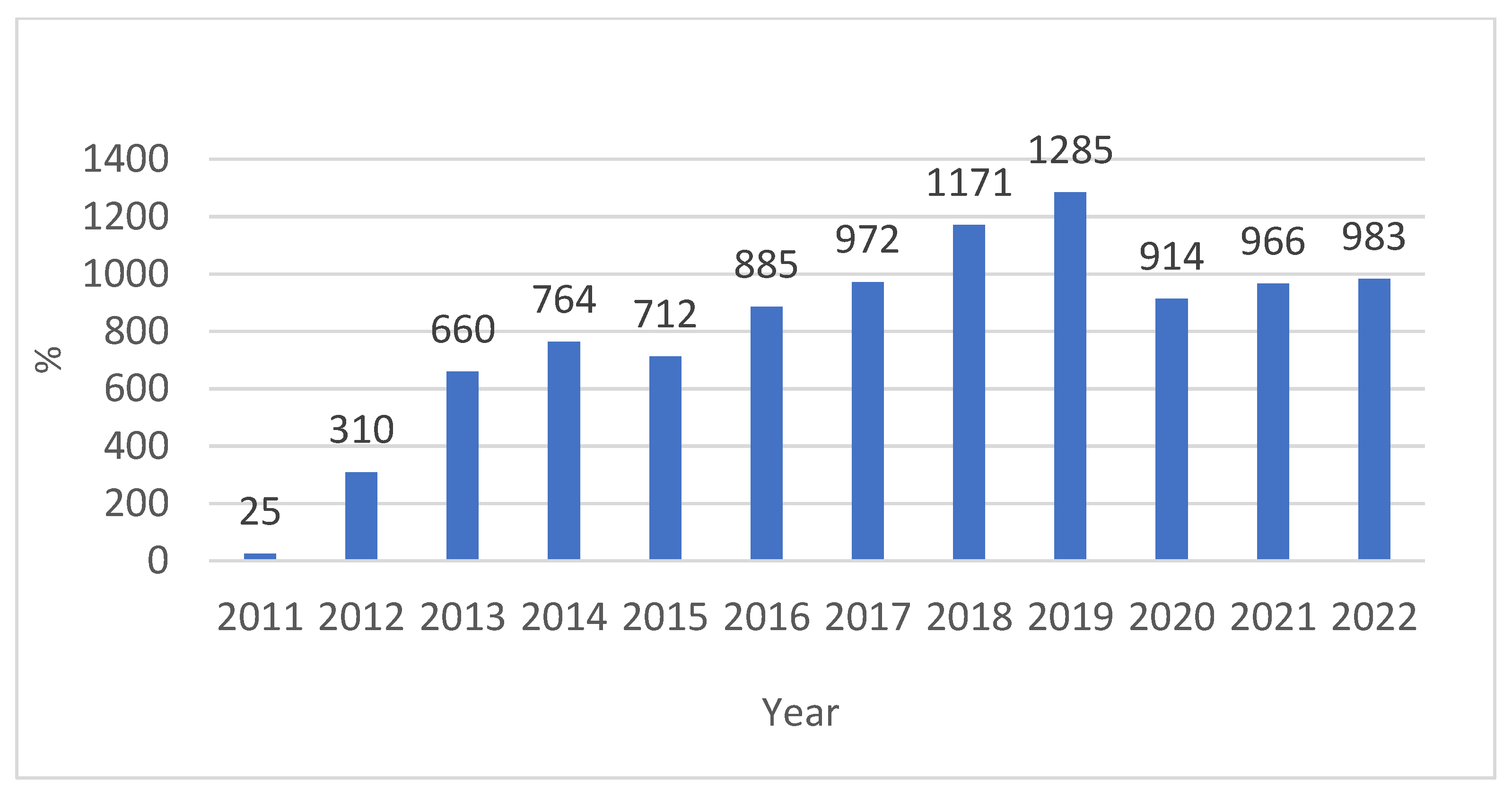
3. Results
4. Discussion
4.1. The Possible Role of Retrotransposition-L1 and Local Stem Cells in Increasing HPV Proliferative Capacity in Young Population Infection
4.2. HPV Relationship with Different Pathogens and the Mutual Benefits
4.3. The Role of Microbiota in the Carcinogenesis Process Induced by HPV in Adults and the Elderly
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Manini, I.; Montomoli, E. Epidemiology and prevention of Human Papillomavirus. Ann. Ig. 2018, 30, 28–32. [Google Scholar] [PubMed]
- International Agency for Research on Cancer. Agents Classified by the IARC Monographs; IARC: Lyon, France, 2018. [Google Scholar]
- Schiffman, M.; Wentzensen, N.; Wacholder, S.; Kinney, W.; Gage, J.C.; Castle, P.E. Human papillomavirus testing in the prevention of cervical cancer. J. Natl. Cancer Inst. 2011, 103, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Hughes, J.P.; Feng, Q.; Xi, L.F.; Cherne, S.; O’Reilly, S.; Kiviat, N.B.; Koutsky, L.A. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol. Biomark. Prev. 2011, 20, 699–707. [Google Scholar] [CrossRef]
- Woodman, C.B.; Collins, S.I.; Young, L.S. The natural history of cervical HPV infection: Unresolved issues. Nat. Rev. Cancer 2007, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Quick, M.C.; Hanamornroongruang, S.; Lai, K.; A Doyle, L.; McKeon, F.D.; Xian, W.; Crum, C.P.; Herfs, M. Microanatomy of the cervical and anorectal squamocolumnar junctions: A proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 2015, 28, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, A.C.; Gurgel, A.P.A.D.; Chagas, B.S.; Coimbra, E.C.; do Amaral, C.M.M. Susceptibility to cervical cancer: An overview. Gynecol. Oncol. 2012, 126, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.; Cheung, T.; Lin, C.K.; Siu, S.N.; Yim, S.; Lo, K.W.; Cheung, J.L.K.; Tam, A.O.; Tang, J.W. Association between HLA-DRB1 polymorphism, high-risk HPV infection and cervical neoplasia in southern Chinese. J. Med. Virol. 2007, 79, 970–976. [Google Scholar] [CrossRef]
- Chan, P.K.; Cheung, J.L.; Cheung, T.-H.; Lin, C.; Siu, S.-S.N.; Yu, M.M.; Tang, J.W.; Lo, K.W.; Yim, S.-F.; Wong, Y.; et al. HLA-DQB1 polymorphisms and risk for cervical cancer: A case-control study in a southern Chinese population. Gynecol. Oncol. 2007, 105, 736–741. [Google Scholar] [CrossRef]
- Del Prete, R.; Ronga, L.; Magrone, R.; Addati, G.; Abbasciano, A.; Di Carlo, D.; Miragliotta, G. Epidemiological evaluation of human papillomavirus genotypes and their associations in multiple infections. Epidemiol. Infect. 2019, 147, e132. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.G.; Rodriguez, A.C.; Castle, P.E.; Herrero, R.; Hildesheim, A.; Katki, H.; Kim, J.J.; Wacholder, S.; Morales, J.; Burk, R.D.; et al. Persistence of concurrent infections with multiple human papillomavirus types: A population-based cohort study. J. Infect. Dis. 2011, 203, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Kjær, S.K.; Frederiksen, K.; Munk, C.; Iftner, T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: Role of persistence. J. Natl. Cancer Inst. 2010, 102, 1478–1488. [Google Scholar] [CrossRef]
