Lifetime Attributable Risk in Mammography Screenings in Dubai: The Influence of Breast Thickness and Age on Radiation Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Scanning Acquisition
2.2. Dose Measurement
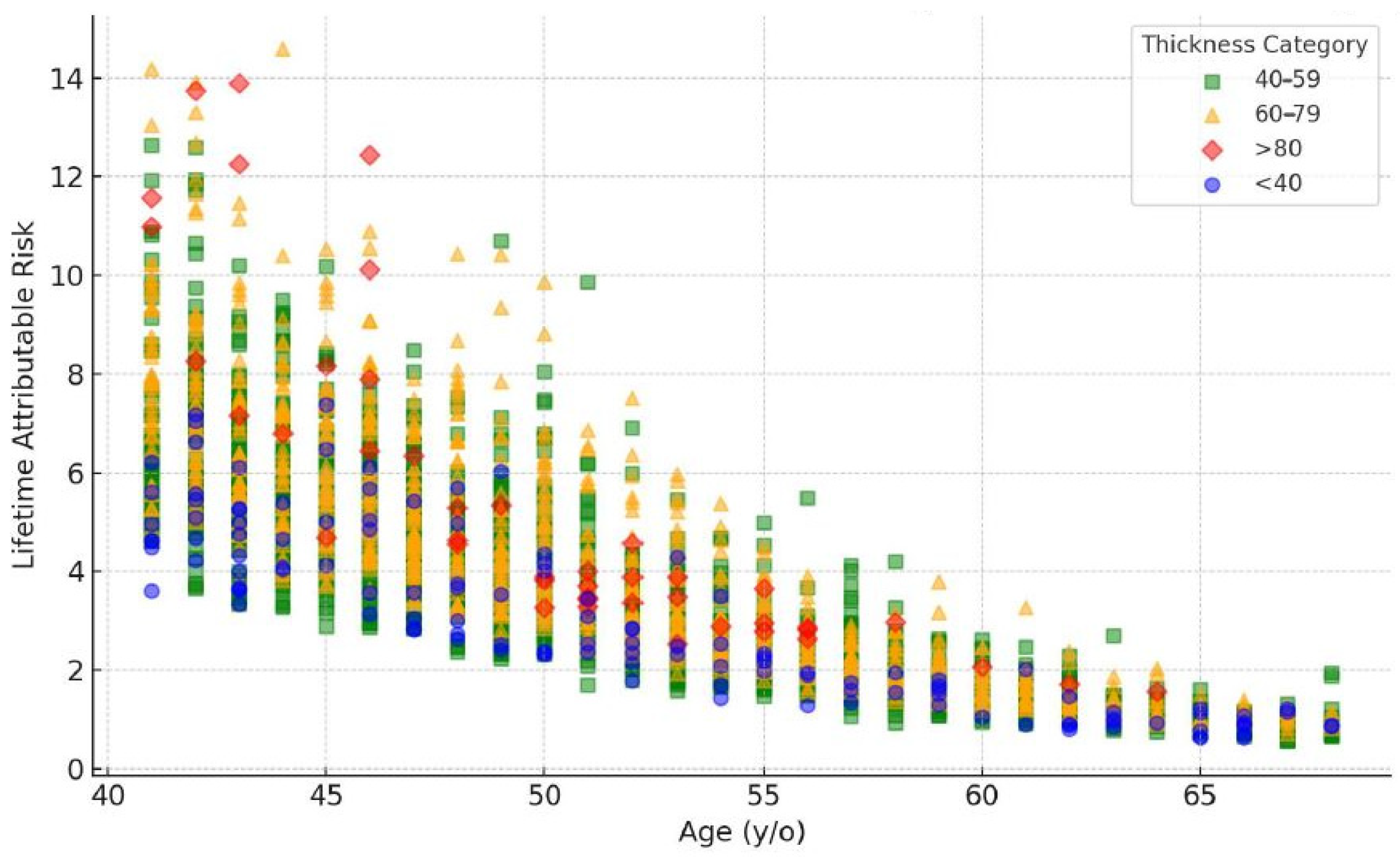
2.3. Radiation Risks
2.4. Data Analysis
3. Results
3.1. Patient Demographic
3.2. Dose–Thickness Correlation
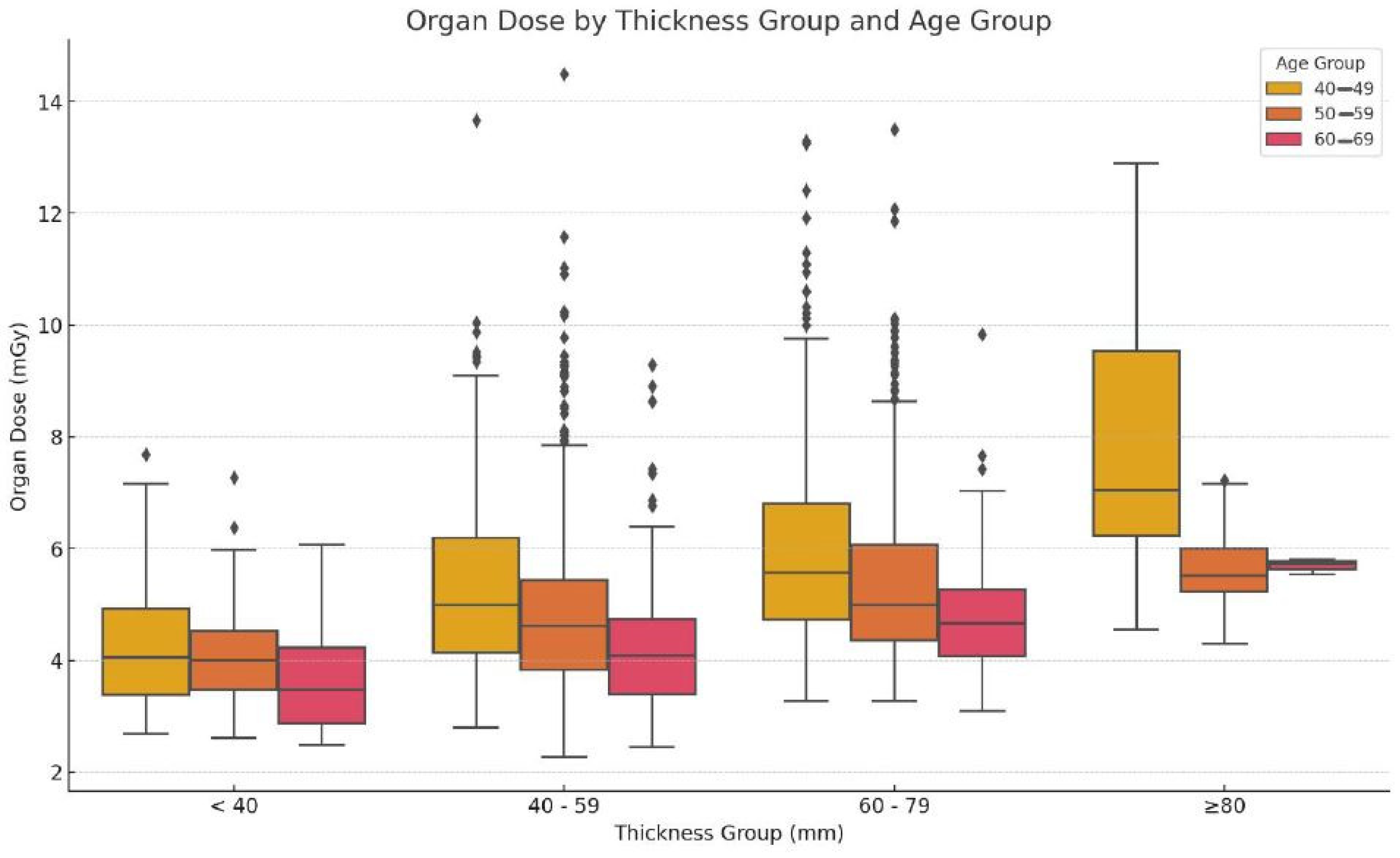
3.3. Dose Distribution across Age and Thickness
3.4. Statistical Analysis
3.5. Summery of Key Results
4. Discussion
4.1. Interpretation of Findings
4.2. Comparison with the Literature
4.3. Clinical Implications
4.4. Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alorabi, L.; Algorashi, E. Accuracy of breast cancer screening using film mammography in comparison to digital mammography. Egypt. J. Hosp. Med. 2018, 70, 1947–1951. [Google Scholar] [CrossRef]
- Miglioretti, D.L.; Zhu, W.; Kerlikowske, K.; Sprague, B.L.; Onega, T.; Buist, D.S.M. Breast cancer screening in the era of density notification legislation: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2020, 112, 1144–1154. [Google Scholar] [CrossRef]
- Yaffe, M.J.; Mainprize, J.G. Risk of radiation-induced breast cancer from mammographic screening. Radiology 2011, 258, 98–105, Erratum in Radiology 2012, 264, 306. [Google Scholar] [CrossRef] [PubMed]
- Feig, S.A.; Hendrick, R.E. Radiation risk from screening mammography of women aged 40–49 years. J. Natl. Cancer Inst. Monogr. 1997, 22, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sechopoulos, I.; Teuwen, J.; Mann, R. Artificial intelligence for breast cancer detection in mammography and digital breast tomosynthesis: State of the art. Semin. Cancer Biol. 2021, 72, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Albariqi, S.M.S. Preliminary Investigations of Radiation Dose and Cancer Risks from Breast Imaging Studies. Ph.D. Thesis, Alfaisal University, Riyadh, Saudi Arabia, 2023. [Google Scholar]
- Hendrick, R.E. Radiation doses and risks in breast screening. J. Breast Imaging 2020, 2, 188–200. [Google Scholar] [CrossRef]