- Mangieri, L.F.L.; Cezar-dos-Santos, F.; Trugilo, K.P.; Watanabe, M.A.E.; de Jaime Curti, R.R.; Castilha, E.P.; Moretto, S.L.; Fernandes, C.Y.M.; de Oliveira, J.N.; de Oliveira, K.B. Cross-Sectional Analysis of Human Papillomavirus Infection and Cytological Abnormalities in Brazilian Women. Pathogens 2023, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, W.-Y.; Lin, T.-W.; Tseng, Y.-J.; Wang, Y.-C.; Yu, J.-R.; Chung, C.-R.; Wang, H.-Y. Trends of HPV Molecular Epidemiology in the Post-Vaccine Era: A 10-Year Study. Viruses 2023, 15, 2015. [Google Scholar] [CrossRef]
- Han, X.; Song, G.; Li, Y.; Dong, Z.; Yan, X.; Wang, S.; Tian, H.; Wu, X.; Li, C.; Huo, Y. Prevalence and genotype distribution of human papillomavirus infection among women aged 30–65 years in Xi’an, China: A population-based study of 14,655 women. Hum. Vaccines Immunother. 2021, 17, 5439–5446. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Herrero, R.; Clifford, G.M.; Snijders, P.J.; Arslan, A.; Anh, P.T.; Bosch, F.X.; Ferreccio, C.; Hieu, N.T.; Lazcano-Ponce, E.; et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int. J. Cancer 2006, 119, 2677–2684. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bagchi, P. Host Cell–Virus Interaction 2.0: Viral Stratagems of Immune Evasion, Host Cellular Responses and Antiviral Counterattacks. Viruses 2023, 15, 1717. [Google Scholar] [CrossRef]
- Stephenson-Tsoris, S.; Liang, T.J. Hepatitis Delta Virus–Host Protein Interactions: From Entry to Egress. Viruses 2023, 15, 1530. [Google Scholar] [CrossRef]
- Becker, N.; Maisner, A. Nipah Virus Impairs Autocrine IFN Signaling by Sequestering STAT1 and STAT2 into Inclusion Bodies. Viruses 2023, 15, 554. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Zhang, J.; Sun, J. Bacterial-Viral Interactions in Human Orodigestive and Female Genital Tract Cancers: A Summary of Epidemiologic and Laboratory Evidence. Cancers 2022, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.P.; Carvalho, B.M.; Coimbra, E.C. A Comprehensive View of the Cancer-Immunity Cycle (CIC) in HPV-Mediated Cervical Cancer and Prospects for Emerging Therapeutic Opportunities. Cancers 2023, 15, 1333. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, H.; Ha, K.T.; Pham, T.T.; Espinoza, J.L. Genetic Predisposition to Persistent Human Papillomavirus-Infection and Virus-Induced Cancers. Microorganisms 2021, 9, 2092. [Google Scholar] [CrossRef] [PubMed]
- Latsuzbaia, A.; Wienecke-Baldacchino, A.; Tapp, J.; Arbyn, M.; Karabegović, I.; Chen, Z.; Fischer, M.; Mühlschlegel, F.; Weyers, S.; Pesch, P.; et al. Characterization and Diversity of 243 Complete Human Papillomavirus Genomes in Cervical Swabs Using Next Generation Sequencing. Viruses 2020, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Olusola, P.; Banerjee, H.N.; Philley, J.V.; Dasgupta, S. Human Papilloma Virus-Associated Cervical Cancer and Health Disparities. Cells 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Escarcega-Tame, M.A.; López-Hurtado, M.; Escobedo-Guerra, M.R.; Reyes-Maldonado, E.; Castro-Escarpulli, G.; Guerra-Infante, F.M. Co-infection between genotypes of the human papillomavirus and Chlamydia trachomatis in Mexican women. Int. J. STD AIDS 2020, 31, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef]