- Ali, R.M.K.M.; England, A.; McEntee, M.F.; Mercer, C.E.; Tootell, A.; Hogg, P. Effective lifetime radiation risk for a number of national mammography screening programmes. Radiography 2018, 24, 240–246. [Google Scholar] [CrossRef]
- Houssami, N.; Zackrisson, S.; Blazek, K.; Hunter, K.; Bernardi, D.; Lång, K.; Hofvind, S. Meta-analysis of prospective studies evaluating breast cancer detection and interval cancer rates for digital breast tomosynthesis versus mammography population screening. Eur. J. Cancer 2021, 148, 14–23. [Google Scholar] [CrossRef]
- Kritsaneepaiboon, S.; Jutiyon, A.; Krisanachinda, A. Cumulative radiation exposure and estimated lifetime cancer risk in multiple-injury adult patients undergoing repeated or multiple CTs. Eur. J. Trauma Emerg. Surg. 2018, 44, 19–27. [Google Scholar] [CrossRef]
- Hooshmand, S.; Reed, W.M.; Suleiman, M.E.; Brennan, P.C. A review of screening mammography: The benefits and radiation risks put into perspective. J. Med. Imaging Radiat. Sci. 2022, 53, 147–158. [Google Scholar] [CrossRef]
- Boyd, N.F.; Martin, L.J.; Bronskill, M.; Yaffe, M.J.; Duric, N.; Minkin, S. Breast tissue composition and susceptibility to breast cancer. J. Natl. Cancer Inst. 2010, 102, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.V.; Williams, M.B.; Patrie, J.T.; Harvey, J.A. Do women with dense breasts have higher radiation dose during screening mammography? Breast J. 2018, 24, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Miglioretti, D.L.; Lange, J.; van den Broek, J.J.; Lee, C.I.; van Ravesteyn, N.T.; Ritley, D.; Kerlikowske, K.; Fenton, J.J.; Melnikow, J.; de Koning, H.J.; et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: A modeling study. Ann. Intern. Med. 2016, 164, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Mohamad, M.A.; Md Zin, R.R. Histopathological correlation of breast carcinoma with breast imaging-reporting and data system. Malays. J. Med. Sci. 2022, 29, 65–74. [Google Scholar] [CrossRef]
- Ennab, F.; Tsagkaris, C.; Babar, M.S.; Tazyeen, S.; Kokash, D.; Mago, A.; Nawaz, F.A.; Al-Shamsi, H.O. A potential rise of breast cancer risk in the UAE post-COVID-19 lockdown: A call for action. Ann. Med. Surg. 2022, 79, 103976. [Google Scholar] [CrossRef]
- Freer, P.E.; Slanetz, P.J.; Haas, J.S.; Tung, N.M.; Hughes, K.S.; Armstrong, K.; Semine, A.A.; Troyan, S.L.; Birdwell, R.L. Breast Cancer Screening in the Era of Density Notification Legislation: Summary of 2014 Massachusetts Experience and Suggestion of an Evidence-Based Management Algorithm by Multi-Disciplinary Expert Panel. Breast Cancer Res. Treat. 2015, 153, 455–464. [Google Scholar] [CrossRef][Green Version]
- Conant, E.F.; Barlow, W.E.; Herschorn, S.D.; Weaver, D.L.; Beaber, E.F.; Tosteson, A.N.A.; Haas, J.S.; Lowry, K.P.; Stout, N.K.; Trentham-Dietz, A.; et al. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol. 2019, 5, 635–642. [Google Scholar] [CrossRef]
- Demb, J.; Abraham, L.; Miglioretti, D.L.; Sprague, B.L.; O’meara, E.S.; Advani, S.; Henderson, L.M.; Onega, T.; Buist, D.S.M.; Schousboe, J.T.; et al. Screening mammography outcomes: Risk of breast cancer and mortality by comorbidity score and age. J. Natl. Cancer Inst. 2020, 112, 599–606. [Google Scholar] [CrossRef]
- Lokate, M.; Stellato, R.K.; Veldhuis, W.B.; Peeters, P.H.; van Gils, C.H. Age-related changes in mammographic density and breast cancer risk. Am. J. Epidemiol. 2013, 178, 101–109. [Google Scholar] [CrossRef]
- Hooshmand, S.; Reed, W.M.; Suleiman, M.E.; Brennan, P.C. RRIMS: Radiation risk in mammography screening—Model evaluation. Br. J. Radiol. 2023, 96, 20230250. [Google Scholar] [CrossRef]
- Halid, B.; Karim, M.; Sabarudin, A.; Bakar, K.; Shariff, N.D. Assessment of lifetime attributable risk of stomach and colon cancer during abdominal CT examinations based on Monte Carlo Simulation. In 6th International Conference on the Development of Biomedical Engineering (BME6) in Vietnam; Springer: Singapore, 2018; pp. 455–459. [Google Scholar] [CrossRef]
- Mohd Norsuddin, N.; Mei Sin, J.G.; Ravintaran, R.; Arasaratnam, S.; Abdul Karim, M.K. Impact of age and breast thickness on mean glandular dose of standard digital mammography and digital breast tomosynthesis. Appl. Radiat. Isot. 2023, 192, 110525. [Google Scholar] [CrossRef] [PubMed]