- Chen, D.; Cui, T.; Ek, W.E.; Liu, H.; Wang, H.; Gyllensten, U. Analysis of the genetic architecture of susceptibility to cervical cancer indicates that common SNPs explain a large proportion of the heritability. Carcinogenesis 2015, 36, 992–998. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Sekine, M.; Hanley, S.J.B.; Kudo, R.; Hara, M.; Adachi, S.; Ueda, Y.; Miyagi, E.; Enomoto, T. Risk factors for HPV infection and high-grade cervical disease in sexually active Japanese women. Sci. Rep. 2021, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Law, M.H.; Neale, R.E.; Coman, W.B.; Pryor, D.I.; Porceddu, S.V.; Whiteman, D.C. Variants of EVER1 and EVER2 (TMC6 and TMC8) and human papillomavirus status in patients with mucosal squamous cell carcinoma of the head and neck. Cancer Causes Control 2016, 27, 809–815, Erratum in: Cancer Causes Control 2016, 27, 951. [Google Scholar] [CrossRef] [PubMed]
- El Kettani, A.; Ailal, F.; El Bakkouri, J.; Zerouali, K.; Béziat, V.; Jouanguy, E.; Casanova, J.-L.; Bousfiha, A.A. HPV-Related Skin Phenotypes in Patients with Inborn Errors of Immunity. Pathogens 2022, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Rajesh, A.; Innes, C.; van der Griend, R.; Fitzgerald, P.; Simcock, B.; Sykes, P.; Hibma, M. The High-Risk Human Papillomavirus Type Influences the Tissue Microenvironment in Cervical Intraepithelial Neoplasia Grade 2. Viruses 2023, 15, 1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, H.; Jia, Y.; Tang, F.; Zhou, H.; Li, X.; Zhou, J.; Huang, K.; Zhang, Q.; Hu, T.; et al. Association of 42 SNPs with genetic risk for cervical cancer: An extensive meta-analysis. BMC Med. Genet. 2015, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Leong, C.M.; Baxter, L.; Inglis, E.; Yun, K.; Backstrom, B.T.; Doorbar, J.; Hibma, M. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J. Virol. 2003, 77, 8378–8385. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejadian, E.; Zelini, P.; Cassaniti, I.; Baldanti, F.; Dominoni, M.; Gritti, A.; Gardella, B. Immunological Aspects of Human Papilloma Virus-Related Cancers Always Says, “I Am like a Box of Complexity, You Never Know What You Are Gonna Get”. Vaccines 2022, 10, 731. [Google Scholar] [CrossRef]
- Ferguson, R.; Ramanakumar, A.V.; Richardson, H.; Tellier, P.P.; Coutlée, F.; Franco, E.L.; Roger, M. Human leukocyte antigen (HLA)-E and HLA-G polymorphisms in human papillomavirus infection susceptibility and persistence. Hum. Immunol. 2011, 72, 337–341. [Google Scholar] [CrossRef]
- Evans, A.M.; Salnikov, M.; Tessier, T.M.; Mymryk, J.S. Reduced MHC Class I and II Expression in HPV-Negative vs. HPV-Positive Cervical Cancers. Cells 2022, 11, 3911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Jong, S.J.; Créquer, A.; Matos, I.; Hum, D.; Gunasekharan, V.; Lorenzo, L.; Jabot-Hanin, F.; Imahorn, E.; Arias, A.A.; Vahidnezhad, H.; et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to β-papillomaviruses. J. Exp. Med. 2018, 215, 2289–2310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Condrat, C.E.; Cretoiu, D.; Radoi, V.E.; Mihele, D.M.; Tovaru, M.; Bordea, C.I.; Voinea, S.C.; Suciu, N. Unraveling Immunological Dynamics: HPV Infection in Women—Insights from Pregnancy. Viruses 2023, 15, 2011. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells 2021, 10, 3104. [Google Scholar] [CrossRef]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune Responses against Human Papillomavirus (HPV) Infection and Evasion of Host Defense in Cervical Cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Hohenstein, E.; Rady, P.L.; Hergersberg, M.; Huber, A.R.; Tyring, S.K.; Bregenzer, T.; Streit, M.; Itin, P. Epidermodysplasia verruciformis in an HIV-positive patient homozygous for the c917A-->T polymorphism in the TMC8/EVER2 gene. Dermatology 2009, 218, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Chaberek, K.; Mrowiec, M.; Kaczmarek, M.; Dutsch-Wicherek, M. The Creation of the Suppressive Cancer Microenvironment in Patients with HPV-Positive Cervical Cancer. Diagnostics 2022, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Smola, S. RIPK3—A predictive marker for personalized immunotherapy? Oncoimmunology 2015, 5, e1075695. [Google Scholar] [CrossRef]
- Giacobbi, N.S.; Mullapudi, S.; Nabors, H.; Pyeon, D. The Chemokine CXCL14 as a Potential Immunotherapeutic Agent for Cancer Therapy. Viruses 2024, 16, 302. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Nakahama, Y.; Nguyen, V.; Espinoza, J.L. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms 2019, 7, 199. [Google Scholar] [CrossRef]
- Wang, Q.; Steger, A.; Mahner, S.; Jeschke, U.; Heidegger, H. The Formation and Therapeutic Update of Tumor-Associated Macrophages in Cervical Cancer. Int. J. Mol. Sci. 2019, 20, 3310. [Google Scholar] [CrossRef]
- DeFeo, C.J.; Weiss, C.D. Escape from Human Immunodeficiency Virus Type 1 (HIV-1) Entry Inhibitors. Viruses 2012, 4, 3859–3911. [Google Scholar] [CrossRef]
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624–2642. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef] [PubMed]
- Ouda, A.M.; Elsabagh, A.A.; Elmakaty, I.M.; Gupta, I.; Vranic, S.; Al-Thawadi, H.; Al Moustafa, A.-E. HPV and Recurrent Respiratory Papillomatosis: A Brief Review. Life 2021, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef]
- Ilboudo, M.; Zohoncon, T.M.; Traore, I.M.A.; Traore, E.M.A.; Kande, A.; Obiri-Yeboah, D.; Djigma, F.W.; Gyebre, Y.M.C.; Simpore, J. Implication of low-risk human papillomaviruses, HPV6 and HPV11 in laryngeal papillomatosis in Burkina Faso. Am. J. Otolaryngol. 2019, 40, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Chiantera, V.; Gerli, S.; Proietti, S.; Lepore, E.; Unfer, V.; Carugno, J.; Favilli, A. Preventing Persistence of HPV Infection with Natural Molecules. Pathogens 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Rositch, A.F.; Koshiol, J.; Hudgens, M.G.; Razzaghi, H.; Backes, D.M.; Pimenta, J.M.; Franco, E.L.; Poole, C.; Smith, J.S. Patterns of Persistent Genital Human Papillomavirus Infection among Women Worldwide: A Literature Review and Meta-Analysis. Int. J. Cancer 2013, 133, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Kim, M.K.; Seo, S.; Lee, D.O.; Chung, Y.K.; Lim, M.C.; Kim, J.; Lee, C.W.; Park, S. Alcohol Consumption and Persistent Infection of High-Risk Human Papillomavirus. Epidemiol. Infect. 2015, 143, 1442–1450. [Google Scholar] [CrossRef]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Lifsics, A.; Cistjakovs, M.; Sokolovska, L.; Deksnis, R.; Murovska, M.; Groma, V. The Role of the p16 and p53 Tumor Suppressor Proteins and Viral HPV16 E6 and E7 Oncoproteins in the Assessment of Survival in Patients with Head and Neck Cancers Associated with Human Papillomavirus Infections. Cancers 2023, 15, 2722. [Google Scholar] [CrossRef]