- Sechopoulos, I. A review of breast tomosynthesis. Part II. Image reconstruction, processing and analysis, and advanced applications. Med. Phys. 2013, 40, 014302. [Google Scholar] [CrossRef]
- Mohd Norsuddin, N.; Segar, S.; Ravintaran, R.; Mohd Zain, N.; Abdul Karim, M.K. Local diagnostic reference levels for full-field digital mammography and digital breast tomosynthesis in a tertiary hospital in Malaysia. Healthcare 2022, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, R.E.; Baker, J.A.; Helvie, M.A. Breast cancer deaths averted over 3 decades. Cancer 2019, 125, 1482–1488. [Google Scholar] [CrossRef]
- Houssami, N.; Bernardi, D.; Pellegrini, M.; Valentini, M.; Fantò, C.; Ostillio, L.; Tuttobene, P.; Luparia, A.; Macaskill, P. Breast cancer detection using single-reading of breast tomosynthesis (3D-mammography) compared to double-reading of 2D-mammography: Evidence from a population-based trial. Cancer Epidemiol. 2017, 47, 94–99. [Google Scholar] [CrossRef]
- Abdulwahid Noor, K.; Mohd Norsuddin, N.; Abdul Karim, M.K.; Che Isa, I.N.; Alshamsi, W. Estimating local diagnostic reference levels for mammography in Dubai. Diagnostics 2023, 14, 8. [Google Scholar] [CrossRef]
- Gennaro, G.; Ferro, F.; Contento, G.; Fornasin, F.; di Maggio, C. Automated analysis of phantom images for the evaluation of long-term reproducibility in digital mammography. Phys. Med. Biol. 2007, 52, 1387–1407. [Google Scholar] [CrossRef]
- Da Silva, S.D.; Joana, G.S.; Oliveira, B.B.; De Oliveira, M.A.; Leyton, F.; Nogueira, M.D.S. Dosimetry and image quality in digital mammography facilities in the State of Minas Gerais, Brazil. Radiat. Phys. Chem. 2015, 116, 292–299. [Google Scholar] [CrossRef]
- Wang, Y.; Kung, L.; Byrd, T.A. Big data analytics: Understanding its capabilities and potential benefits for healthcare organizations. Technol. Forecast. Soc. Chang. 2018, 126, 3–13. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Edwards, S.A.; Prummel, M.V.; Muradali, D.; Majpruz, V.; Done, S.J.; Brown, P.; Shumak, R.S.; Yaffe, M.J. Digital compared with screen-film mammography: Performance measures in concurrent cohorts within an organized breast screening program. Radiology 2013, 268, 684–693. [Google Scholar] [CrossRef]
- Sechopoulos, I.; Boone, J.M.; Dance, D.; van Engen, R.; Russo, P.; Young, K.C. Mammography dose estimates do not reflect any specific patient’s breast dose. Eur. J. Radiol. 2020, 131, 109216. [Google Scholar] [CrossRef] [PubMed]
- Dalah, E.Z.; Alkaabi, M.K.; Al-Awadhi, H.M.; Antony, N.A. Screening mammography diagnostic reference level system according to compressed breast thickness: Dubai Health. J. Imaging 2024, 10, 188. [Google Scholar] [CrossRef] [PubMed]


| Category | n | Parameter (Mean ± SD) (Min–Max) | ||||||
|---|---|---|---|---|---|---|---|---|
| Age | Age | Tube Voltage (kV) | Tube Current (mAs) | Compression Force (N) | Thickness (mm) | Entrance Dose (mGy) | Organ Dose (mGy) | |
| 40–49 | 1147 | 45.09 ± 2.55 (41.00–49.00) | 29.31 ± 1.1 (26–32) | 108.83 ± 39.34 (39–305) | 92.89 ± 39.46 (28.4–196.9) | 57.3 ± 10.96 (24–93) | 4.98 ± 2.05 (1.44–16.02) | 5.54 ± 1.66 (2.69–13.66) |
| 50–59 | 1073 | 54.00 ± 2.84 (50.00–59.00) | 29.36 ± 1.05 (26–32) | 98.7 ± 34.52 (33–299) | 92.69 ± 38.41 (29.1–194.3) | 57.64 ± 10.25 (22–96) | 4.57 ± 1.86 (1.15–16.46) | 5.09 ± 1.54 (2.27–14.49) |
| 60–69 | 381 | 63.16 ± 2.59 (60.00–68.00) | 29.13 ± 1.1 (24–32) | 81.52 ± 24.66 (34–199) | 90.66 ± 38.96 (29.3–190.4) | 55.28 ± 10.46 (15–82) | 3.78 ± 1.32 (0.98–9.7) | 4.38 ± 1.12 (2.45–9.83) |
| All Patients | 2601 | 51.41 ± 6.91 (41.00–68.00) | 29.30 ± 1.08 (24.00–32.00) | 100.65 ± 36.70 (33.00–305.00) | 92.48 ± 38.95 (28.40–196.90) | 57.15 ± 10.62 (15.00–96.00) | 4.64 ± 1.92 (0.98–16.46) | 5.19 ± 1.59 (2.27–14.49) |
| Breast Thickness (mm) | Organ Dose (mGy) by Age Group | One-Way ANOVA | Pair-Wise Comparison |
|---|---|---|---|
| 40–49 years | 50–59 years | 60–69 years | 40–49 vs. 50–59 |
| <40 | 4.31 ± 1.17 | 4.15 ± 0.98 | 3.64 ± 0.95 |
| 40–59 | 5.25 ± 1.49 | 4.87 ± 1.51 | 4.21 ± 1.12 |