- Milan, G.; Guarducci, G.; Nante, N.; Montomoli, E.; Manini, I. Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap? Vaccines 2023, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Harshithkumar, R.; More, A.; Mukherjee, A. Human Papilloma Virus: An Unraveled Enigma of Universal Burden of Malignancies. Pathogens 2023, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 2019, 7, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Cosper, P.F.; Bradley, S.; Luo, L.; Kimple, R.J. Biology of HPV Mediated Carcinogenesis and Tumor Progression. Semin. Radiat. Oncol. 2021, 31, 265–273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flockerzi, A.; Ruggieri, A.; Frank, O.; Sauter, M.; Maldener, E.; Kopper, B.; Wullich, B.; Seifarth, W.; Muller-Lantzsch, N.; Leib-Mosch, C.; et al. Expression patterns of transcribed human endogenous retrovirus HERV-K(HML-2) loci in human tissues and the need for a HERV Transcriptome Project. BMC Genom. 2008, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Curty, G.; Menezes, A.N.; Brant, A.C.; de Mulder Rougvie, M.; Moreira, M.Â.M.; Soares, M.A. Expression of Retroelements in Cervical Cancer and Their Interplay with HPV Infection and Host Gene Expression. Cancers 2021, 13, 3513. [Google Scholar] [CrossRef]
- Zappacosta, R.; Lattanzio, G.; Viola, P.; Ianieri, M.M.; Gatta, D.M.; Rosini, S. A very rare case of HPV-53-related cervical cancer, in a 79-year-old woman with a previous history of negative Pap cytology. Clin. Interv. Aging 2014, 9, 683–688, Erratum in: Clin. Interv. Aging 2014, 9, 2103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Syed, A.S.; Marcuzzi, G.P.; Miller-Lazic, D.; Hess, J.; Hufbauer, M.; Akgül, B. HPV8 Reverses the Transcriptional Output in Lrig1 Positive Cells to Drive Skin Tumorigenesis. Cancers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Chénais, B. Transposable Elements and Human Diseases: Mechanisms and Implication in the Response to Environmental Pollutants. Int. J. Mol. Sci. 2022, 23, 2551. [Google Scholar] [CrossRef]
- Dewannieux, M.; Harper, F.; Richaud, A.; Letzelter, C.; Ribet, D.; Pierron, G.; Heidmann, T. Identification of an Infectious Progenitor for the Multiple-Copy HERV-K Human Endogenous Retroelements. Genome Res. 2006, 16, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Chénais, B. Transposable Elements in Cancer and Other Human Diseases. Curr. Cancer Drug Targets 2015, 15, 227–242. [Google Scholar] [CrossRef]
- Hanks, D.C.; Kazazian, H.H. Roles for Retrotransposon Insertions in Human Disease. Mob. DNA 2016, 7, 9. [Google Scholar] [CrossRef]
- Smola, S. Human Papillomaviruses and Skin Cancer. Adv. Exp. Med. Biol. 2020, 1268, 195–209. [Google Scholar] [PubMed]
- Akgül, B.; Ghali, L.; Davies, D.; Pfister, H.; Leigh, I.M.; Storey, A. HPV8 early genes modulate differentiation and cell cycle of primary human adult keratinocytes. Exp. Dermatol. 2007, 16, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Podgorska, M.; Oldak, M.; Marthaler, A.; Fingerle, A.; Walch-Ruckheim, B.; Lohse, S.; Muller, C.S.L.; Vogt, T.; Ustav, M.; Wnorowski, A.; et al. Chronic Inflammatory Microenvironment in Epidermodysplasia Verruciformis Skin Lesions: Role of the Synergism Between HPV8 E2 and C/EBPbeta to Induce Pro-Inflammatory S100A8/A9 Proteins. Front. Microbiol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Stumbrytė-Kaminskienė, A.; Gudlevičienė, Ž.; Dabkevičienė, D.; Mackevičienė, I. Combined Effect of HPV and Several Gene SNPs in Laryngeal Cancer. Medicine 2020, 56, 81. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. Human Papillomavirus and the Stroma: Bidirectional Crosstalk during the Virus Life Cycle and Carcinogenesis. Viruses 2017, 9, 219. [Google Scholar] [CrossRef]
- Russ, E.; Iordanskiy, S. Endogenous Retroviruses as Modulators of Innate Immunity. Pathogens 2023, 12, 162. [Google Scholar] [CrossRef]
- Sun, J.; Xiang, J.; An, Y.; Xu, J.; Xiong, Y.; Wang, S.; Xia, Q. Unveiling the Association between HPV and Pan-Cancers: A Bidirectional Two-Sample Mendelian Randomization Study. Cancers 2023, 15, 5147. [Google Scholar] [CrossRef]
- Liu, L.; Xie, Y.; Yang, H.; Lin, A.; Dong, M.; Wang, H.; Zhang, C.; Liu, Z.; Cheng, Q.; Zhang, J.; et al. HPVTIMER: A shiny web application for tumor immune estimation in human papillomavirus-associated cancers. iMeta 2023, 2, e130. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chiou, H.L.; Sheu, G.T.; Hsieh, L.L.; Chen, J.T.; Chen, C.Y.; Su, J.M.; Lee, H. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 2001, 61, 2799–2803. [Google Scholar]
- Tachezy, R.; Hrbacek, J.; Heracek, J.; Salakova, M.; Smahelova, J.; Ludvikova, V.; Svec, A.; Urban, M.; Hamsikova, E. HPV persistence and its oncogenic role in prostate tumors. J. Med. Virol. 2012, 84, 1636–1645. [Google Scholar] [CrossRef]
- Guerendiain, D.; Grigorescu, R.; Kirk, A.; Stevenson, A.; Holden, M.T.G.; Pan, J.; Kavanagh, K.; Graham, S.V.; Cuschieri, K. HPV status and HPV16 viral load in anal cancer and its association with clinical outcome. Cancer Med. 2022, 11, 4193–4203. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Ghasemian, E.; Harding-Esch, E.; Mabey, D.; Holland, M.J. When Bacteria and Viruses Collide: A Tale of Chlamydia trachomatis and Sexually Transmitted Viruses. Viruses 2023, 15, 1954. [Google Scholar] [CrossRef]
- Turman, B.J.; Darville, T.; O’Connell, C.M. Plasmid-mediated virulence in Chlamydia. Front. Cell Infect. Microbiol. 2023, 13, 1251135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gargiulo Isacco, C.; Balzanelli, M.G.; Garzone, S.; Lorusso, M.; Inchingolo, F.; Nguyen, K.C.D.; Santacroce, L.; Moscow, A.; Del Prete, R. Alterations of Vaginal Microbiota and Chlamydia trachomatis as Crucial Co-Causative Factors in Cervical Cancer Genesis Procured by HPV. Microorganisms 2023, 11, 662. [Google Scholar] [CrossRef]
- Sangpichai, S.; Patarapadungkit, N.; Pientong, C.; Ekalaksananan, T.; Chaiwiriyakul, S.; Thongbor, R.; Sirivech, P.; Jangsiriwitayakorn, P.; Triamwittayanon, T. Chlamydia trachomatis infection in high-risk human papillomavirus based on cervical cytology specimen. Asian Pac. J. Cancer Prev. 2019, 20, 3843–3847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ssedyabane, F.; Amnia, D.A.; Mayanja, R.; Omonigho, A.; Ssuuna, C.; Najjuma, J.N.; Freddie, B. HPV-Chlamydial coinfection, prevalence, and association with cervical intraepithelial lesions: A pilot study at Mbarara Regional Referral Hospital. J. Cancer Epidemiol. 2019, 2019, 9092565. [Google Scholar] [CrossRef]