| 60–79 | 5.95 ± 1.68 | 5.44 ± 1.57 | 4.78 ± 1.00 |
| ≥80 | 7.93 ± 2.36 | 5.69 ± 0.81 | 5.69 ± 0.14 |
| Series | Parameter (Mean ± SD, (Min–Max)) | |||||
|---|---|---|---|---|---|---|
| Tube Voltage (kV) | Tube Current (mAs) | Compression Force (N) | Thickness (mm) | Entrance Dose (mGy) | Organ Dose (mGy) | |
| LCC | 29.3 ± 1.1 (24–32) | 100.7 ± 36.7 (33–305) | 92.5 ± 38.9 (28.4–196.9) | 57.1 ± 10.6 (15–96) | 4.63 ± 1.9 (0.98–16.46) | 1.21 ± 0.39 (0.56–3.9) |
| LMLO | 29.8 ± 1.2 (24–32) | 118.2 ± 45.4 (17–418) | 110.6 ± 44.2 (28.2–213.4) | 61.9 ± 11.9 (16–100) | 5.6 ± 2.5 (1.01–24.2) | 1.4 ± 0.5 (0.31–4.66) |
| RCC | 29.7 ± 1.03 (28–32) | 109.3 ± 44.2 (50–245) | 76.5 ± 20.3 (41.6–119.7) | 60.0 ± 11.5 (41–90) | 4.9 ± 2.4 (1.83–11.4) | 1.24 ± 0.45 (0.65–2.65) |
| RMLO | 29.8 ± 1.17 (24–32) | 118.4 ± 46.7 (33–430) | 114.4 ± 42.9 (28–198.1) | 62.2 ± 11.9 (13–102) | 5.7 ± 2.5 (0.89–24.1) | 1.38 ± 0.46 (0.57–4.43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, K.A.M.; Norsuddin, N.M.; Isa, I.N.C.; Abdul Karim, M.K. Lifetime Attributable Risk in Mammography Screenings in Dubai: The Influence of Breast Thickness and Age on Radiation Exposure. Diagnostics 2025, 15, 83. https://doi.org/10.3390/diagnostics15010083
Noor KAM, Norsuddin NM, Isa INC, Abdul Karim MK. Lifetime Attributable Risk in Mammography Screenings in Dubai: The Influence of Breast Thickness and Age on Radiation Exposure. Diagnostics. 2025; 15(1):83. https://doi.org/10.3390/diagnostics15010083
Chicago/Turabian StyleNoor, Kaltham Abdulwahid Mohd, Norhashimah Mohd Norsuddin, Iza Nurzawani Che Isa, and Muhammad Khalis Abdul Karim. 2025. "Lifetime Attributable Risk in Mammography Screenings in Dubai: The Influence of Breast Thickness and Age on Radiation Exposure" Diagnostics 15, no. 1: 83. https://doi.org/10.3390/diagnostics15010083
APA StyleNoor, K. A. M., Norsuddin, N. M., Isa, I. N. C., & Abdul Karim, M. K. (2025). Lifetime Attributable Risk in Mammography Screenings in Dubai: The Influence of Breast Thickness and Age on Radiation Exposure. Diagnostics, 15(1), 83. https://doi.org/10.3390/diagnostics15010083