- Rocca, A.; Braga, L.; Volpe, M.C.; Maiocchi, S.; Generali, D. The Predictive and Prognostic Role of RAS–RAF–MEK–ERK Pathway Alterations in Breast Cancer: Review of the Literature and Comparison with the Analysis of Cancer Genomic Datasets. Cancers 2022, 14, 5306. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, M.; Pruski, D.; Wszołek, K.; de Mezer, M.; Żurawski, J.; Jach, R.; Millert-Kalińska, S. Prevalence of HPV and Assessing Type-Specific HPV Testing in Cervical High-Grade Squamous Intraepithelial Lesions in Poland. Pathogens 2023, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Chaban, B.; Links, M.G.; Jayaprakash, T.P.; Wagner, E.C.; Bourque, D.K.; Lohn, Z.; Albert, A.Y.; van Schalkwyk, J.; Reid, G.; Hemmingsen, S.M.; et al. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome 2014, 2, 23. [Google Scholar] [CrossRef]
- de Paz-Silava, S.L.M.; Tabios, I.K.B.; Tantengco, O.A.G.; Climacosa, F.M.M.; Velayo, C.L.; Lintao, R.C.V.; Cando, L.F.T.; Perias, G.A.S.; Idolor, M.I.C.; Francisco, A.G.; et al. Determinants of Acquisition, Persistence, and Clearance of Oncogenic Cervical Human Papillomavirus Infection in the Philippines Using a Multi-Omics Approach: DEFEAT HPV Study Protocol. Healthcare 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef]
- Lewis, F.M.T.; Bernstein, K.T.; Aral, S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Gardella, B.; Pasquali, M.F.; La Verde, M.; Cianci, S.; Torella, M.; Dominoni, M. The Complex Interplay between Vaginal Microbiota, HPV Infection, and Immunological Microenvironment in Cervical Intraepithelial Neoplasia: A Literature Review. Int. J. Mol. Sci. 2022, 23, 7174. [Google Scholar] [CrossRef]
- Myers, E.R.; McCrory, D.C.; Nanda, K.; Bastian, L.; Matchar, D. Mathematical Model for the Natural History of Human Papillomavirus Infection and Cervical Carcinogenesis. Am. J. Epidemiol. 2000, 151, 1158–1171. [Google Scholar] [CrossRef]
- Głowienka-Stodolak, M.; Bagińska-Drabiuk, K.; Szubert, S.; Hennig, E.E.; Horala, A.; Dąbrowska, M.; Micek, M.; Ciebier, M.; Zeber-Lubecka, N. Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies. Cancers 2024, 16, 399. [Google Scholar] [CrossRef]
- Park, M.G.; Cho, S.; Oh, M.M. Menopausal Changes in the Microbiome—A Review Focused on the Genitourinary Microbiome. Diagnostics 2023, 13, 1193. [Google Scholar] [CrossRef]
- de Oliveira, N.S.; de Lima, A.B.F.; de Brito, J.C.R.; Sarmento, A.C.A.; Gonçalves, A.K.S.; Eleutério, J., Jr. Postmenopausal Vaginal Microbiome and Microbiota. Front. Reprod. Health 2021, 3, 780931. [Google Scholar] [CrossRef]
- Yang, M.; Wen, S.; Zhang, J.; Peng, J.; Shen, X.; Xu, L. Systematic Review and Meta-analysis: Changes of Gut Microbiota before and after Menopause. Dis. Mrk. 2022, 2022, 3767373. [Google Scholar] [CrossRef]
- Coscia, M.F.; Monno, R.; Ballini, A.; Mirgaldi, R.; Dipalma, G.; Pettini, F.; Cristallo, V.; Inchingolo, F.; Foti, C.; de Vito1, D. Human papilloma virus (HPV) genotypes prevalence in a region of South Italy (Apulia). Ann. Ist. Super. Sanità 2015, 51, 248–251. [Google Scholar] [CrossRef]
- Nickel, J.C.; Stephens-Shields, A.J.; Landis, J.R.; Mullins, C.; van Bokhoven, A.; Lucia, M.S.; Henderson, J.P.; Sen, B.; Krol, J.E.; Ehrlich, G.D. A Culture-Independent Analysis of the Microbiota of Female Interstitial Cystitis/Bladder Pain Syndrome Participants in the MAPP Research Network. J. Clin. Med. 2019, 8, 415. [Google Scholar] [CrossRef]
- Vaughan, M.H.; Mao, J.; Karstens, L.A.; But, L.; Amundsen, C.L.; Schmader, K.E.; Siddiqui, N.Y. The Urinary Microbiome in Postmenopausal Women with Recurrent Urinary Tract Infections. J. Urol. 2021, 206, 1222–1231. [Google Scholar] [CrossRef]
- Cascardi, E.; Cazzato, G.; Daniele, A.; Silvestris, E.; Cormio, G.; Di Vagno, G.; Malvasi, A.; Loizzi, V.; Scacco, S.; Pinto, V.; et al. Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology 2022, 11, 1114. [Google Scholar] [CrossRef]


| Total | Male | Female | |||
|---|---|---|---|---|---|
| Infected | % of All M | Infected | % of All F | ||
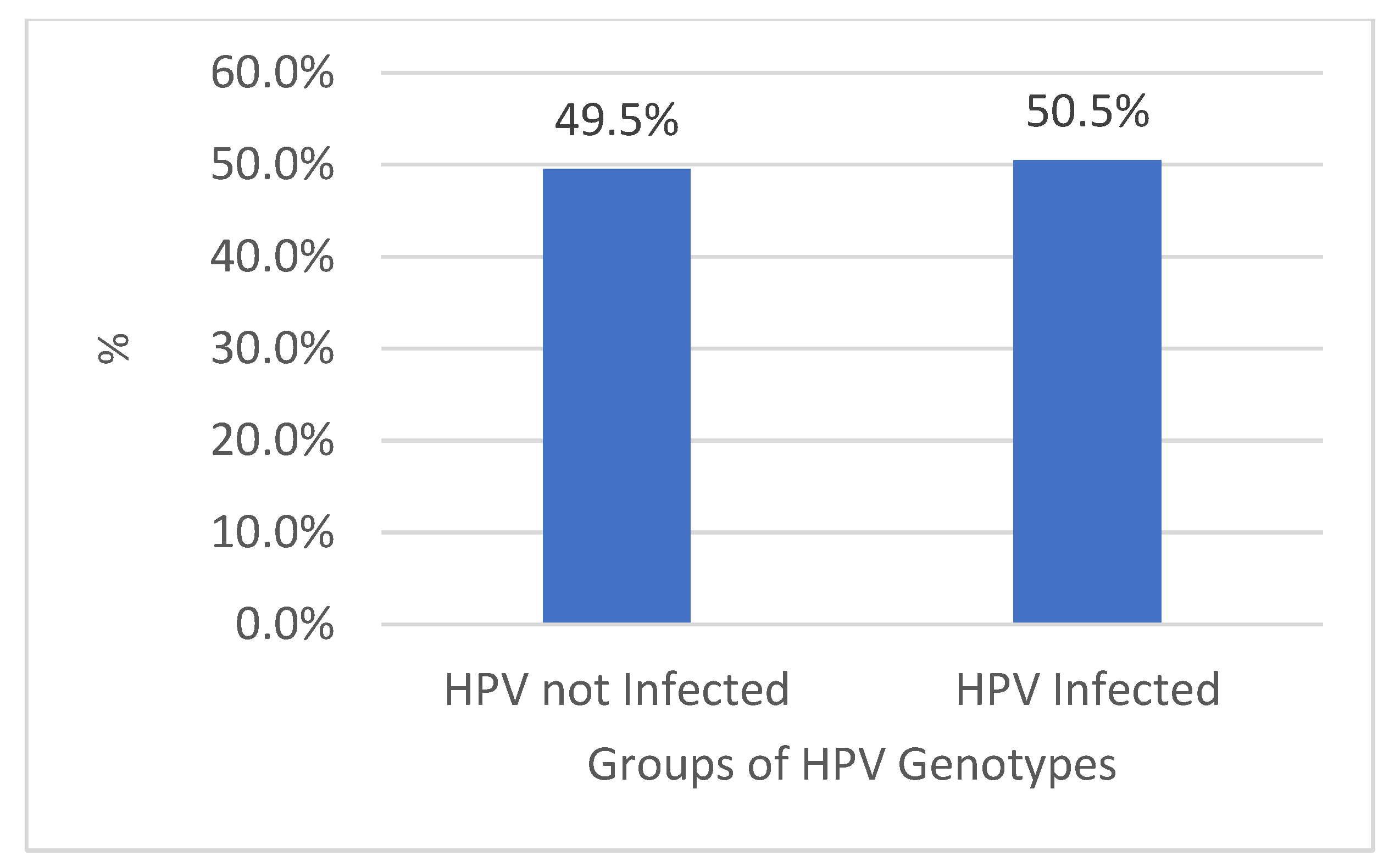
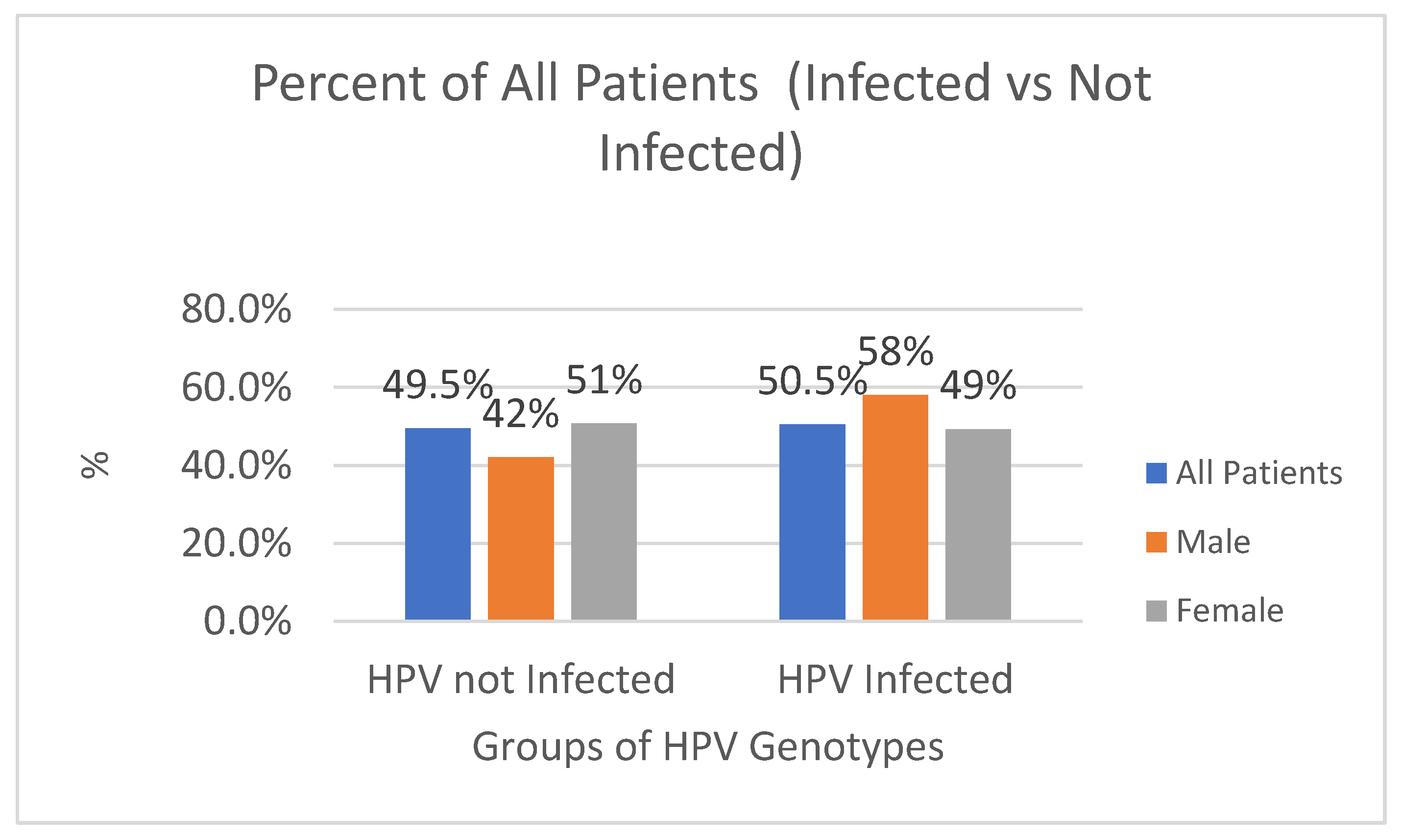
| HPV not Infected | 4778 | 554 | 42% | 4224 | 51% |
| HPV Infected | 4869 | 764 | 58% | 4105 | 49% |
| Total | 9647 | 1318 | 100% | 8329 | 100% |
| All Genders | Male | Female | |||||
|---|---|---|---|---|---|---|---|
| Genotypes | Number | % All Genders | Number | % from Males | Number | % from Females | |
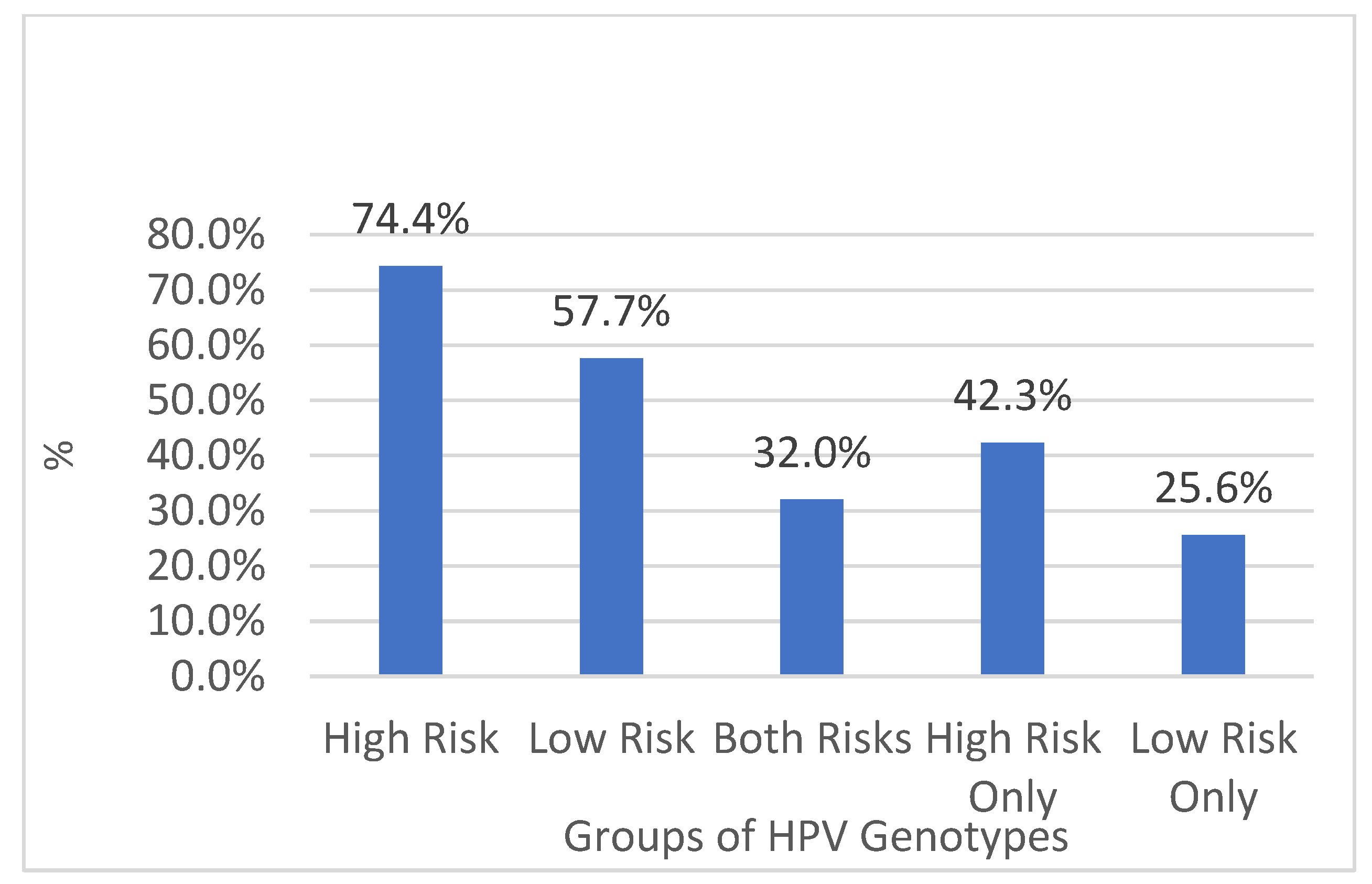
| HPV High-Risk Genotypes | High Risk | 3621 | 74% | 445 | 58% | 3176 | 77% |
| HPV Low-Risk Genotypes | Low Risk | 2807 | 58% | 554 | 73% | 2253 | 55% |
| HPV Both Risk Genotypes | Both | 1559 | 32% | 235 | 31% | 1324 | 32% |
| HPV High-Risk Genotypes Only | High Only | 2062 | 42% | 210 | 27% | 1852 | 45% |
| HPV Low-Risk Genotypes Only | Low Only | 1248 | 26% | 319 | 42% | 929 | 23% |
| HPV Infected Total | Total Infected | 4869 | 100% | 764 | 100% | 4105 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Prete, R.; Nesta, D.; Triggiano, F.; Lorusso, M.; Garzone, S.; Vitulano, L.; Denicolò, S.; Indraccolo, F.; Mastria, M.; Ronga, L.; et al. Human Papillomavirus Carcinogenicity and the Need of New Perspectives: Thoughts from a Retrospective Analysis on Human Papillomavirus Outcomes Conducted at the Hospital University of Bari, Apulia, Italy, between 2011 and 2022. Diagnostics 2024, 14, 968. https://doi.org/10.3390/diagnostics14090968
Del Prete R, Nesta D, Triggiano F, Lorusso M, Garzone S, Vitulano L, Denicolò S, Indraccolo F, Mastria M, Ronga L, et al. Human Papillomavirus Carcinogenicity and the Need of New Perspectives: Thoughts from a Retrospective Analysis on Human Papillomavirus Outcomes Conducted at the Hospital University of Bari, Apulia, Italy, between 2011 and 2022. Diagnostics. 2024; 14(9):968. https://doi.org/10.3390/diagnostics14090968
Chicago/Turabian StyleDel Prete, Raffaele, Daniela Nesta, Francesco Triggiano, Mara Lorusso, Stefania Garzone, Lorenzo Vitulano, Sofia Denicolò, Francesca Indraccolo, Michele Mastria, Luigi Ronga, and et al. 2024. "Human Papillomavirus Carcinogenicity and the Need of New Perspectives: Thoughts from a Retrospective Analysis on Human Papillomavirus Outcomes Conducted at the Hospital University of Bari, Apulia, Italy, between 2011 and 2022" Diagnostics 14, no. 9: 968. https://doi.org/10.3390/diagnostics14090968
APA StyleDel Prete, R., Nesta, D., Triggiano, F., Lorusso, M., Garzone, S., Vitulano, L., Denicolò, S., Indraccolo, F., Mastria, M., Ronga, L., Inchingolo, F., Aityan, S. K., Nguyen, K. C. D., Tran, T. C., Gargiulo Isacco, C., & Santacroce, L. (2024). Human Papillomavirus Carcinogenicity and the Need of New Perspectives: Thoughts from a Retrospective Analysis on Human Papillomavirus Outcomes Conducted at the Hospital University of Bari, Apulia, Italy, between 2011 and 2022. Diagnostics, 14(9), 968. https://doi.org/10.3390/diagnostics14090968









